Abstract
Objective
Synuclein-γ (SNCG) is a marker for adverse and aggressive disease in breast cancer. In previous study, we found SNCG mRNA to be overexpressed in uterine serous carcinoma compared to uterine endometrioid adenocarcinoma. The aim of this study is to explore the prognostic value of SNCG in patients with endometrial cancer.
Methods
279 endometrial cancer patients were retrieved from the archives. The tissue paraffin blocks were stained for SNCG antibody and its expression was correlated with clinicopathological prognostic factors.
Results
There was a positive association between SNCG+ immunoexpression and tumor grade, tumor stage, type II carcinomas, deep myometrial invasion and lymphovascular invasion. A correlation between SNCG+ and adverse outcomes, such as shorter overall survival (OS) and disease free survival (DFS), was also detected. Following adjuvant therapy (radiation and chemotherapy or chemotherapy alone), we observed a difference in 5 years DFS rate between SNCG+ (41.6%) and SNCG− patients (59.5%).
Conclusion
Overexpression of SNCG seemed to be a predictor biomarker for aggressive tumor behavior and adverse outcome in patients with endometrial cancer. Future exploration of SNCG as a potential therapeutic target for selected patients could be of interest.
Keywords: Synuclein-γ, SNCG, Endometrial adenocarcinoma, Clinical outcome
Introduction
Endometrial carcinoma is the most common gynecologic malignancy in the United States [1]. Endometrioid adenocarcinomas (EAC) account for more than 80% of cases, and they tend to present as low grade, early stage tumors with favorable outcomes. While uterine serous carcinomas (USC) represent a minority (3–10%) of total endometrial cancer cases, these are usually high grade tumors with deep myometrial invasion, lymphovascular involvement, and a more aggressive clinical course [2]. USC is responsible for a disproportionate number of deaths due to the fact that most of these tumors have already spread outside the uteri corpus. The 5-year survival rate for stages I–II EAC is estimated to range between 75 and 87%, and between 44 and 50% for stages I–II USC [3–4]. Numerous prognostic parameters have been implicated in endometrial carcinoma including tumor grade, tumor subtypes, tumor stage, presence of lymphovascular invasion (LVI), and depth of myometrial invasion [5,6,7]. High tumor grade, advanced stage disease, presence of LVI, deep myometrial invasion (outer third) and type II (USC and clear cell subtypes) endometrial adenocarcinoma constitute a high-group risk for recurrence and aggressive outcome in comparison to low-risk group defined by low tumor grade, tumor confined to uterine corpus at presentation, absence of LVI, superficial myometrial invasion (inner third), and type I (EAC and mucinous subtypes) endometrial adenocarcinoma. Besides these clinico-pathological factors, numerous biomarkers such as estrogen/progesterone receptors, bcl-2, catenin, Her2/neu and p53 were shown to predict prognosis in women with endometrial carcinoma [8,9].
Synuclein-γ (SNCG) (also known as breast cancer-specific protein 1) was initially cloned from infiltrating breast carcinoma cells [10]. This gene is located at the 10q23.2 locus and highly expressed in several cancer types such as advanced stage of ovarian, breast, liver, prostate and colon cancer [11–15]. It has been shown to promote cell growth, tumor invasiveness and metastasis and to interfere with drug-induced apoptosis [15,16]. In a recent study, we demonstrated the overexpression of SNCG mRNA in a number of USC tissue samples in comparison to EAC [17]. These findings suggested that SNCG merits further investigation both as a prognostic factor and as a therapeutic target. Thus, we examined SNCG protein expression using immunohistochemistry in paraffin-embedded tissues from 279 patients with endometrial cancers, all diagnosed and treated in one institution. We also analyzed the relationship between SNCG protein expression and clinicopathologic factors.
Materials and methods
Patients population
After obtaining the IRB approval, the Pathology archive at Ros-well Park Cancer Institute, Buffalo-NY was searched for endometrial adenocarcinoma cases from January 2000 to December 2010. A chart review was conducted with extraction of clinical information including the patients’ age at the time of diagnosis, the surgical stage, the post-operative therapy, the disease free survival (DFS), the site of recurrence, the cause and the time of death. All patients underwent a surgical staging procedure including an abdominal hysterectomy with bilateral salpingo-oophorectomy, with or without pelvic and para-aortic lymph nodes dissection and pelvic washing, depending on the tumor grade and the tumor stage. Patients were treated according to the National Comprehensive Cancer Network (NCCN) guidelines (www.cancer.gov).
Histological evaluation
Tumor grade was assessed using the International Federation of Gynecology and Obstetrics (FIGO) system and tumor stage was assigned based on 1992 FIGO surgical staging guidelines [18]. All slides were examined by an expert gynecologic pathologist for confirmation of the tumor type, tumor size, tumor grade, depth of myometrial invasion (MI) and presence of lymphovascular invasion (LVI).
Immunohistochemistry
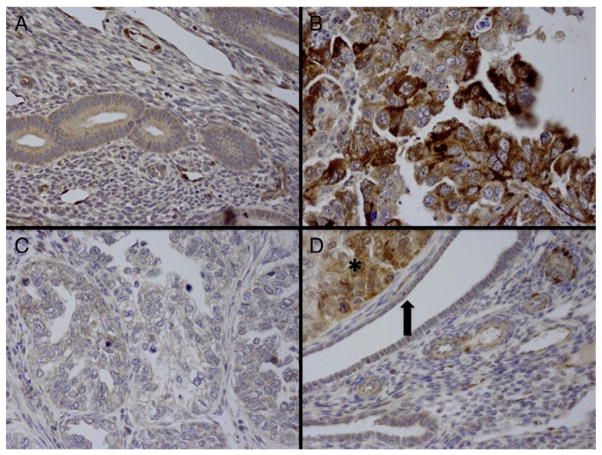
Four micrometer thick sections were deparaffinized with xylene, and washed with ethanol. Sections were cooled 20 min and incubated 10 min with 3% H2O2 to quench endogenous peroxidase activity. Blocking was performed using serum-free protein block, Dakocytomation (Carpenteria, CA) for 30 min. The sections were pretreated with an EDTA buffer saline solution, steamed for 20 min and then sections were incubated with synuclein-γ antibody (monoclonal; 1:20 dilution; R&D systems MN, USA) for 1 h at room temperature. The diaminobenzidine complex was used as a chromogen. We chose invasive ductal carcinoma as positive control and negative control slides omitting the primary antibody were included in all assays. The stain intensity was diffuse and homogenous throughout the tumor. The extent of immunochemical reactivity was graded based on intensity as follows: 0 (negative), 1+ (weak), 2+ (moderate), 3+ (strong). For the sake of statistical analysis, negative and weak stains were grouped as group I (negative) and moderate and strong as group II (positive). 10 cases of normal endometrium were included to evaluate synuclein expression. Examples of normal endometrium, positive and negative cases are illustrated in Fig. 1A–D.
Fig. 1.
A: Normal endometrium showed negative expression for synuclein (magnification ×40). B: Uterine serous carcinoma with strong positivity for SNCG (magnification ×40). C: Clear cell carcinoma with negative immunoexpression for SNCG (magnification ×40). D: Endometrioid adenocarcinoma FIGOIII (left upper corner marked by asterisk) strongly expressing SNCG immunostain, while the normal glands (marked in an arrow) are negative for SNCG expression (×40).
Statistical analyses
Statistical analyses were performed by R (http://www.r-project.org/). The clinical parameters used for modeling are age, tumor size, histologic subtypes, myometrial depth of invasion, LVI, FIGO grade, recurrence, status, and survival time. To test the association between the biomarker and the clinical parameters, Fisher’s exact test was performed for categorical parameters and Welch t- test was used for the continuous ones. For survival analysis, Kaplan–Meier method with log-rank test was used to calculate the cumulative survival time, and check both the overall survival (OS) and disease free survival (DFS) difference between the patients with the different biomarker status. Multivariate cox proportional hazard model was used to determine the hazard ratio that represents the relative risk of death among patients with SNCG+ compared with those with SNCG−. All reported p values are two sided.
Results
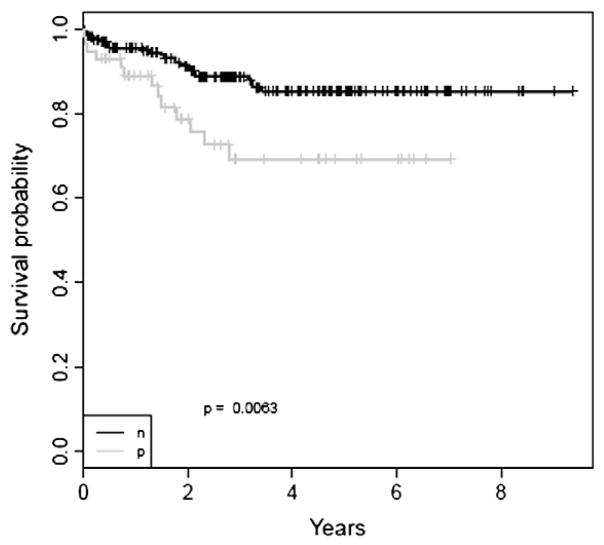
The clinical and pathologic features of 279 patients with endometrial adenocarcinoma are summarized in Table 1. All patients (n=279) had surgery for endometrial cancer with no previous chemotherapy or radiation therapy, and all had a complete follow-up information with median of 2.7 years. The distribution of clinical factors in relation to the status of SNCG expression is illustrated in Table 2. As summarized in Table 3, there was a strong association between SNCG+ and advanced tumor stage (p=0.032), high tumor grade (p=<0.001), presence of LVI (p<0.001) and deep myometrial invasion (>50%) (p=0.031). Strong association between SNCG expression and tumor subtype (p<0.001) was also noted. Specifically, serous and clear cell carcinomas, as well as carcinosarcoma were more likely to express SNCG than endometrioid adenocarcinomas. Furthermore, SNCG+ patients were more likely to have shorter DFS (p=0.006) and to have a worse outcome (p=0.006), with a 5 years DFS probability rate of 54.9% for SNCG+ patients versus 81.0% for SNCG− patients. Kaplan survival analysis revealed a strong association of SNCG+ with OS (p=0.0063). SNCG+ tumors were more likely to have shorter OS rate in comparison to those with negative expression, with a 5 year estimated OS rate of 69.2% for SNCG+ patients versus 85.4% for SNCG− patients (Fig. 2). Multivariate cox regression analysis incorporating age, stage, tumor size, LVI, recurrence, depth of invasion, subtype, and grade demonstrated that SNCG had a trend toward significance to predict OS (p=0.1, HR=1.97, CI: 0.87–4.45) with a 1.97-fold increased relative risk for SNCG+ patients in comparison to SNCG− patients (Table 4).
Table 1.
Clinical and pathologic features of patients. (Data in parentheses are percentages).
| No. of patients | 279 |
|---|---|
| Follow up time, year | |
| Median | 2.74 |
| Age, year | |
| Median | 65 |
| Range | 29–97 |
| Stage | |
| I | 180(65) |
| II | 37(13) |
| III | 41(15) |
| IV | 21(7) |
| Subtype | |
| Endometrioid | 200(72) |
| USC(n=46)+CCC(n=14) | 60(21) |
| Carcinosarcoma | 19(7) |
| Grade(FIGO) | |
| 1 | 118(42) |
| 2 | 52(19) |
| 3 | 109(39) |
| Depth of myometrial invasion | |
| Median(range) | 38.98(0–100) |
| <=50 | 177(63) |
| >50 | 102(37) |
| LVI | |
| No | 201(72) |
| Yes | 78(28) |
| Lymph nodes dissection | 202(72) |
| Recurrence | |
| No | 217(78) |
| Yes | 44(16) |
| Persistent | 13(4) |
| Progression | 5(2) |
| SNCG immunoexpression | |
| Negative | 222(80) |
| Positive | 57(20) |
| Status | |
| ANED | 209(75) |
| AWED | 33(12) |
| DOD | 23(8) |
| DNED | 7(2.5) |
| Others | 7(2.5) |
Table 2.
The distribution of clinic factors in relation to dichotomous status of SNCG expression.
| SNCG+ | SNCG− | p value | |
|---|---|---|---|
| FIGO grade | |||
| G1+2 | 17 (6) | 153 (55) | <0.001 |
| G3 | 40 (14) | 69 (25) | |
| Tumor subtype | <0.001 | ||
| USC (n=46)+CCC(n=14) | 29 (10) | 31 (11) | |
| Endometrioid | 23 (8) | 177 (63) | |
| Carcinosarcoma | 5 (2) | 14 (5) | |
| Depth of invasion | 0.031 | ||
| =<50% | 29 (10) | 148 (53) | |
| >50% | 28 (10) | 74 (27) | |
| Tumor stage | 0.032 | ||
| I+II | 38 (14) | 179 (64) | |
| III+IV | 19 (7) | 43 (15) | |
| LVI | <0.001 | ||
| Yes | 29 (10) | 49 (18) | |
| No | 28 (10) | 173 (62) | |
| Lymph nodes | 0.169 | ||
| Pos (51) | 15 (5) | 36 (13) | |
| Negative (151) | 29 (10) | 122 (44) | |
| Missing(77) | 13 (5) | 64 (23) | |
| Recurrence | 0.006 | ||
| Yes | 17 (6) | 27 (10) | |
| No | 38 (14) | 179 (64) | |
| Others | 2 (0.7) | 16 (6) | |
| Status | 0.006 | ||
| ANED | 34 (12) | 175 (63) | |
| All others | 23 (8) | 47 (17) | |
Table 3.
Association between SNCG immunoexpression and the clinical variables.
| Variables (group 2 vs group 1) | p value | Odds ratio (p vs. n) |
|---|---|---|
| Age | 0.002 | |
| Stage | 0.032 | 2.075 |
| Tumor size | 0.586 | 1.33 |
| LVI | <0.001 | 0.275 |
| Depth of myometrial invasion | 0.031 | 1.926 |
| Grade_FIGO | <0.001 | 5.183 |
| Subtype | <0.001 | 0.139 |
| Recurrence | 0.006 | 0.337 |
| Status | 0.006 | 2.509 |
Age as continuous variable.
Stage: Group 1: stages I and II; Group 2: stages III and IV.
Lympho-vascular invasion (LVI): Group 1: no; Group 2: yes.
Depth of myometrial invasion: Group 1: <=50%; Group 2: >50%.
Histologic Grade: Group 1: FIGO Grades 1 and 2; Group 2: FIGO Grade 3.
Subtype: Group 1: endometrioid; Group 2: others.
Recurrence: Group 1: no; Group 2: all others.
Status: Group 1: ANED; Group 2: all others.
Fig. 2.
Kaplan survival analysis revealed a strong association of SNCG with OS (p=0.0063). The black curve is for SNCG− patients, while the gray curve is for SNCG+ patients.
Table 4.
Multivariate cox proportional-hazards analysis of overall survival.
| Variable | Hazard ratio | CI of hazard ratio | p value |
|---|---|---|---|
| Age | 1.02 | 0.99–1.05 | 0.2213 |
| Grade_FIGO (3 vs 1 and 2) | 4.28 | 1.69–10.82 | 0.0021 |
| Stage (III/IV vs I/II) | 1.64 | 0.7–3.81 | 0.2519 |
| Tumor size (>2 cm vs <=2 cm) | 3.33 | 0.99–11.26 | 0.0525 |
| LVI (N vs Y) | 1.27 | 0.5–2.89 | 0.5751 |
| Recurrence | |||
| N vs Y | 0.26 | 0.11–0.62 | 0.0023 |
| Others vs Y | 3.93 | 1.43–10.82 | 0.008 |
| Depth of Invasion (>50 vs <=50) | 2.06 | 0.95–4.47 | 0.0686 |
| Subtype | |||
| Endometrioid vs USC+CCC | 2.75 | 1.01–7.51 | 0.0484 |
| SNCG (P vs N) | 1.97 | 0.87–4.45 | 0.1023 |
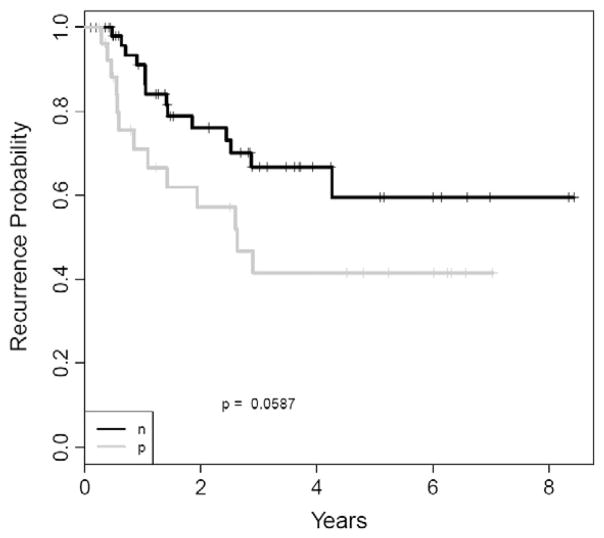
We further explored the impact of SNCG on chemotherapy response. Of the 80 (among 279) patients who received radiation+ chemotherapy or chemotherapy alone, 53 were SNCG−, while 27 were SNCG+. There was no significant independent value of SNCG in predicting OS, DFS, or DOD between these two groups. However, a difference in DFS probability rate was observed with 5 years DFS probability of 41.6% for the 27 SNCG+ patients versus 59.5% for the 53 SNCG− patients (Fig. 3).
Fig. 3.
Kaplan survival analysis revealed that for patients who received radiation+ chemotherapy or chemotherapy alone there is no significant independent value of SNCG in predicting DFS. The black curve is for SNCG− patients who received radiation + chemotherapy or chemotherapy alone, while the gray curve is for SNCG+ patients who received radiation + chemotherapy or chemotherapy alone. However, a difference in DFS probability rate was observed with 5 years DFS probability of 41.6% for the 27 SNCG+ patients and of 59.5% for the 53 SNCG− patients.
Discussion
The overexpression of SNCG in several types of cancer raises questions about the involvement of SNCG in tumorigenesis and tumor metastasis [11–15]. In breast cancer, its expression was correlated with advanced stage and unfavorable outcome as well as resistance to microtubule-disrupting agents such as paclitaxel [19,20]. Furthermore, de novo expression of synuclein-γ in synuclein-γ negative breast cancer cell lines caused significant increase in cell motility and invasiveness in vitro and an increased in the amount of metastasis in nude mice [16]. The mechanism of cell invasion and metastasis was attributed by numerous studies to the strong induction by SNCG of matrix metalloproteinases (MMPs) such as MMP9, and of tissue inhibitors of MMP1s and MMP2s expressions [21]. Recently synuclein-γ mRNA was found to be more highly expressed in a uterine serous carcinoma cell line (SPEC2) in comparison to an endometrioid cancer cell line [22]. Using cDNA microarray and RT-PCR method on fresh frozen tissue samples, we demonstrated that SNCG was upregulated and SNCG-mRNA was overexpressed in USC in comparison to EAC samples [17]. These compelling lines of evidence suggest that SNCG might have a prognostic value in endometrial cancer.
In our series of 279 patients with endometrial cancer, we found that the expression of SNCG protein was about 20%, a percentage close to the 38% expression seen in breast cancer [19]. In addition, and similar to the findings in normal breast and colon tissue, there was no expression of SNCG in normal endometrial tissue. Similar to other malignancies, there was a significant association between SNCG+ expression and high tumor stage, indicating that SNCG might not be an indicator and even involved in disease progression [11–15]. Similar to the findings in patients with breast cancer, we found a strong association between SNCG expression and probability for survival. Our data indicates that SNCG+ patients have a significantly poorer outcome including shorter OS and DFS than those SNCG− patients. In addition, we detected a significant association between SNCG expression and high risk clinicopathological prognostic factors for recurrence. In the only previous report on endometrial cancer, Morgan et al. found a correlation between SNCG expression, advanced stage disease and decreased DFS in 20 patients with USC [22]. By evaluating much larger series and studying different endometrial cancer subtypes, our results confirm the preliminary findings by Morgan et al.
SNCG protein expression was significantly associated with OS in univariate analysis, but it did not reach an independent statistical significance in multivariate analysis. However, because patients with SNCG+ expression have a higher likelihood (HR 1.97) for death in comparison to those patients with negative expression, SNCG still has a considerable implication in endometrial cancer patients.
Evidence showed that SNCG has a role in drug resistance to chemotherapy such as paclitaxel and vimblastine [20,22]. Microtubule inhibitors are used as adjuvant chemotherapeutic agents in addition to carboplatin to treat patients with advanced endometrial adenocarcinoma, type II uterine carcinomas and endometrial cancer recurrences. The microtubule inhibitor agents are thought to arrest cells in mitosis by triggering the mitotic checkpoint activation, resulting in cell arrest in the mitotic phase. Thus, prolonged treatments usually lead to cell death by apoptosis. To accomplish this, the whole mitotic checkpoint machinery including BubR1 which is a critical component, should function normally [23–25]. Numerous studies have shown that the inhibitory effect of SNCG on BubR1 function could explain the tumor resistance to microtubule inhibiting agents such as paclitaxel [20,22]. Furthermore, Morgan et al. showed that knockdown synuclein-γ in SPEC2 cells resulted in a significant decrease in cell proliferation and an increased sensitivity to paclitaxel-induced apoptosis. This is consistent with our findings that the patients with SNCG+ expression treated with radiation and chemotherapy (paclitaxel and carboplatin) or chemotherapy alone had shorter DFS in comparison to patients with SNCG− expression. This led us to speculate that SNCG expression could be an indicator for tumor resistance to adjuvant therapy in patients with endometrial cancer.
One limitation of our study was that the majority of our tumors were endometrioid type, well differentiated, and presented at very early stages, which is a frequent occurrence in endometrial cancer. Because these tumors have favorable outcome, it is expected that the majority of the patient population will be alive at the time of last follow-up. The resulting fewer numbers of unfavorable outcome in these patients might limit our statistical power in predicting survival.
In summary, using a monoclonal antibody, our work is the first to comprehensively study the expression of SNCG in a large series of patients with endometrial cancer in correlation with the clinical outcome. Our results indicate that SNCG+ endometrial cancers cases have worse clinical outcome compared to SNCG− cases. Patients with SNCG+ tumors had a statistically shorter DFS and a high probability of death. These findings support the potential clinical utility of incorporating SNCG IHC in the evaluation of endometrial cancer patients in order to identify those who are at higher risk of recurrence and progression and to plan targeted therapy.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Kitchener HC, Trimble EL. Endometrial cancer state of the science meeting. Int J Gynecol Cancer. 2009;19:134–40. doi: 10.1111/IGC.0b013e3181995f90. [DOI] [PubMed] [Google Scholar]
- 3.Naumann RW. Uterine papillary serous carcinoma: state of the state. Curr Oncol Rep. 2008;10:505–11. doi: 10.1007/s11912-008-0076-x. [DOI] [PubMed] [Google Scholar]
- 4.Benito V, Lubrano A, Arencibia O, et al. Pure papillary serous tumors of the endometrium: a clinicopathological analysis of 61 cases from a single institution. Int J Gynecol Cancer. 2009;19:1364–9. doi: 10.1111/IGC.0b013e3181b7a1d5. [DOI] [PubMed] [Google Scholar]
- 5.Part J. Prognostic parameters of endometrial carcinoma. Hum Pathol. 2004;35:649–62. doi: 10.1016/j.humpath.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Figge DC, Otto PM, Tamimi HK, Greer BE. Treatment variables in the management of endometrial cancer. Am J Obstet Gynecol. 1983;146:495–500. doi: 10.1016/0002-9378(83)90787-1. [DOI] [PubMed] [Google Scholar]
- 7.Keys HM, Roberts JA, Brunetto VL. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adeno-carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–51. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 8.Bansal N, Yendluri V, Wenham RW. The molecular biology of endometrial cancers and the implications of pathogenesis, classification, and targeted therapies. Cancer Control. 2009;16:8–13. doi: 10.1177/107327480901600102. [DOI] [PubMed] [Google Scholar]
- 9.Doll A, Abal M, Rigau M, et al. Novel molecular profiles of endometrial cancer —new light through old windows. J Steroid Biochem Mol Biol. 2008;108:221–9. doi: 10.1016/j.jsbmb.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Ji H, Liu YE, Jia T, et al. Identification of a breast cancer-specific gene, BCSG1, by different differential cDNA sequencing. Cancer Res. 1997;57:759–64. [PubMed] [Google Scholar]
- 11.Wu K, Weng Z, Tao Q, et al. Stage-specific expression of breast cancer-specific gene gamma-synuclein. Cancer Epidemiol Biomarkers Prev. 2003;12:920–5. [PubMed] [Google Scholar]
- 12.Bruening W, Giasson BI, Klein-Szanto AJ, Lee VM, Trojanowski JQ, Godwin AK. Synucleins are expressed in the majority of breast and ovarian carcinomas and in preneoplastic lesions of the ovary. Cancer. 2000;88:2154–63. [PubMed] [Google Scholar]
- 13.Li Z, Sclabas GM, Peng B, et al. Overexpression of synucelin-gamma in pancreatic adenocarcinoma. Cancer. 2004;101:58–65. doi: 10.1002/cncr.20321. [DOI] [PubMed] [Google Scholar]
- 14.Ye Q, Feng B, Peng YF, et al. Expression of γ-synucelin in colorectal cancer tissues and its role on colorectal cancer cell line HCT116. Worl J Gastroenterol. 2009;28:5035–43. doi: 10.3748/wjg.15.5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad M, Attoub S, Singh MN, Martin FL, El-Agnaf OMA. γ-Synuclein and the progression of cancer. FASEB J. 2007;21:3419–30. doi: 10.1096/fj.07-8379rev. [DOI] [PubMed] [Google Scholar]
- 16.Jia T, Liu YE, Liu J, Shi YE. Stimulation of breast cancer invasion and metastasis by synuclein gamma. Cancer Res. 1999;59:742–7. [PubMed] [Google Scholar]
- 17.Mhawech-Fauceglia P, Wang D, Kesterson J, et al. Gene expression profiles in stage I uterine serous carcinoma in comparison to grade 3 and grade 1 stage I endometrioid adenocarcinoma. PLoS One. 2011 Mar 23;6(3):e18066. doi: 10.1371/journal.pone.0018066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creasman WT. Revision in classification by International Federation of Gynecology and Obstetrics. Am J Obstet Gynecol. 1992;167:857–8. doi: 10.1016/s0002-9378(11)91607-x. [DOI] [PubMed] [Google Scholar]
- 19.Guo J, Shou C, Meng L, et al. Neuronal protein synuclein γ predicts poor outcome in breast cancer. Int J Cancer. 2007;121:1296–305. doi: 10.1002/ijc.22763. [DOI] [PubMed] [Google Scholar]
- 20.Singh VK, Zhou Y, Marsh JA, et al. Synuclein-γ targeting peptide inhibitor that enhances sensitivity of breast cancer cells to antimicrotubule drugs. Cancer Res. 2007;67:626–33. doi: 10.1158/0008-5472.CAN-06-1820. [DOI] [PubMed] [Google Scholar]
- 21.Surgucheva IG, Sivak JM, Fini ME, Palazzo RE, Surguchov AP. Effect of gamma-synuclein overexpression on matrix metalloproteinases in retinoblastoma Y79 cells. Arch Biochem Biophys. 2003;410:167–76. doi: 10.1016/s0003-9861(02)00664-1. [DOI] [PubMed] [Google Scholar]
- 22.Morgan J, Hoekstra AV, Chapman-Davis E, Hardt JL, Kim JJ, Buttin BM. Synuclein γ (SNCG) may be a novel prognostic biomarker in uterine papillary serous cracinoma. Gynecol Oncol. 2009;114:293–8. doi: 10.1016/j.ygyno.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Blajeski AL, Phan VA, Kottle TJ, Kaufmann SH. G(1) and G(2) cell cycle arrest following microtubule depolymerization in human breast cancers. J Clin Invest. 2002;110:91–9. doi: 10.1172/JCI13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin HJ, Baek KH, Jeon AH, et al. Dual roles of human BubR1, a mitotic checkpoint kinase, in the monitoring of chromosomal instability. Cancer Cell. 2003;4:483–97. doi: 10.1016/s1535-6108(03)00302-7. [DOI] [PubMed] [Google Scholar]
- 25.Pan ZZ, Bruening W, Giasson BI, Lee VM, Godwin AK. Gamma-synuclein promotes cancer cell survival and inhibits stress- and chemotherapy drug-induced apoptosis by modulating MAPK pathways. J Biol Chem. 2002;277:35050–60. doi: 10.1074/jbc.M201650200. [DOI] [PubMed] [Google Scholar]