Sir
Endometrial cancer (EC) is the most common gynecologic malignancy in developed countries. Recently, glycolytic enzymes have gained considerable attention in human cancers where glucose is the primary source of energy, and a high rate of glycolysis, which is the hallmark of cancer cells, provides the tumor with metabolic and survival advantages.1,2 Aldolase is an enzyme that is critical for the glycolytic pathway. Furthermore, an increased aldolase expression was found in various tumor types including cervical, endometrial, kidney, lung cancers and most recently in EC.3–6 An important regulator of glycolysis, namely clotrimazole (antifungal azole and calmodulin antagonist), was found to manipulate the binding of glycolytic enzymes to the cytoskeleton, which in turn modulates cell function, proliferation and differentiation.7,8 Herein, we evaluated cases of EC for aldolase mRNA expression and the effect of clotrimazole on EC cell lines.
RNA was extracted from 70 patients with the diagnosis of endometrial adenocarcinoma. The expression of aldolaseC was determined using Taqman. qRT-PCR gene expression Assay On Demand Probe ⁄ Primers (Applied Biosystems, Foster City, CA), with housekeeping gene GAPDH as an endogenous control. Samples were run, with three replicate assays for each gene in each sample. For each assay, the average GAPDH Ct (Cycle threshold) value in the TaqMan qPCR assay was subtracted from the Ct aldolaseC to obtain a ΔCt value (aldolaseC – GAPDH).
Endometrial cancer cell lines including HEC1 and RL95 and colon cancer cell line (CT-26) (chosen as a control) were purchased from the American Tissue Culture Collection (ATCC, Manassas, VA) and they were cultivated according to the supplier’s recommendations. The cells were incubated in 5% CO2 at 37C in PBS containing 5 mM glucose in the absence and presence of 50uM clotrimazole (Sigma, St-Louis, Missouri). Clotrimazole was added to the cultures at different time points (1 h, 4 h, 8 h, 12 h, 18 h and 24 h). Untreated and clotrimazole treated cells were done in triplicate and washed one time with PBS and then harvested with trypsin. The cells were counted in a hemocytometer. Cell viability was determined by trypan blue dye exclusion. Transmission electron microscopy was done on the treated ⁄ and non-treated cells as described previously.9 For each assay, the average GAPDH Ct (Cycle threshold) value in the TaqMan qPCR assay was subtracted from the Ct of Aldolase to obtain a delta Ct value (Aldolase – GAPDH). Linear regression analyses were used to examine the association between the aldolaseC qRT-PCR levels and the patients’ clinicopathologic characteristics. Cox regression analyses were used to examine the relation of aldolaseC expression on survival. All statistical analysis was performed using the computing environment R (http://www.r-project.org/).
The clinical and pathology data of the 70 patients with the qRT-PCR measurement are summarized in Table 1. High aldolaseC mRNA levels were positively associated with high nuclear and FIGO grades (P = 0.0566 each). Aldolase mRNA levels were not associated with tumor subtypes (P = 0.1057). We found that aldolaseC overexpression showed a potential predictive value for shorter overall survival. However, statistical significance threshold was not reached (P = 0.285), probably due to the relatively small sample size (70 in total). We found no evidence that aldolaseC mRNA levels to be associated with other patient outcomes such as recurrence. HEC1 and CT26 were successfully treated with clotrimazole resulting in detachment of cancer cells from the culture plates. Tryptan blue dye showed that clotrimazole induced time-dependent reduction in cell viability in CT26 cell line and HEC1 cell lines (Figure 1). Treatment of cells with clotrimazole affected the morphology of the cells as seen with examination by TEM. These same cells at the 4 h time point showed a greatly enlarged and highly vacuolated subcellular compartment and enlarged and swollen mitochondria with loss of cristae (Figure 2C–D). At the 24 h time point, cell death has occurred, which was evidenced by loss of integrity of the plasma membrane which resulted in cell rupture (Figure 2E,F).
Table 1.
Clinical and pathologic features of 70 patients. (Data in parentheses are percentages)
| No. of evaluable patients | 70 |
|
| |
| Age, year | |
| Median | 71 |
|
| |
| Range | 36–92 |
|
| |
| Follow up time, months | |
| Median | 69.25 |
|
| |
| Range | 0–285.25 |
|
| |
| Stage | |
| I | 36 (51) |
|
| |
| II | 10 (14) |
|
| |
| III | 19 (27) |
|
| |
| IV | 5 (7) |
|
| |
| Subtype | |
| Endometriod | 42 (60) |
|
| |
| CCC+serous | 28 (40) |
|
| |
| Grade FIGO | |
| 1 | 16 (23) |
|
| |
| 2 | 16 (23) |
|
| |
| 3 | 38 (54) |
|
| |
| Grade Nuclear | |
| 1 | 10 (14) |
|
| |
| 2 | 22 (32) |
|
| |
| 3 | 38 (54) |
|
| |
| Tumor size, cm | |
| <=2 | 6 (9) |
|
| |
| >2 | 64 (91) |
|
| |
| Depth of invasion | |
| Median | 38 |
|
| |
| Range | (0–100) |
|
| |
| Recurrence | |
| N | 43 (61) |
|
| |
| Y | 23 (33) |
|
| |
| others | 4 (6) |
|
| |
| Status | |
| Alive with no evidence of disease (ANED) | 49 (70) |
|
| |
| Alive with evidence of disease (AWED) | 12 (17) |
|
| |
| Death of disease (DOD) | 7 (10) |
|
| |
| Death form other causes | 2 (1) |
Figure 1.
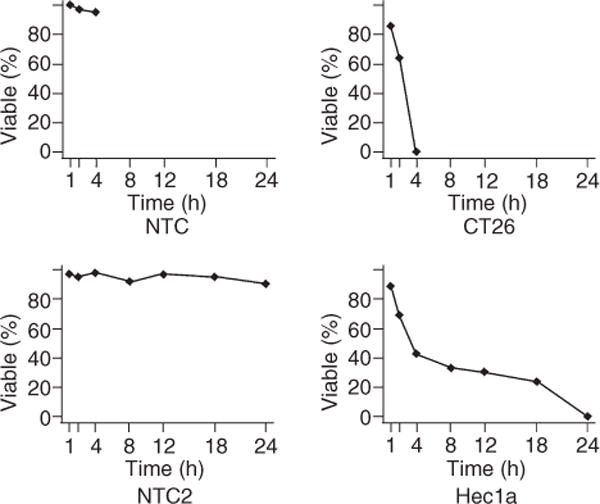
The cell viability of CT26 and HEC1a of treated and untreated with clotrimazole.
Figure 2.
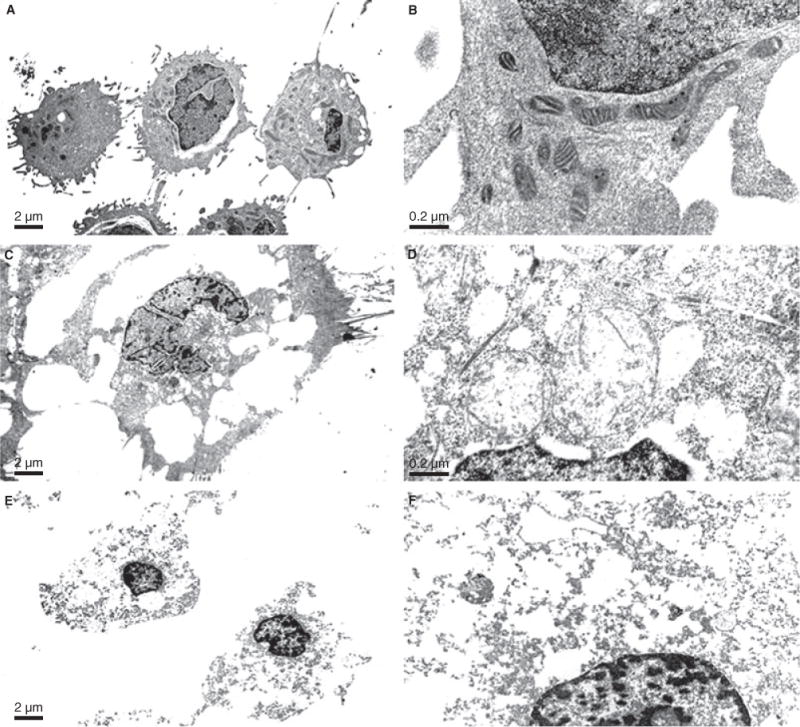
Composite TEM micrograph of HEC1 Control cells (A,B), HecA1 4 h treated (C,D) and HEC1 24 h treated (E,F,) at magnifications of 4000 and 40 000 respectively. TEM of untreated HecA1 cells demonstrate rounded cells containing numerous mitochondria with occasional vacuoles (A) and on higher magnification demonstrate thin cisternae of rough endoplasmic reticulum and perinuclear pleomorphic electron dense mitochondria with intact cristae (B). These same cells at the 8 h time point show a greatly enlarged and highly vacuolated subcellular compartment at low magnification (C) and enlarged and swollen mitochondria with loss of cristae at the higher magnification (D). At the 24 h time point, cell death has occurred, which is evidenced by loss of integrity of the plasma membrane with resulting cell rupture (E). At higher magnification, most subcellular organelles are gone with only a few remnants of distended cisternae of rough endoplasmic reticulum and rare distorted mitochondria remaining (F).
Our work showed that aldolaseC was overexpressed in a subset of human samples and in endometrial cancer cell lines. The overexpression of aldolaseC by tumor cells is an indication that human endometrial cancer, like other tumor types, depends on glycolysis for its energy supply to ensure its survival.
Binding glycolytic enzymes to the cytoskeleton provides local ATP and it also affects cell structure which has led to the hypothesis that inhibition of glycolysis may severely abolish ATP generation in cancer cells leading to their death.10,11 Clotrimazole is an antifungal azole derivate that has been recognized as a calmodulin antagonist which induces inhibition of cell proliferation. Recent studies on melanoma, lung, and colon cancer cell lines showed that clotrimazole induces a significant dose-and time dependent reduction in aldolase levels, ATP and cell viability.7,8 Our experiment revealed that treating EC cell line by clotrimazole caused detachment of cancer cells from the plates. Furthermore, we found that clotrimazole treatment of endometrial cancer cell line had an extreme impact on tumor cell shape and viability. The decrease of cell viability was cancer cell type-dependent. In addition, clotrimazole treatment had devastating effects on cell structure and these effects were time-dependent. Therefore, clotrimazole could be a promising targeted therapy in subset of endometrial cancer patients who are resistant or not suitable candidates for conventional therapy. Thus, future studies will be aimed at testing the anti-tumor effects of clotrimazole in vivo, potentially leading to clinical trial of this strategy.
References
- 1.Saw RJ. Glucose metabolism and cancer. Curr Opin Cell Biol. 2006;18:598–08. doi: 10.1016/j.ceb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Beckner ME, Stracke ML, Liotta LA, Schiffmann E. Glycolysis as primary energy source in tumor chemotaxis. J Natl Cancer Inst. 1990;82:1836–1840. doi: 10.1093/jnci/82.23.1836. [DOI] [PubMed] [Google Scholar]
- 3.Marshall MJ, Goldberg DM, Neal FE, Millar DR. Enzymes of glucose metabolism in carcinoma of the cervix and endometrium of the human uterus. Br J Cancer. 1978;37:990–01. doi: 10.1038/bjc.1978.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takashi M, Haimoto H, Koshikawa T, Kato K. Expression of aldolaseC isozyme in renal cell carcinoma. Am J Clin Pathol. 1990;93:631–636. doi: 10.1093/ajcp/93.5.631. [DOI] [PubMed] [Google Scholar]
- 5.Ojika T, Imaizumi M, Abe T, Kato K. Immunochemical and immunohistochemical studies on three aldolase isozymes in human lung cancer. Cancer. 1991;67:2153–2158. doi: 10.1002/1097-0142(19910415)67:8<2153::aid-cncr2820670825>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Cao QJ, Belbin T, Socci N, et al. Distinctive gene expression profiles by cDNA microarrays in endometrioid and serous caricnomas of the endometrium. Int J Gynecol Pathol. 2004;23:321–329. doi: 10.1097/01.pgp.0000139646.32997.3a. [DOI] [PubMed] [Google Scholar]
- 7.Penso J, Beitner R. Detachment of glycolytic enzymes from cytoskeleton of lewis lung carcinoma and colon adenocarcinoma cells induced by clotrimazole and its correlation to cell viability and morphology. Mol Genet Metab. 2002;76:181–188. doi: 10.1016/s1096-7192(02)00046-x. [DOI] [PubMed] [Google Scholar]
- 8.Penso J, Beitner R. Clotrimazole decreases glycolysis and the viability of lung carcinoma and colon adenocarcinoma cells. Eur J Pharmacol. 2002;451:227–235. doi: 10.1016/s0014-2999(02)02103-9. [DOI] [PubMed] [Google Scholar]
- 9.de Mesy Jensen Bentley KL. “Pop-off” technique for FNA smears for diagnostic electron microscopy. Tech. Sample CY-1. Amer Soc Clin Pathol. 1987 [Google Scholar]
- 10.Walsh JL, Keith TJ, Knull HR. Glycolytic muscle interactions with tubulin and microtubules. Biochim Biophys Acta. 1989;59:949–999. doi: 10.1016/0167-4838(89)90031-9. [DOI] [PubMed] [Google Scholar]
- 11.Bronstein WW, Knull HR. Interaction of muscle glycolytic enzymes with thin filament proteins. Can J Biochem. 1981;59:494–499. doi: 10.1139/o81-069. [DOI] [PubMed] [Google Scholar]


