Abstract
Background
The potential role of bevacizumab in the treatment of ovarian granulosa-cell tumors has not been evaluated.
Case
An 82 year old woman with refractory ovarian granulosa-cell carcinoma was treated with bevacizumab with symptomatic relief of ascites.
Conclusion
Bevacizumab may have a role in the management of malignant ascites in the patient with refractory granulosa-cell carcinoma of the ovary which should be confirmed in a larger series of well selected patients.
Keywords: Bevacizumab, Ovarian granulosa-cell carcinoma, Ascites
Introduction
Recent attention has focused on the role of bevacizumab in the treatment of epithelial ovarian cancer [1]. However, the potential role of bevacizumab in the treatment of sex-cord tumors such as ovarian granulosa-cell tumors has not been evaluated. We present a case of refractory ovarian granulosa-cell carcinoma treated with bevacizumab with symptomatic relief of ascites.
Case report
An 82 year old woman originally presented to her gynecologist with post-menopausal bleeding. An endometrial biopsy revealed simple hyperplasia. A pelvic ultrasound showed a complex left adnexal mass measuring 8×5×8 cm. The patient was referred to our institution. Pelvic exam was consistent with ultrasound findings. On review of systems the patient’s only complaint in addition to vaginal bleeding was breast tenderness. Significant laboratory values included a CA125 of 41 U/mL and an inhibin >1000 pg/mL. Pathology review of her endometrial biopsy confirmed a minor focus of simple hyperplasia. These findings were suspicious for a hormonally active granulosa-cell tumor of the ovary.
The patient underwent an exploratory laparotomy with hysterectomy, bilateral salpingoophorectomy including resection of a cystic and solid left ovarian mass, pelvic and periaortic lymph node sampling, and partial omentectomy. Her postoperative course was uneventful. She was discharged home on post-operative day three. Final pathology was consistent with an ovarian granulosa-cell tumor, Stage IA and focal simple endometrial hyperplasia. Furthermore, the histology of the ovarian tumor revealed that the neoplastic cells had a very high mitotic rate 13/10HPF and flow cytometry revealed a diploid population. Tumor cells were strongly positive for VEGF monoclonal antibody by immunohistochemistry (Fig. 1).
Fig. 1.
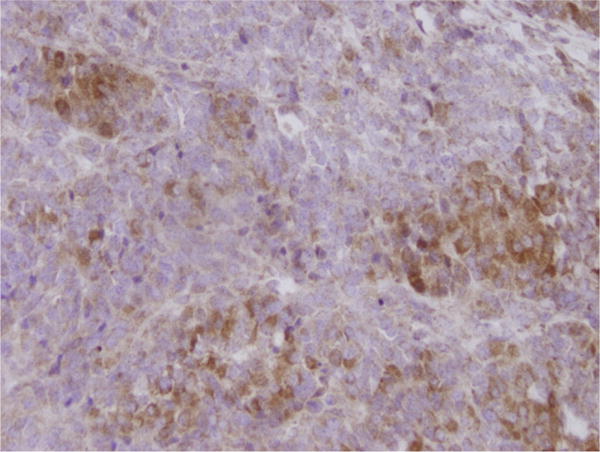
The ovarian granulosa-cell tumor cells expressed VEGF protein in a strong and diffuse pattern. The positivity was cytoplasmic. (×40).
The patient was followed with serial inhibin-A levels which were noted to have decreased to <0.3 pg/mL 1 month postoperatively. Seven months after surgery she presented with a complaint of abdominal distention. Her inhibin-A level was noted to have risen to 994.9 pg/mL. A CT showed evidence of carcinomatosis and ascites with a left-sided cystic pelvic lesion and nodules adjacent to the spleen. She was started on bleomycin 10 U days 1–3, etoposide 100 mg/m2 days 1–3 and cisplatin 75 mg/m2 on day 1, to be administered every 4 weeks. She required paracenteses for symptomatic ascites prior to and following her first cycle of chemotherapy with removal of 4.9 L and 4.8 L, respectively. Her chemotherapy course was complicated by treatment interruption and dosage reduction secondary to bleomycin-induced pulmonary toxicity and renal insufficiency. After completing six cycles of BEP chemotherapy a CT showed interval resolution of multiple peritoneal nodules and near complete resolution of ascites with only a small amount in the pelvis. Her post-treatment inhibin-A was 3.8 pg/mL. Three months later her inhibin-A increased to 51.8 pg/mL. A CT at that time showed peritoneal nodules and ascites. Paclitaxel and then cisplatin with etoposide were each discontinued after two doses because her ascites increased, requiring repeated paracenteses. She was switched to weekly paclitaxel 80 mg/m2 with bevacizumab 15 mg/kg every 3 weeks. A CT after eight doses of taxol and three doses of bevacizumab showed stable disease with minimal ascites. The patient has now received 8 doses of bevacizumab in combination with weekly paclitaxel. Her inhibin-A level has decreased from 111.0 pg/mL prior to initiation of bevacizumab to 37.9 pg/mL. She has not required any further paracenteses, has no evidence of ascites or disease on physical exam and has experienced no bevacizumab related toxicity. She has been able to maintain her physical activity, has a good appetite with maintenance of her nutritional status (albumin 3.6 g/dL).
Discussion
Tumor growth is dependent on angiogenesis. Vascular endothelial growth factor (VEGF) is a potent mitogen for vascular endothelial cells [2]. Bevacizumab is a humanized monoclonal antibody directed against VEGF. In phase II trials, single-agent bevacizumab has been shown to be active in the setting of persistent, recurrent, and platinum-resistant ovarian cancer [3,4]. Both of these studies enrolled only patients with epithelial ovarian carcinoma and those with peritoneal-origin carcinoma. Monk et al. observed a 16% response rate with single-agent bevacizumab in patients with advanced refractory epithelial ovarian cancer [5]. Cohn et al. reported an improvement in cancer-related symptoms in patients with refractory epithelial ovarian cancer treated with biweekly bevacizumab and weekly taxane chemotherapy [6]. Gynecologic Oncology Group Study 218 is currently underway to evaluate whether the addition of bevacizumab to standard chemotherapy concurrently and as consolidation therapy improves overall survival in patients with Stage III or IV epithelial ovarian or primary peritoneal cancer.
The role of bevacizumab in recurrent granulosa-cell of the ovary is unknown. In this case report, our patient with refractory granulosa-cell carcinoma of the ovary treated with paclitaxel and concurrent bevacizumab had symptomatic improvement in her malignant ascites. She has not required a paracentesis after starting paclitaxel and bevacizumab. Despite the bevacizumab being given in combination with paclitaxel, a known antiangiogenic in ovarian cancer cell lines [7], we believe the patient’s symptomatic improvement is due primarily to bevacizumab as she earlier had received single-agent paclitaxel with persistence of symptomatic ascites.
Recent evidence suggests that bevacizumab may have a potential role in the treatment of granulosa-cell tumors of the ovary. Schmidt et al. analyzed 32 granulosa-cell tumor specimens for immunohistochemical expression of VEGF [8]. Expression of VEGF was noted in 94% of granulosa-cell tumor specimens and in none of the ten normal ovary controls. The presence of VEGF implies that these tumors may be susceptible to targeting by bevacizumab.
The goal in managing malignant ascites in the recurrent ovarian cancer setting is to obtain symptom control with minimal side-effects of therapy. Cytotoxic agents as well as paracenteses are associated with inherent risks and discomfort. The use of paclitaxel with bevacizumab in our patient was undertaken with minimal side-effects and resulted in symptomatic relief of her ascites, abrogating the need for paracentesis. Similarly, Numnum et al. reported on their use of bevacizumab in the palliative setting for ovarian carcinoma patients with symptomatic relief of ascites in the four patients in their series [9].
Secondary to the rarity of ovarian granulosa-cell tumors, a randomized, placebo-controlled trial investigating the addition of bevacizumab to standard cisplatin-based chemotherapy is highly improbable. Considering that a majority of ovarian granulosa-cell carcinomas may express VEGF, bevacizumab’s mechanism of action, and the symptomatic improvement in our patient’s ascites, it is possible that bevacizumab may have a role in the management of malignant ascites in the patient with refractory granulosa-cell carcinoma of the ovary. Further investigation is needed to evaluate the efficacy and safety of bevacizumab in the setting of refractory ovarian granulosa-cell carcinoma in carefully selected patients.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- 1.Aghajanian C. The role of bevacizumab in ovarian cancer—an evolving story. Gyn Oncol. 2006;102:131–3. doi: 10.1016/j.ygyno.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–9. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 3.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2007;25(33):5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 4.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25(33):5180–6. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 5.Monk BJ, Han E, Josephs-Cowan CA, Pugmire G, Burger RA. Salvage bevacizumab (rhuMAB VEGF)-based therapy after multiple prior cytotoxic regimens in advanced refractory epithelial ovarian cancer. Gyn Oncol. 2006;102:140–4. doi: 10.1016/j.ygyno.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Cohn DE, Valmadre S, Resnick KE, Eaton LA, Copeland LJ, Fowler JM. Bevacizumab and weekly taxane chemotherapy demonstrates activity in refractory ovarian cancer. Gyn Oncol. 2006;102:134–9. doi: 10.1016/j.ygyno.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Hata K, Osaki M, Dhar DK, Nakayam K, Fujiwaki R, Ito H, et al. Evaluation of the antiangiogenic effect of Taxol in a human epithelial ovarian carcinoma cell line. Cancer Chemother Pharmacol. 2004;53(1):68–74. doi: 10.1007/s00280-003-0693-x. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M, Kammerer U, Segerer S, Cramer A, Kohrenhagen N, Dietl J, et al. Glucose metabolism and angiogenesis in granulosa cell tumors of the ovary: Activation of M2PK, TKTL1 and VEGF. Eur J Obstet Gynecol Reprod Biol. doi: 10.1016/j.ejogrb.2008.02.009. in press. [Corrected Proof. Available online 3 April, 2008] [DOI] [PubMed] [Google Scholar]
- 9.Numnum TM, Rocconi RP, Whitworth J, Barnes MN. The use of bevacizumab to palliate symptomatic ascites in patients with refractory ovarian carcinoma. Gyn Oncol. 2006;102:425–8. doi: 10.1016/j.ygyno.2006.05.018. [DOI] [PubMed] [Google Scholar]


