Abstract
Aims
To evaluate the components of the transforming growth factor (TGF)-β–Smad signalling pathway in human endometrial cancer (EC).
Methods and results
TGF-β1, TGF-β receptor type I, TGF-β receptor type II, Smad2, Smad3, Smad4, Skil and Disabled-2 (DAB2) mRNA levels were determined by reverse transcriptase polymerase chain reaction on EC cell lines and in 70 EC tissues. Immunohistochemistry for Skil and DAB2 antibodies was performed on 362 EC cases. Decreased mRNA levels of all eight components of the TGF-β pathway tested were found in the majority of 70 cases. For DAB2, the mRNA level was correlated with protein expression level (P = 0.04). The Skil mRNA level was associated with tumour stage (P = 0.03), and the Smad2/3/4 mRNA level with tumour grade (P = 0.03, P = 0.02, and P = 0.00, respectively). The Smad4 mRNA level was also associated with tumour size (P = 0.05), subtype (P = 0.04), and disease-free survival (DFS) (P = 0.05). The TGF-β1 mRNA level was associated with DFS (P = 0.04). Finally, tumours with positive Skil protein expression had a shorter recurrence time, whereas, those with positive DAB2 protein expression had a longer recurrence time.
Conclusions
Down-regulation of the TGF-β–Smad signalling pathway might be responsible for the pathogenesis of human EC, and some of its components appeared to be prognostic factors. Exploration of future therapy targeting the TGF-β–Smad pathway is warranted in EC.
Keywords: endometrial cancer, mRNA level, patient prognosis, protein immunoexpression, TGF-β–Smad signalling pathway
Introduction
Transforming growth factor (TGF)-β is a multifunctional polypeptide that controls many aspects of cell function, such as cell proliferation, differentiation, migration, apoptosis, adhesion, angiogenesis, wound healing, immune surveillance, and survival. TGF-β has three isoforms (TGF-β1, TGFβ2, and TGF-β3), with TGF-β1 being the most prevalent. TGF-β has been shown to have a tumour suppressive function at early stages of tumorigenesis, and a tumour promoter function at advanced stages. Therefore, the growth-inhibitory function of TGF-β is selectively lost in advanced cancers, and instead it induces growth, invasion and metastasis of cancer cells. TGF-β exerts its effect by binding directly to TGF-β receptor type II (TβRII), a constitutively active transmembrane serine/threonine kinase that is recognized by TGFβ receptor type I (TβRI), leading to the formation of an oligomeric complex. Subsequently, TβRI becomes activated via phosphorylation by TβRII, and leads to further propagation of TGF-β signalling by the Smad family of proteins. Thus, phosphorylation of the cytoplasmic Smad2 and Smad3 proteins allows for the formation of a heteromeric complex with Smad4. The cytoplasmic Smad complex is then translocated to the nucleus, where it binds to DNA in a sequence-specific manner, and regulates gene transcription.1–4 However, TGF-β signalling pathways are negatively regulated by numerous regulators, among them Ski/Skil (avian sarcoma viral oncogene homologue) and Ski-related gene (Sno). Ski and Sno interact with Smad proteins, resulting in repression of their transcriptional activity.5,6 Up-regulation of SnoK and Ski in numerous human cancer cell lines suggested that they could be considered as oncogenes.5,6 However, their up-regulation and down-regulation in colonic cancer suggested that they may have both oncogenic and tumour suppressive functions in human colonic carcinogenesis.7 Another regulator of TGF-β signalling pathway is human Disabled-2 (DAB2), which is a putative tumour suppressor gene (TSG), discovered in ovarian carcinoma.8,9 DAB2 exerts its tumour-suppressive activity by mediating TGF-β-induced growth inhibition; it does this by directly binding to Smad2 and Smad3 through the phosphotyrosine interacting domain (PID) domain. DAB2 is lost in 80–90% of ovarian/breast cancer cell lines.9,10 Resistance to TGF-β growth-inhibitory effects early in tumorigenesis has been demonstrated in numerous studies.11–14 In endometrial cancer (EC), decreased levels of TβRII mRNA and a frameshift mutation of TβRII via mismatch repair deficiency are both frequently present in endometrioid-type adenocarcinoma, and this is likely to result in loss of receptor function and unresponsiveness of TGF-β signalling. Furthermore, concomitant deregulation of TβRII and Smad4 were found to be present in endometrioid-type adenocarcinoma. These data suggest that deregulation of the TGF-β signalling pathway might play a role in endometrial carcinogenesis of the endometrioid type. However, these reports are often limited to only a few components of the TGF-β pathway or by their small sample size and/or evaluation of endometrioid histological subtypes only.15–17
The goal of our study was to evaluate a large panel of components of the TGF-β pathway and its regulators in various EC histological subtypes. We then correlated their value with histological – clinical data and patient outcome. To do so, we evaluated TGF-β, TβRI, TβRII, Smad2, Smad3, Smad4, Skil and DAB2 mRNA levels in two EC cell lines and 70 samples from 70 patients with newly diagnosed EC, using a reverse transcriptase polymerase chain reaction (RT-PCR) TaqMan Low Density Custom Array (TLDA) format. We also sought to correlate their expression with clinical data and patient outcomes. Finally, we explored the protein expression of two major regulators, Skil and DAB2, by immunohistochemistry (IHC) in 362 patients, and correlated this with mRNA levels and patient outcomes.
Materials and methods
PATIENT POPULATION
After Institutional Review Board approval had been obtained, the pathology archives of Roswell Park Cancer Institute (RPCI) were searched for EC cases from January 2000 to December 2009. A chart review was conducted, with extraction of clinical information, including patient age at the time of diagnosis, surgical stage, postoperative therapy, disease-free survival (DFS), recurrence time and site, death of disease, or death from unrelated causes. All patients underwent a complete surgical staging procedure, including an abdominal hysterectomy with bilateral salpingo-oophorectomy, with or without pelvic and para-aortic lymph node dissection and pelvic washing, depending on the tumour grade and the tumour stage. Patients were treated according to the National Comprehensive Cancer Network guidelines (http://www.cancer.gov). Three hundred and sixty-two patients were found to be suitable for evaluation.
HISTOLOGY AND IMMUNOHISTOCHEMISTRY
Tumour grade was assessed with two methods – nuclear grade and the International Federation of Gynecology and Obstetrics (FIGO) system – and tumour stage was assigned on the basis of the 1992 FIGO surgical staging guidelines.18 All slides were examined by an expert gynaecological pathologist for confirmation of the tumour type, tumour size, tumour grade, depth of myometrial invasion (MI), and presence of lympho vascular invasion (LVI). IHC for DAB2 and Skil antibodies on paraffin-block tissues from 362 patients was performed as follows. Sections were cut at 5 μm, and dried in a 60°C oven for 1 h. Slides were then deparaffinized in xylene, and rehydrated with graded alcohols. Endogenous peroxidase was quenched with aqueous 3% H2O2 for 10 min, and washed with phosphate-buffered saline/Tween-20 (PBS/T). An antigen retrieval was performed with citrate buffer and in the microwave oven for 10 min. Slides were incubated with the primary antibodies against DAB2 (polyclonal; 1:100; Protein Tech Group, Chicago, IL, USA) and Skil (polyclonal; 1:50; Sigma, St Louis, MO, USA) for 1 h. An isotype-matched control (1 μg/ml rabbit (Rb) IgG) was used on a duplicate slide in place of the primary antibody as a negative control. A PBS/T wash was followed by incubation with anti-rabbit biotinylated secondary antibody and ABC Elite reagent (Vector Laboratories, Burlingame, CA, USA) for 30 min each, with PBS/T washes in between. PBS/T was used as a wash, the chromogen 3,3′-diaminobenzidine was applied for 5 min, and counterstaining was performed with haematoxylin. Both Skil and DAB2 were weakly positive in normal endometrium. In tumour samples, the results were scored on the basis of staining intensity, and categorized into two groups: negative (negative/weak intensity) and positive (moderate/strong intensity). For both Skil and DAB2 antibody, the staining had a cytoplasmic pattern.
CELL LINES, SAMPLE COLLECTION, AND RNA PREPARATION
Two EC cell lines (HEC1) and (RL95) were purchased from the American Tissue Culture Collection (ATCC, Manassas, VA, USA), and cultivated according to the supplier's recommendations. In addition, adequate fresh frozen (FF) specimens from 70 of 362 patients who had undergone surgery for uterine cancer at RPCI were available for analysis. FF tissues were cut and examined to ensure that the tissue contained >80% tumour. Finally, six FF normal endometrium samples from patients who had undergone hysterectomy surgery for fibroids (leiomyoma) were analysed and used as a normal endometrial control. The frozen sections were diced and pestled on dry ice. The RNA was extracted with the RNeasy Mini kit (Qiagen, Valencia, CA, USA), and quantitated with a Nanodrop (Nano Drop Products, Wilmington, DE, USA). The RNA was immediately converted to cDNA with the use of dNTPs, random hexamers (Qiagen), and Superscript II (Qiagen). The cDNA was stored for later use at−80°C.
QUANTITATIVE REVERSE TRANSCRIPTASE PCR TLDA FORMAT
The mRNA expression levels of Smad2, Smad3, Smad4, TGF-β1, TβRI, TβRII, Skil and DAB2 were selected for validation, and determined with a TLDA as a 384-well microfluidic card preloaded with TaqMan Gene Expression Assays. The refrigerated card was brought to room temperature. Each cDNA was diluted to a final concentration of 25 ng/μl. A mix was made for each gene of interest, consisting of Universal Master Mix (UMM) (both Applied Biosystems, Foster City, CA, USA) and PCR-grade water (Sigma). Ten microlitres of the diluted 25 ng/μl cDNA was added to each UMM mixture. The mixture was mixed and centrifuged for 2 min. One hundred microlitres of the cDNA/UMM was added to each port (250 ng of cDNA per port in total). The TLDA card was centrifuged (Thermal Sorvall Legend T) at 1000 g for 1 min, the centrifugation step was repeated, the TLDA card was sealed, and the upper portion of the card was trimmed away. With the relative quantification (RQ) Manager Software SDS V2.2.2 (Applied Biosystems), the data were analysed, and the baseline and the threshold were verified for each gene of interest. With the cycle thresholds (CTs) in Excel, the RQs were calculated by use of the 2−ΔΔCT method [gene of interest samples were relative to the endogenous control glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and were then normalized to an average of four normal endometrial tissues].
STATISTICAL ANALYSIS
Statistical analyses were performed with software (http://www.r-project.org/). The clinical parameters used for modelling were the tumour stage, size, histological subtypes, myometrial depth of invasion, LVI, FIGO grade, nuclear grade, recurrence status, and DFS. Fisher's exact test was performed to test the association between IHC and the clinical parameters. Univariate and multivariate logistic regression models were used to determine the association of each clinical parameter with the gene expression (RT-PCR) value. The P-value for each parameter was derived from the likelihood ratio test. To investigate the impact of gene expression on recurrence, univariate and multivariate survival analyses were performed with a Cox proportional hazards model. Real-time PCR data were the normalized expression values with the housekeeping gene GAPDH as the reference gene. For each assay, the average GAPDH CT value in the TaqMan qPCR assay was subtracted from the CT of the gene of interest to obtain a ΔCT value (gene of interest – GAPDH). Comparison between a normal sample and a cell line was performed by subtracting the CT in the cell line from the CT in the normal sample (ΔΔCt value). A lower ΔΔCT value indicates a lower expression level of the gene of interest in the cell line than in the normal sample. A two-sided sample Student's t-test was used to check the relationships between IHC and gene expression. Kaplan–Meier survival curves were stratified by IHC to check the recurrence difference between patients with different IHC status.
Results
PATIENTS AND IHC DATA
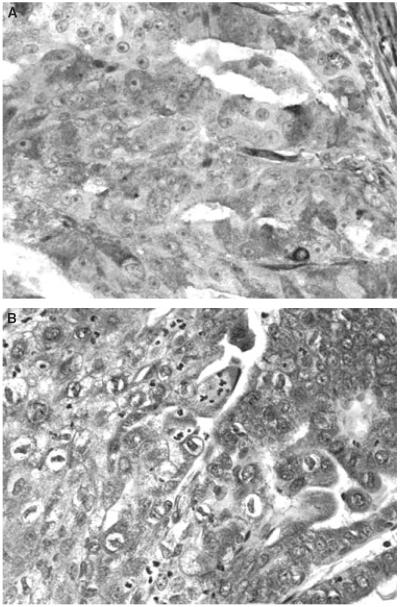
The characteristics of 362 patients are summarized in Table 1. The median age of the patients was 65 years. Seventy-seven percentage of cases were of the endometrioid type, 22% of the serous and clear cell types, and 1% of the undifferentiated type. Forty-four percent of cases were FIGO grade 1, 20% grade 2, and 36% grade 3, whereas 67% were stage I, 12% stage II, 15% stage III, and 6% stage IV. The median follow-up period was 2.14 years. Illustrations of immunoreactivity for each of DAB2 and Skil are given in Figure 1A,B. Sixteen percentage of cases were DAB2-positive, and 84% were DAB2-negative; and 66% of cases were Skil-positive, and 34% were Skil-negative. The association of DAB2 and Skil staining with patient clinical parameters is further illustrated in Table 2. Univariate analysis showed that DAB2 immunoexpression was strongly associated with tumour size (P = 0.027), FIGO grade (P = 0.0004), nuclear grade (P = 0.0008), histological subtype (P = 0.0002), and DFS (P = 0.040). We found that Skil immunoexpression did not have significant associations with any clinical parameters or outcome status.
Table 1.
Clinical and histopathological characteristics of the 362 patients
| Age (years) | |
| Median | 65 |
|
| |
| Range | 29–97 |
|
| |
| Stage, n (%) | |
| I | 242 (67) |
|
| |
| II | 43 (12) |
|
| |
| III | 55 (15) |
|
| |
| IV | 22 (6) |
|
| |
| Subtype, n (%) | |
| Endometrioid | 279 (77) |
|
| |
| CCC + serous | 80 (22) |
|
| |
| Others | 3 (1) |
|
| |
| Grade (FIGO), n (%) | |
| I | 160 (44) |
|
| |
| II | 72 (20) |
|
| |
| III | 130 (36) |
|
| |
| Grade (nuclear) | |
| 1 | 123 (34) |
|
| |
| 2 | 104 (29) |
|
| |
| 3 | 135 (37) |
|
| |
| Tumour size (mm) | |
| ≤20 | 86 (24) |
|
| |
| >20 | 276 (76) |
|
| |
| Depth of myometrial invasion (%) | |
| ≤50 | 239 (66) |
|
| |
| >50 | 123 (34) |
|
| |
| LVI | |
| No | 264 (73) |
|
| |
| Yes | 98 (27) |
|
| |
| Recurrence | |
| No | 264 (73) |
|
| |
| Yes | 98 (27) |
|
| |
| Skil | |
| Negative | 123 (34) |
|
| |
| Positive | 237 (66) |
|
| |
| DAB2 | |
| Negative | 303 (84) |
|
| |
| Positive | 57 (16) |
|
| |
| Status | |
| Alive with no evidence of disease | 279 (77) |
|
| |
| Alive with evidence of disease | 37 (10) |
|
| |
| Dead of disease | 29 (8) |
|
| |
| Dead with no evidence of disease | 10 (3) |
|
| |
| Others | 11 (3) |
CCC, clear cell carcinoma; FIGO, International Federation of Gynecology and Obstetrics; LVI, lymphovascular invasion.
Figure 1.
Immunohistochemistry for DAB2 (A) and Skil (B) shows strong cytoplasmic immunoreactivity of tumour cells.
Table 2.
Association between Skil and DAB2 protein levels and clinical parameters
| Variables | Skil, P-value | DAB2, P-value |
|---|---|---|
| Age | 0.438104 | 0.31167 |
| Stage | 0.496313 | 0.214462 |
| Tumour size | 0.695067 | 0.026841 |
| LVI | 0.454003 | 0.193674 |
| MI | 0.351787 | 0.361266 |
| Grade (FIGO) | 0.202524 | 0.000484 |
| Grade (nuclear) | 0.357179 | 0.000883 |
| Subtype | 0.144599 | 0.000244 |
| Recurrence | 0.454003 | 0.193674 |
| DFS | 0.11452 | 0.039739 |
DFS, Disease-free survival; FIGO, International Federation of Gynecology and Obstetrics; LVI, lymphovascular invasion; MI, myometrial invasion.
The P-values are for Fisher's exact test, for testing the associations between protein levels of Skil and DAB2 and the clinical parameters. For this analysis, we considered the cut-off for each of the variables as follows. Age: group 1 (G1) <65 years; group 2 (G2) ≥65 years. Stage: G1, stage I and II; G2, stage III and IV. Tumour size: G1, ≤20 mm; G2, >20 mm. LVI: G1, absent; G2, present. MI: G1 ≤50%; G2, >50%. Grade: G1, grade 1 and 2; G2, grade 3. Histological subtype: G1, clear cell carcinoma (CCC) and serous; G2, endometrioid subtype. Recurrence: G1, negative; G2, positive. Status: G1, DFS; G2, all other outcomes.
PATIENT AND MRNA DATA
The characteristics of 70 patients and their samples are shown in Table 3. The median age of patients was 71 years. The median follow-up period was 1.75 years. Sixty-four percentage of cases were positive for Skil immunoexpression, and 36% were negative. Furthermore, 77% of cases showed immunoexpression of DAB2, and 23% did not show any expression. With the criteria of more than two-fold changes for significantly increased or decreased mRNA level, Smad3 mRNA was normally expressed in both endometrial cell lines, HEC1 and RL95, as compared with normal patient sampless. However, Skil, DAB2, Smad2, Smad4, TGF-β1, TβRI and TβRII showed decreased mRNA levels (Figure 2). As shown in Figure 3, when DAB2 and Skil protein expression determined by IHC was correlated with mRNA levels determined by RT-PCR, we found a significant association between DAB2 expression determined by IHC and DAB2 mRNA level determined by RT-PCR (P = 0.04). However, no such association was seen with Skil (P = 0.85). In 70 patient samples, decreased mRNA level was a frequent finding: DAB2 was down-regulated by at least two-fold in 77.1% of cases, Skil in 71.4%, Smad2 in 71.4%, Smad3 in 78.6%, Smad4 in 78.57%, TGF-β1 in 81.4%, TβRI in 78.6%, and TβRII in 84.3%. We also found a strong association among the mRNA levels of Skil, DAB2, Smad2, Smad3, Smad4, TGF-β1, TβRI and TβRII in these 70 samples (data not shown). Table 4 shows a multivariate analysis of mRNA levels and the clinical data and patient outcome. The Skil mRNA level was significantly associated with tumour stage (P = 0.03), Smad2 and Smad3 mRNA levels were associated with nuclear and FIGO grades (P = 0.03 and P = 0.02, respectively), and the Smad4 mRNA level was significantly associated with tumour size (P = 0.050), tumour subtype (P = 0.04), LVI (P = 0.03), nuclear and FIGO grade (P = 0.00 each), and DFS (P = 0.05). Finally, the TGF-β1 mRNA level was associated with DFS (P = 0.04), and the TβR2 level was associated with LVI (P = −0.05). There were no associations between any of the mRNA levels and the disease recurrence or time to recurrence on multivariate analysis. Even though the P-value did not reach statistical significance, Figure 4 clearly shows that tumours with positive expression of Skil protein as detected by IHC had a shorter recurrence time than those with negative Skil expression, making it clinically relevant. The inverse was true for DAB2; tumours with negative DAB2 protein expression had a shorter recurrence time than those with positive DAB2 expression.
Table 3.
Clinical and histopathological characteristics of the 70 patients analysed for mRNA level by reverse transcriptase PCR
| Age (years) | |
| Median | 71 |
|
| |
| Range | 36–92 |
|
| |
| Stage, n (%) | |
| I | 34 (49) |
|
| |
| II | 12 (17) |
|
| |
| III | 17 (24) |
|
| |
| IV | 7 (10) |
|
| |
| Subtype, n (%) | |
| Endometrioid | 42 (60) |
|
| |
| CCC + serous | 28 (40) |
|
| |
| Grade (FIGO), n (%) | |
| I | 16 (23) |
|
| |
| II | 16 (23) |
|
| |
| III | 38 (54) |
|
| |
| Grade (nuclear), n (%) | |
| 1 | 10 (14) |
|
| |
| 2 | 22 (32) |
|
| |
| 3 | 38 (54) |
|
| |
| Tumour size (mm), n (%) | |
| ≤20 | 6 (9) |
|
| |
| >20 | 64 (91) |
|
| |
| Depth of myometrial invasion (%), n (%) | |
| ≤50 | 41 (59) |
|
| |
| ×50 | 29 (41) |
|
| |
| LVI, n (%) | |
| No | 41 (59) |
|
| |
| Yes | 29 (41) |
|
| |
| Skil, n (%) | |
| Negative | 25 (36) |
|
| |
| Positive | 45 (64) |
|
| |
| DAB2, n (%) | |
| Negative | 54 (77) |
|
| |
| Positive | 16 (23) |
|
| |
| Recurrence, n (%) | |
| No | 43 (61) |
|
| |
| Yes | 23 (33) |
|
| |
| Others | 4 (6) |
|
| |
| Status, n (%) | |
| Alive with no evidence of disease | 47 (67) |
|
| |
| Alive with evidence of disease | 13 (18) |
|
| |
| Dead of disease | 8 (11) |
|
| |
| Others | 2 (3) |
CCC, clear cell carcinoma; FIGO, International Federation of Gynecology and Obstetrics; LVI, lymphovascular invasion.
Figure 2.
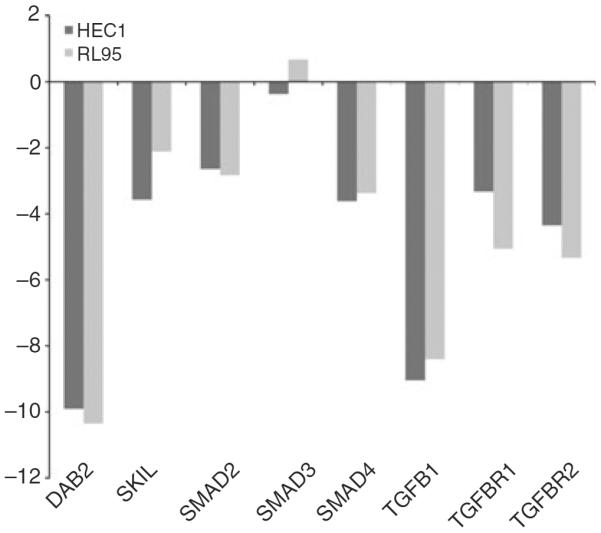
Bar plots showing the mRNA levels of Smad2, Smad3, Smad4, transforming growth factor (TGF)-β1, TGF-β receptor type I (TβRI), TGF-β receptor type II (TβRII), Skil and DAB2 in each of the HEC1 and RL95 cancer cell lines as compared with normal endometrial tissue.
Figure 3.
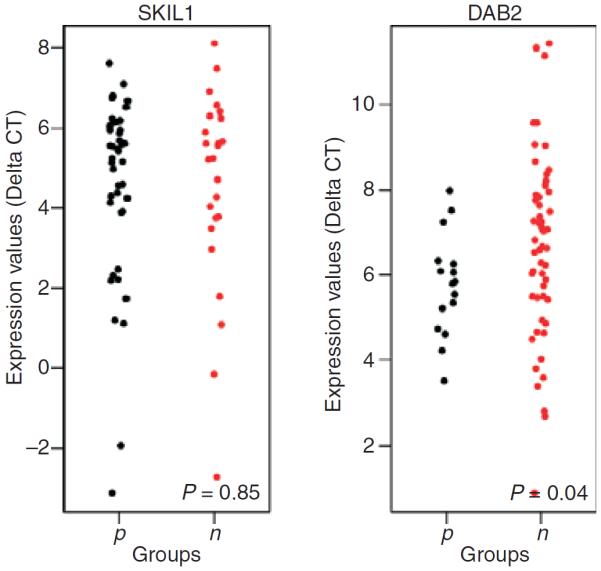
Scatter plots showing the association between the mRNA levels and immunohistochemical expression for Skil and DAB2 in 70 samples. A significant association between mRNA level and immunohistochemical expression was seen for DAB2 (P = 0.04). However, no such association was found for Skil (P = 0.85).
Table 4.
Multivariate analysis among ΔCTs of genes and clinical variables
| Stage |
Tumour size |
Subtype |
MI |
LVI |
Grade (FIGO) |
Grade (nuclear) |
Recurrence |
Good outcome |
|
|---|---|---|---|---|---|---|---|---|---|
| P-value | P-value | P-value | P-value | P-value | P-value | P-value | P-value | P-value | |
| DAB2 | 0.77 | 0.51 | 0.61 | 0.11 | 0.25 | 0.65 | 0.65 | 0.7 | 0.58 |
|
| |||||||||
| Skil | 0.03 | 0.92 | 0.83 | 0.41 | 0.24 | 1 | 1 | 0.88 | 0.99 |
|
| |||||||||
| Smad2 | 0.7 | 0.06 | 0.16 | 0.98 | 0.07 | 0.03 | 0.03 | 0.83 | 0.32 |
|
| |||||||||
| Smad3 | 0.33 | 0.24 | 0.15 | 0.19 | 0.52 | 0.02 | 0.02 | 0.34 | 0.45 |
|
| |||||||||
| Smad4 | 0.24 | 0.05 | 0.04 | 0.64 | 0.03 | 0 | 0 | 0.5 | 0.05 |
|
| |||||||||
| TGF-β1 | 0.84 | 0.65 | 0.97 | 0.15 | 0.22 | 0.12 | 0.12 | 0.47 | 0.04 |
|
| |||||||||
| TβRI | 0.88 | 0.56 | 0.68 | 0.74 | 0.09 | 0.78 | 0.78 | 0.25 | 0.38 |
|
| |||||||||
| TβRII | 0.36 | 0.43 | 0.09 | 0.33 | 0.05 | 0.81 | 0.81 | 0.15 | 0.35 |
CT, Cycle threshold; FIGO, International Federation of Gynecology and Obstetrics; LVI, lymphovascular invasion; MI, myometrial invasion; TβRI, transforming growth factor-β receptor type I; TβRII, transforming growth factor-β receptor type II; TGF, transforming growth factor.
P-values are for for testing of the association among mRNA levels of the eight genes and the clinical variables. Logistic regression was performed on the two groups, and the grouping that we considered for this analysis was similar to the one used in Table 2.
Figure 4.
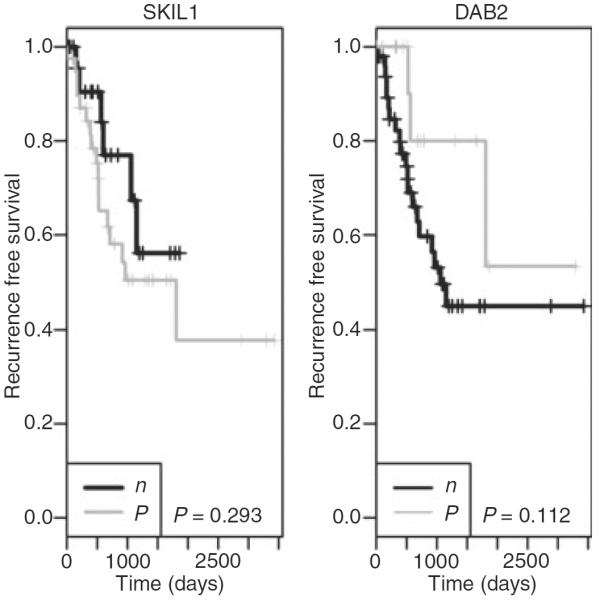
The survival curve showing the recurrence time of 70 patients stratified by Skil and DAB2 protein expression. Even though the P-value is not significant (owing to the relatively small sample size), patients with Skil-positive tumours tend to have longer recurrence times than those with negative Skil expression. On the other hand, patients with DAB2-positive tumours tend to have shorter recurrence times than those with negative DAB2 expression.
Discussion
TGF-β signalling is a very complex pathway, with alteration of a single component being sufficient to render cancer cells unresponsive to TGF-β. Impairment of the TGF-β–Smad pathway can result in the prevention of growth inhibition, initiation of cell proliferation, and carcinogenesis. Our results show that Smad2, Smad3, Smad4, TGF-β1, TβRI, TβRII, Skil and DAB2 were down-regulated in the vast majority of tumour samples. Apart from Smad3, this was also the case in two separate EC cancer cell lines. These results confirm that alterations of components of the TGFβ Smad-dependent signalling pathway and their regulators play a role in the pathogenesis of human EC. We also found a strong correlation among Smad2, Smad3, Smad4, TGF-β1, TβRI, TβRII, Skil and DAB2 mRNAs (data not shown), confirming their close networking at the transcriptional level in human EC.
Smad4 was first identified as a TSG of pancreatic cancer in 1996, and it was designated DPC4 (homozygously deleted in pancreatic carcinoma, locus four).19–21 Inactivation of the Smad4 gene through the loss of heterozygosity or intragenic mutations such as missense, nonsense and frameshift mutations at the Mad homology two regions is frequent in pancreatic and colonic cancers. Smad4 proved to be a prognostic factor in numerous cancers, where loss of Smad4 expression in gastric, colorectal and breast cancers correlated with poor survival.22–24 In published reports, low Smad4 mRNA levels were associated with tumours with deep MI. However, our study does not support this finding.17 On multivariate analysis, we found that the Smad4 mRNA level was significantly associated with both nuclear and FIGO grades, tumour subtype, tumour size, LVI, and DFS. Thus, tumours with higher Smad4 mRNA levels were more likely to be >20 mm, to be of endometrioid histological subtype, and to lack LVI, whereas tumours with lower Smad4 mRNA levels were more likely to be of high grade, and to have a worse clinical outcome. These data suggest that Samd4 may serve as a prognostic factor in women with EC; this should be confirmed by larger studies.
TGF-β1 plays an important role in promoting tumour progression. Studies have shown that TGF-β1 mRNA is usually down-regulated in endometrioid adenocarcinoma.17 In our study, TGF-β1 was down-regulated in EC cell lines and a high percentage of EC samples. Furthermore, high TGF-β1 mRNA level was an independent factor for predicting poor prognosis, lending credence to the theory that the TGF-β1 could be a target molecule for functional inactivation in EC. A possible explanation of why tumours with high TGF-β1 levels have a poor prognosis may be that, as tumour cells escape the growth-inhibitory response of TGF-β1, they are more prone to produce massive amounts of these proteins, leading to tumour acquisition of an advantage for tumour cell survival.25
TβRI and TβRII have been shown to be down-regulated in numerous cancer types, and the loss of their protein expression as determined by IHC is a marker for poor prognosis in oesophageal squamous cell carcinoma.11–13 Decreased levels of TβRI and TβRII mRNA have been reported previously in endometrioid-type adenocarcinoma.15,16 Both TβRI and TβRII mRNA were down-regulated in EC cell lines and in the vast majority of our samples. As previously seen, protein expression of both TβRI and TβRII as determined by IHC was reduced in endometrioid adenocarcinoma in comparison with normal endometrium. The aforementioned data suggest that TβRI and TβRII were lost at the transcriptional level of the gene. In those studies and in our study, neither TβRI nor TβRII had an impact on patient outcome. Previous methylation studies have demonstrated that promoter hypermethylation is not the major cause of this loss, but rather that it is caused by a frameshift mutation via mismatch repair deficiency.16
Disabled-2 is a regulator protein that acts as an adaptor molecule, serving to bridge the TGF-β receptor complex to the Smad pathway. Accumulating evidence highlights the role of DAB2 as a potent regulator of cancer cell growth. Loss of DAB2 expression has been reported as an early occurrence in ovarian, oesophageal and breast carcinoma, but its clinical significance remains to be determined.6,26,27 In our series, we found that DAB2 mRNA was down-regulated in EC cell lines and EC human samples in comparison with normal endometrium. There was also an association between DAB2 mRNA level as determined by real-time PCR and protein expression as determined by IHC. On univariate analysis, tumours with reduced/loss of DAB2 protein most often presented as high-grade tumours with serous histological subtypes, and had a poor prognosis and a shorter disease-free time before recurrence. Thus, loss of DAB2 could be an indicator of poor outcome in patients diagnosed with EC.
Skil is a potent negative regulator of the TGF-β signalling pathway, and has been implicated in the regulation of cell differentiation. Skil simultaneously interacts with Samd2/3 and with Smad4, blocking the ability of the Smad complex to activate transcription of TGF-β target genes. Skil and Sno have been long considered to be tumour promoters. However, recent studies have shown that they can have a complex function, having both pro-oncogenic and anti-oncogenic properties in cancers.5 Loss of Skil has been seen as an early event in colorectal and oesophageal carcinomas.7,28 Skil was down-regulated in EC cell lines and in most of our human samples. A statistically significant association between Skil mRNA levels and tumour stage was also seen. Therefore, tumours that exhibit high Skil mRNA levels are more likely to present at an advanced stage. This suggests an oncogenic property of Skil in EC. No association was found between the Skil mRNA level and protein expression. This finding could be attributable to the Skil gene itself and/or to the IHC methodology, including protein degradation, post-translational modifications, and method of antigen retrieval. However, the lack of studies in the literature on DAB2 and Skil in EC made comparison of our study with other studies impossible.
In summary, we have found a profound deregulation of the TGF-β–Smad signalling pathway in EC that most likely leads to cancer growth, invasion, and poor patient prognosis. In future studies, we would like to: (i) further evaluate protein expression of the remaining components of this pathway, such as Smad2, Smad3, Smad4, TGF-β1, TβRI, and TβRII, and correlate their expression with the clinicopathological data of the 365 patients; and (ii) perform a knockdown analysis of a specific gene in this pathway, using RNA interference, to evaluate its function in endometrial carcinogenesis. Through better understanding of the precise mechanisms that regulate TGF-β and its cascade in EC, we could successfully develop therapies targeting the TGF-β signalling pathway. This targeted therapy could be of great benefit for patients with tumours that are resistant to conventional chemo-therapy, especially patients with recurrence and widespread disease.
Acknowledgements
The authors would like to thank Mary Vaughn for performing the IHC, and Timothy Dolan for his search of the pathology archives. We also thank Heidi Wagner for her review of the manuscript.
Abbreviations
- CT
cycle threshold
- DAB2
Disabled-2
- DFS
disease-free survival
- EC
endometrial cancer
- FF
fresh frozen
- FIGO
International Federation of Gynecology and Obstetrics
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IHC
immunohistochemistry
- LVI
lymphovascular invasion
- MI
myometrial invasion
- PBS/T
phosphate-buffered saline/Tween-20
- PCR
polymerase chain reaction
- PRCI
Roswell Park Cancer Institute
- RQ
relative quantification
- TβRI
transforming growth factor-β receptor type I
- TβRII
transforming growth factor-β receptor type II
- TGF
transforming growth factor
- TLDA
TaqMan Low Density Custom Array
- TSG
tumour suppressor gene
- UMM
Universal Master Mix
Footnotes
Author contributions All authors contributed to the present study as follows: P. Mhawech-Fauceglia: designed and conducted the study, and wrote the manuscript. J. Kesterson and S. Akers: collected patient data and updated the follow-up. D. Wang: conducted statistical analysis. S. Lele: supervised and monitored the statistical analysis and the experiments. K. Clark: collaborated in the study design and performed the TLDA analysis. S. Lele and N. C. DuPont: helped with study design and assisted with manuscript review.
Conflict of interest This manuscript is an original contribution that has not been previously published in any form and is not under consideration for publication elsewhere. All authors have participated in the study to a significant extent. We are unaware of any conflicts of interest or any ethical issues.
The Health Sciences Institutional Review Board (HSIRB) of RPCI has authorized this research. We have adhered strictly to the Human Research Protections Program, and we have followed the authorization template for the use and disclosure of identifiable health information for research purposes.
P. Mhawech-Fauceglia is a full-time professor. All of her income comes from her New York State professional salary and RPCI. She has no other investments or sources of income or alliances.
References
- 1.Jaowlew SB. Transforming growth factor-β in cancer and metastasis. Cancer Metastasis Rev. 2006;25:435–457. doi: 10.1007/s10555-006-9006-2. [DOI] [PubMed] [Google Scholar]
- 2.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat. Rev. Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 3.Joshi A, Cao D. TGF-beta signaling, tumor microenvironment and tumor progression: the butterfly effect. Front. Biosci. 2010;15:180–194. doi: 10.2741/3614. [DOI] [PubMed] [Google Scholar]
- 4.Moustakas A, Souchelnytskyl S, Heldin C-H. Smad regulation in TGF-β signal transduction. J. Cell Sci. 2001;114:4359–4369. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- 5.Deheuninck J, Luo K. Ski and SnoN, potent regulators of TGF β signaling. Cell Res. 2009;19:47–57. doi: 10.1038/cr.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hocevar BA, Smine A, Xu X-X, Howe PH. The adaptor molecule Disabled-2 links the transforming factor β receptors to the Smad pathway. EMBO J. 2001;20:2789–2801. doi: 10.1093/emboj/20.11.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bravou V, Antonacopoulou A, Papadaki H, et al. TGF β repressors SnoN and Ski are implicated in human colorectal carcinogenesis. Cell Oncol. 2009;31:41–51. doi: 10.3233/CLO-2009-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mok SC, Chan WY, Wong KK, et al. DOC-2, a candidate tumor suppressor gene in human epithelial ovarian cancer. Oncogene. 1998;16:2381–2387. doi: 10.1038/sj.onc.1201769. [DOI] [PubMed] [Google Scholar]
- 9.Fazili Z, Sun W, Mittelstaedt S, Cohen C, Xu XX. Disabled-2 inactivation is an early step in ovarian tumorigenicity. Oncogene. 1999;18:3104–3113. doi: 10.1038/sj.onc.1202649. [DOI] [PubMed] [Google Scholar]
- 10.Santin AD, Zhan F, Bellone S, et al. Gene expression profiles in primary ovarian serous papillary tumors and normal ovarian epithelium: identification of candidate molecular markers for ovarian cancer diagnosis. Int. J. Cancer. 2004;112:14–25. doi: 10.1002/ijc.20408. [DOI] [PubMed] [Google Scholar]
- 11.Friedman E, Gold LI, Klimstra D, Zeng ZS, Winawer S, Cohen A. High levels of transforming growth factor-beta 1 correlate with disease progression in human colon cancer. Cancer Epidemiol. Biomarkers Prev. 1995;4:549–554. [PubMed] [Google Scholar]
- 12.Freiss H, Yamanaka Y, Buchler M, et al. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlated with decreased survival. Gastroenterology. 1993;105:1846–1856. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- 13.Fukai Y, Fukuchi M, Masuda N, et al. Reduced expression of transforming growth factor-β receptors is an unfavorable prognostic factor in human esophageal squamous cell carcinoma. Int. J. Cancer. 2003;104:161–166. doi: 10.1002/ijc.10929. [DOI] [PubMed] [Google Scholar]
- 14.Rozenblum E, Schutte M, Goggins SA, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731–1734. [PubMed] [Google Scholar]
- 15.Parekh TV, Gama P, Wen X, et al. Transforming growth factor β signaling is disabled early in human endometrial carcinogenesis concomitant with loss of growth inhibition. Cancer Res. 2002;62:2778–2790. [PubMed] [Google Scholar]
- 16.Sakaguchi J, Kyo S, Kanaya T, et al. Aberrant expression and mutations of TGF β receptor type II gene in endometrial cancer. Gynecol. Oncol. 2005;98:427–433. doi: 10.1016/j.ygyno.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Piestrzeniewicz-Ulanska D, Brys M, Semczuk A, Rechberger T, Jakowicki JA, Krajewska WM. TGF β signaling is disrupted in endometrioid-type endometrial carcinomas. Gynecol. Oncol. 2004;95:173–180. doi: 10.1016/j.ygyno.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 18.Creasman WT. Revision in classification by International Federation of Gynecology and Obstetrics. Am. J. Obstet. Gynecol. 1992;167:857–858. doi: 10.1016/s0002-9378(11)91607-x. [DOI] [PubMed] [Google Scholar]
- 19.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor supressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 20.Yang G, Yang X. Smad4-mediated TGFβ signaling in tumorigenesis. Int. J. Biol. Sci. 2010;6:1–8. doi: 10.7150/ijbs.6.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyaki M, Kuroki T. Role of Smad4 (DPC4) inactivation in human cancer. Biochem. Biophys. Res. Commun. 2003;306:799–804. doi: 10.1016/s0006-291x(03)01066-0. [DOI] [PubMed] [Google Scholar]
- 22.Kloth JN, Kenter GG, Spijker HS, et al. Expression of Smad2 and Smad4 in cervical cancer: absent nuclear smad4 expression correlates with poor survival. Mod. Pathol. 2008;21:866–875. doi: 10.1038/modpathol.2008.62. [DOI] [PubMed] [Google Scholar]
- 23.Xie W, Mertens JC, Reiss DJ, et al. Alterations of Smad signaling in human breast carcinoma are associated with poor outcome; a tissue microarray study. Cancer Res. 2002;62:497–505. [PubMed] [Google Scholar]
- 24.Alazzouzi H, Alhopuro P, Salovaara R, et al. Smad4 as a prognostic marker in colorectal cancer. Clin. Cancer Res. 2005;11:2606–2611. doi: 10.1158/1078-0432.CCR-04-1458. [DOI] [PubMed] [Google Scholar]
- 25.Gold LI. The role of transforming growth factor beta (TG-beta) in human cancer. Crit. Rev. Oncog. 1999;10:303–360. [PubMed] [Google Scholar]
- 26.Anupam K, Tusharkant C, Gupta SD, Ranju R. Loss of disabled-2 expression is an early event in esophageal squamous tumorigenesis. World J. Gastroenterol. 2006;12:6041–6045. doi: 10.3748/wjg.v12.i37.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagadi SAR, Prasad CP, Srivastava A, Prashad R, Gupta SD, Ralhan R. Frequent loss of Dab2 protein and infrequent promotor hypermethylation in breast cancer. Breast Cancer Res. Treat. 2007;104:277–286. doi: 10.1007/s10549-006-9422-6. [DOI] [PubMed] [Google Scholar]
- 28.Fukuchi M, Nakajima M, Fukai Y, et al. Increased expression of c-Ski as a co-repressor in transforming growth factor-beta signaling correlates with progression of esophageal squamous cell carcinoma. Int. J. Cancer. 2004;108:818–824. doi: 10.1002/ijc.11651. [DOI] [PubMed] [Google Scholar]