Abstract
Background. Host responses to infection are critical determinants of disease severity and clinical outcome. The development of tools to risk stratify children with malaria is needed to identify children most likely to benefit from targeted interventions.
Methods. This study investigated the kinetics of candidate biomarkers of mortality associated with endothelial activation and dysfunction (angiopoietin-2 [Ang-2], soluble FMS-like tyrosine kinase-1 [sFlt-1], and soluble intercellular adhesion molecule-1 [sICAM-1]) and inflammation (10 kDa interferon γ-induced protein [CXCL10/IP-10] and soluble triggering receptor expressed on myeloid cells-1 [sTREM-1]) in the context of a randomized, double-blind, placebo-controlled, parallel-arm trial evaluating inhaled nitric oxide versus placebo as adjunctive therapy to parenteral artesunate for severe malaria. One hundred eighty children aged 1–10 years were enrolled at Jinja Regional Referral Hospital in Uganda and followed for up to 6 months.
Results. There were no differences between the 2 study arms in the rate of biomarker recovery. Median levels of Ang-2, CXCL10, and sFlt-1 were higher at admission in children who died in-hospital (n = 15 of 180; P < .001, P = .027, and P = .004, respectively). Elevated levels of Ang-2, sTREM-1, CXCL10, and sICAM-1 were associated with prolonged clinical recovery times in survivors. The Ang-2 levels were also associated with postdischarge mortality (P < .0001). No biomarkers were associated with neurodisability.
Conclusions. Persistent endothelial activation and dysfunction predict survival in children admitted with severe malaria.
Keywords: angiopoietin-2, mortality, pediatric, severe malaria, sTREM-1
Malaria remains a leading cause of global morbidity and mortality and results in an estimated 306 000 deaths in African children under 5 years of age annually [1]. Severe malaria, which affects children during critical periods of brain development, is associated with long-term neurologic, cognitive, attention, and memory deficits in survivors [2–5].
Although reductions in malaria mortality are likely to continue from sustained investment in malaria control programs, mortality remains unacceptably high. The development and implementation of tools to rapidly stratify children according to risk-groups are urgently needed. In a recent study, we evaluated the ability of clinical risk scores developed in resource-constrained settings to predict all-cause in-hospital mortality in a cohort of Ugandan children with fever [6–8]. The Lambaréné Organ Dysfunction Score (LODS) was the simplest to compute, did not require equipment, and had the best prediction. Host biomarkers that could be integrated with simple clinical disease severity scores (eg, LODS) to (1) improve patient triage and (2) identify children most likely to benefit from targeted interventions would represent an important advance in improving outcomes in severe malaria. Furthermore, validated biomarkers of disease pathways that contribute to severe malaria would facilitate the design and conduct of clinical trials of pathway-specific interventions to improve outcome.
Investigations into the pathophysiology of severe malaria highlight the importance of host immune response in determining clinical outcome. Excessive proinflammatory responses and systemic endothelial activation have been associated with disease severity and mortality in several studies [9–17]. In this study, we further explore a panel of biomarkers associated with endothelial activation and dysfunction (angiopoietin-2 [Ang-2], soluble FMS-like tyrosine kinase-1 [sFlt-1], and soluble intercellular adhesion molecule-1 [sICAM-1]) and inflammation (10 kDa interferon gamma-induced protein [CXCL10/IP-10] and soluble triggering receptor expressed on myeloid cells-1 [sTREM-1]). These biomarkers represent a panel of candidate biomarkers of mortality previously identified in Ugandan children with severe malaria [14].
METHODS
Trial Design
This study is a secondary analysis of a randomized, double-blind, placebo-controlled trial evaluating inhaled nitric oxide (iNO) as an adjunctive therapy for severe malaria as described elsewhere [18, 19]. All children were treated with intravenous artesunate. The primary objective of this study was to evaluate the association between biomarker levels and trial arm by intention-to-treat analysis. Secondary objectives were to evaluate the association between the biomarkers, disease severity, clinical recovery, and mortality.
The study was approved by Makerere University School of Medicine Research Ethics Committee, Uganda National Council on Science and Technology, Ugandan National Drug Authority, and the University Health Network (Toronto, Canada). All parents or guardians provided written informed consent (ClinicalTrials.gov identifier NCT01255215).
Study Participants
The study was performed at Jinja Regional Referral Hospital in mid-eastern Uganda [20]. Children aged 1–10 years were eligible if they had a rapid diagnostic test positive for both Plasmodium falciparum histidine-rich protein 2 and lactate dehydrogenase ([LDH] First Response Malaria Ag. Premier Medical Corporation Limited, India) [21] as well as selected criteria for severe malaria including the following: repeated seizures (2 or more generalized seizures in 24 hours), prostration (child unable to sit unsupported or stand), impaired consciousness (Blantyre coma score [BCS] <5), and respiratory distress (age-related tachypnea with sustained nasal flaring, deep breathing, or subcostal retractions). Well known severity criteria for severe malaria, including severe anemia and hypoglycemia where not inclusion criterial in this trial, although they may have occurred alongside any of the other inclusion criteria. Exclusion criteria included the following: severe anemia (hemoglobin <5 g/dL or pallor) without any other features of severe malaria; known chronic illness; severe malnutrition, hemoglobinopathy, and clinical suspicion of bacterial meningitis [19].
Laboratory Procedures
Blood was drawn at admission and daily during the first 72 hours of hospital admission. Samples were sent to a central laboratory for quantification of parasite density and for complete blood counts [21]. Ethylenediaminetetraacetic acid plasma was stored at −80°C until biomarker testing, as described previously [21].
Statistical Analysis
Statistical analyses were done with SPSS version 20, Graph Pad Prism version 6, MedCalc version 13.1.2, and R (version 1.3.2, 2014). The longitudinal course of biomarkers was compared between study arms using linear mixed-effects (LME) models according to a prespecified analytic approach [18].
Data are presented as medians (interquartile range) and analyzed non-parametrically. The relationship between continuous variables and mortality was assessed using the Mann-Whitney U test. The Jonckheere-Terpstra test for ordered alternatives was used to assess (1) a linear trend between biomarker levels and BCS or LODS and (2) biomarker levels by quartile and time to clinical recovery. Categorical data were analyzed using Pearson's χ2 test. The Friedman's test with Dunn's multiple comparison test was used to test for changes in biomarker levels across hospitalization. To assess the ability of the clinical and biomarker models to discriminate between survivors and nonsurvivors, logistic regression was used, and the predicted probabilities were used to generate non-parametric receiver operating characteristic (ROC) curves. The areas under ROC (AUC) curves were compared using the method of DeLong.
The LME analysis of the in-hospital longitudinal course of log (Ang-2) over time in patients who died after discharge but within 6 months of illness versus those who were known to be alive at 6 months was performed. Time and survival group (death or survival at 6 months) were entered as fixed effects without an interaction term. Intercepts and slopes were modeled for each subject as random effects. Visual inspection of residual plots did not reveal deviations from homoscedasticity or normality. P values were obtained by likelihood ratio tests of the full model (including survival group) against the model without the survival group.
RESULTS
Longitudinal Assessment of Biomarkers From Admission to Discharge
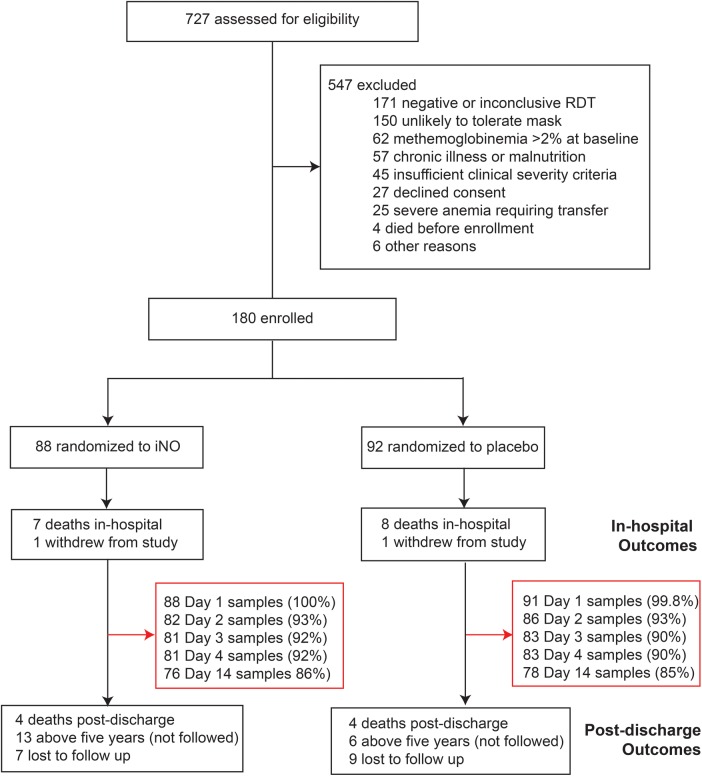
One hundred eighty children were enrolled in the clinical trial and randomized to receive either iNO (n = 88) or placebo (n = 92) (Figure 1). For a description of the trial population, see Table 1.
Figure 1.
Study flowchart for secondary biomarker analysis. Abbreviations: iNO, inhaled nitric oxide; RDT, rapid diagnostic test.
Table 1.
Description of Cohorta
| Characteristics | Cohort (n = 180) | In-hospital Mortality (n = 15) | Postdischarge Mortality (n = 8) |
|---|---|---|---|
| Demographics and History | |||
| Age, years | 2.0 (1.0–3.0) | 1.1 (1.0–2.0) | 1.1 (1.0–2.8) |
| Female sex | 78 (43.3) | 7 (53.3) | 5 (62.5) |
| Weight-for-age z-score | −1 (−2, 0) | −1 (−2, 0) | 0 (−2, 1) |
| Height-for-age z-score | −2 (−3, 0) | −2 (−3, 0) | 0 (−2, 1) |
| Weight-for-height z-score | 0 (−1, 1) | −1 (−2, 0) | 0 (−1, 1) |
| Fever before enrollment, days | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 2.0 (2.0–2.8) |
| Characteristics at admission | |||
| Temperature, °C | 37.9 (37.0–38.8) | 37.0 (36.6–37.8)† | 37.8 (36.9–39.0) |
| Heart rate, bpm | 162 (144–179) | 173 (156–183) | 156 (155–163) |
| Respiratory rate, bpm | 48 (38–62) | 61 (47–77)† | 55 (40–64) |
| Systolic blood pressure, mmHg | 110 (100–120) | 100 (90–126)† | 120 (120–130)† |
| Blantyre coma score (BCS) | 2 (2–3) | 2 (1–2) | 3 (2–4) |
| Lambaréné Organ Dysfunction Score | 2 (1–3) | 3 (2–3) | 1.5 (0.3–2.0) |
| Prostrate | 164/180 (91.1) | 15/15 (100.0) | 6/8 (75.0) |
| Convulsions >6 h after admission | 32/178 (18.0) | 1/15 (6.7) | 2/8 (25.0) |
| Impaired consciousness | 155/180 (86.1) | 13/15 (86.7) | 5/8 (62.5) |
| Comatose (BCS < 3) | 106/180 (58.9) | 14/15 (93.3)† | 2/8 (25.0) |
| Deep breathing | 86/180 (47.8) | 12/15 (80.0)† | 3/8 (37.5) |
| Severe anemia | 113/179 (63.1) | 13/15 (86.7)† | 8/8 (100.0)† |
| Hemoglobinuria | 33/180 (18.3) | 5/15 (33.3) | 2/8 (25.0) |
| Jaundice | 28/179 (15.6) | 3/15 (20.0) | 3/8 (37.5) |
| Shock | 16/180 (8.9) | 4/15 (26.7)† | 1/8 (12.5) |
| Number of severe malaria Criteriab | 4 (3–6) | 7 (6–7)† | 4 (3–5) |
| Laboratory Results | |||
| Parasitemia (parasites/µL) | 26 720 (2434–84 640) | 6600 (360–108 520) | 1460 (400–46 800)† |
| Hemoglobin (g/dL) | 4.8 (3.2–6.5) | 4.7 (2.8–5.0) | 3.4 (2.6–3.6)† |
| White blood cell count | 11.8 (7.6–19.3) | 10.7 (5.2–20.0) | 18.8 (8.7–24.1) |
| Platelet count | 71 (38–128) | 65 (40–133) | 131 (67–243) |
| Glucose | 6.7 (5.5–8.1) | 6.9 (4.4–7.9) | 6.0 (5.5–11.6) |
| Lactate | 3.6 (2.1–6.4) | 6.6 (3.7–10.6)† | 4.3 (2.5–7.3) |
| Base excess | −8 (−12, −4) | −16 (−19, −12)† | −8 (−11, −5) |
| Creatinine, µmol/L | 32 (23–42) | 39 (23–49) | 28 (24–35) |
| BUN, mg/dL | 16 (10–28) | 28 (18–42)† | 23 (10–43) |
| LDH, mU/mL | 482 (313–699) | 795 (482–1496)† | 716 (529–902)† |
| Outcome | |||
| Mortality | |||
| In-hospital | 15/180 (8.3) | — | — |
| 6 moc | 23/143 (16.1) | — | — |
| Neurologic disability at discharge | 13/163 (8.0) | — | 0/7 (0.0) |
Abbreviations: bpm, beats per minute; BUN, blood urea nitrogen; IQR, interquartile range; LDH, lactate dehydrogenase.
a Data are presented as median (IQR) or n (%).
b Criteria assessed: coma (BCS < 3), repeated seizures (>2 generalized seizures in 24 hours), respiratory distress (age-related tachypnea with sustained nasal flaring, deep breathing, or subcostal retractions), prostration (unable to sit unsupported), acute kidney injury (as described previously [22]), jaundice, hypoglycemia (<2.2 mM), hyperlactatemia (>5 mmol/L), severe anemia (Hb < 5 g/dL), shock.
c Children <5 years were observed for 6 months (n = 19 > 5 years, n = 16 lost to follow up, n = 2 withdrew).
† Indicates P < .05 between in-hospital deaths and surviving children; or post-discharge deaths and children known to survive to 6 months.
There were no differences in clinical recovery times, development of complications, or outcomes between trial arms [19]. To determine whether iNO therapy affected biomarker levels, we generated LME models for each biomarker. Because mortality was low and comparable between groups (8.0% and 9.9% in the iNO and placebo groups, respectively), analysis was performed on survivors. Biomarker levels did not differ between treatment arms for any markers (P > .05); therefore, biomarker data were pooled in subsequent analyses.
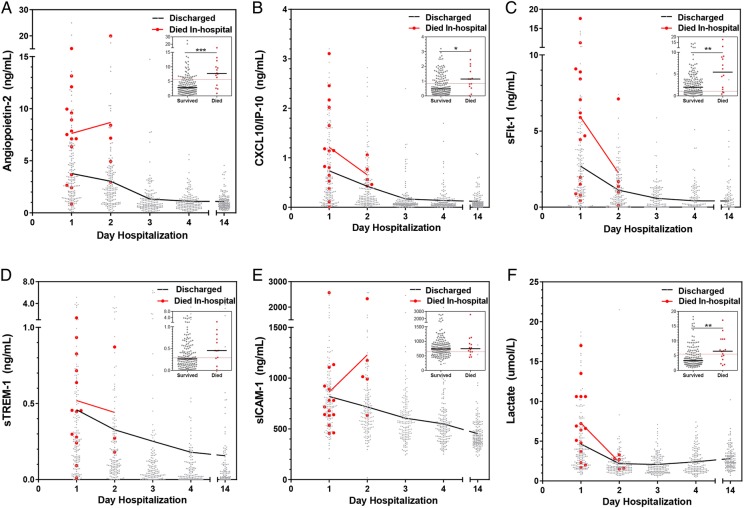
All biomarkers were higher at presentation and decreased over hospitalization ( P < .05; Figure 2). Using the day 14 sample as our normative value (convalescent sample), the majority of markers (Ang-2, CXCL10, sFlt-1, and sTREM-1) returned to baseline by day 3 in hospital (P > .05 day 3 vs day 14). Venous lactate had faster recovery, normalizing within 12 hours of admission, whereas sICAM-1 remained elevated on day 4.
Figure 2.
Biomarker levels in survivors vs nonsurvivors and kinetics of biomarker recovery. Scatter plot showing the distribution and median of biomarker levels on each day of hospitalization and at follow up (day 14). The median trajectory for survivors (black) and nonsurvivors (red) are shown. Inset depicts scatter plot of biomarker levels at admission in survivors and nonsurvivors (Mann-Whitney U test, where *P < .05, **P < .01, ***P < .001). The dotted red line in the mortality scatter plot represents the Youden index generated for that biomarker in the index population [14]. Abbreviations: CXCL10/IP-10, 10 kDa interferon γ-induced protein; sFlt-1, soluble FMS-like tyrosine kinase-1; sICAM-1, soluble intercellular adhesion molecule-1; sTREM-1, soluble triggering receptor expressed on myeloid cells-1.
We evaluated the association between admission biomarker levels and time to clinical recovery comparing the median recovery times across quartiles of biomarkers relative to the following: (1) time to first feed, (2) time to fever resolution, (3) time to localize pain, (4) time to regain consciousness (BCS = 5), (5) time to first sit, and (6) duration of hospitalization (Table 2). Time to first feed, time to first sit, and time to regain consciousness increased across quartiles of Ang-2 and sTREM-1, whereas time to fever resolution increased across quartiles of sICAM-1 and CXCL10 (P < .05 for all comparisons). Increased time to first sit and increased duration of hospitalization were observed across quartiles of sICAM-1.
Table 2.
Association Between Biomarker Levels by Quartile and Time to Clinical Recoverya
| Biomarker Quartile | Time to First Feed (Hour) | Time to Fever Resolution (Hour) | Time to Localize Pain (Hour) | Time to BCS = 5 (Hour) | Time to First Sit (Hour) | Duration Hospitalization (Hour) |
|---|---|---|---|---|---|---|
| Angiopoietin-2 | P = .032 | P = .247 | P = .804 | P = .036 | P = .002 | P = .429 |
| Q1 | 14.5 (10.0–23.3) | 6.2 (0–22.9) | 6.5 (0–14.9) | 11.7 (5.8–19.8) | 24.0 (13.0–46.2) | 64.0 (60.2–82.8) |
| Q2 | 15.2 (8.0–29.0) | 8.0 (0–35.6) | 4.1 (0–11.8) | 14.4 (7.0–25.4) | 27.6 (15.4–65.6) | 63.0 (57.9–81.4) |
| Q3 | 19.0 (10.0–39.6) | 9.4 (2.0–26.0) | 6.0 (0–16.4) | 17.2 (8.2–38.7) | 44.2 (24.4–70.2) | 71.8 (60.3–88.6) |
| Q4 | 22.5 (10.1–37.1) | 8.0 (0–33.3) | 6.5 (0–12.0) | 15.5 (8.5–32.0) | 39.1 (26.8–65.6) | 78.5 (59.0–103.0) |
| CXCL10 | P = .474 | P = .006 | P = .617 | P = .713 | P = .172 | P = .332 |
| Q1 | 16.0 (9.1–39.1) | 5.2 (0–14.3) | 6.0 (0–15.1) | 14.5 (8.3–33.8) | 30.6 (15.0–62.0) | 78.2 (61.8–97.8) |
| Q2 | 14.0 (8.5–35.0) | 10.6 (0–30.1) | 5.8 (0–17.0) | 14.0 (7.0–29.0) | 33.0 (19.0–58.8) | 64.0 (59.7–83.9) |
| Q3 | 15.5 (9.9–21.1) | 8.0 (0–20.7) | 5.8 (0–10.3) | 12.0 (6.0–18.0) | 29.0 (18.0–62.8) | 61.7 (56.5–79.0) |
| Q4 | 23.3 (11.8–43.4) | 16.0 (5.8–41.5) | 7.6 (0–14.8) | 20.5 (10.8–39.5) | 42.9 (25.4–72.0) | 78.3 (61.1–102.7) |
| sFlt-1 | P = .332 | P = .325 | P = .162 | P = .760 | P = .159 | P = .949 |
| Q1 | 16.1 (9.1–39.5) | 7.0 (0–25.4) | 6.3 (1.0–23.0) | 15.5 (5.7–38.9) | 24.7 (12.5–55.6) | 63.0 (59.7–85.3) |
| Q2 | 17.8 (10.7–43.6) | 7.2 (0–28.2) | 6.0 (0–12.1) | 14.5 (8.1–43.6) | 38.7 (20.0–68.2) | 68.8 (57.8–86.5) |
| Q3 | 16.3 (10.3–28.5) | 10.0 (0–30.6) | 4.1 (0–12.3) | 14.0 (7.6–25.0) | 34.3 (20.5–62.8) | 75.4 (59.5–83.5) |
| Q4 | 12.8 (8.1–26.8) | 9.0 (0–31.9) | 6.0 (0–15.0) | 13.5 (8.0–25.4) | 45.0 (18.7–63.8) | 68.4 (59.0–86.0) |
| sICAM-1 | P = .112 | P = .008 | P = .088 | P = .157 | P = .009 | P = .029 |
| Q1 | 16.0 (10.1–36.2) | 6.1 (0–25.7) | 0 (0–11.9) | 14.5 (7.0–32.1) | 26.0 (15.0–62.0) | 63.5 (59.0–86.0) |
| Q2 | 14.3 (8.5–23.8) | 7.1 (0–15.8) | 6.5 (0–11.3) | 11.5 (6.5–21.6) | 29.4 (16.7–48.1) | 62.0 (59.0–79.5) |
| Q3 | 14.8 (9.6–27.7) | 7.2 (0–24.6) | 6.0 (0–14.2) | 13.5 (8.1–21.8) | 32.5 (17.8–59.9) | 77.2 (60.5–84.0) |
| Q4 | 25.0 (10.2–45.3) | 19.8 (3.1–47.9) | 7.9 (0–18.1) | 22.5 (8.5–45.3) | 47.7 (27.9–80.4) | 78.8 (61.3–126.7) |
| sTREM-1 | P = .009 | P = .949 | P = .413 | P = .018 | P = .001 | P = .225 |
| Q1 | 12.7 (6.8–21.0) | 9.4 (0–26.3) | 5.7 (0–13.8) | 12.3 (6.0–19.0) | 25.1 (8.9–49.8) | 62.0 (59.0–83.0) |
| Q2 | 16.0 (8.0–30.3) | 6.6 (0–28.6) | 6.0 (0–18.4) | 12.2 (6.0–28.0) | 24.5 (12.5–39.7) | 63.5 (58.0–93.0) |
| Q3 | 17.8 (12.3–38.2) | 8.3 (0–25.5) | 5.3 (0–14.8) | 16.0 (8.5–31.0) | 46.2 (25.5–68.1) | 78.5 (60.4–86.3) |
| Q4 | 17.8 (11.4–43.0) | 6.8 (0–34.1) | 7.6 (0–13.3) | 14.5 (10.2–40.3) | 41.2 (22.6–69.6) | 76.0 (61.1–90.1) |
Abbreviations: Ang-2, angiopoietin-2; BCS, Blantyre coma score; CXCL10/IP-10, 10 kDa interferon γ-induced protein; IQR, interquartile range; sFlt-1, soluble FMS-like tyrosine kinase-1; sICAM-1, soluble intercellular adhesion molecule-1; sTREM-1, soluble triggering receptor expressed on myeloid cells-1.
a Data are presented as median (IQR). Data were analyzed non-parametrically using the Jonkcheere-Terpstra test for ordered alternatives. Ang-2: Q1, ≤1.41 ng/mL; Q2, 1.42 to ≤3.00 ng/mL; Q3, 3.01 to ≤5.73 ng/mL; Q4, >5.74 ng/mL. CXCL10: Q1, ≤0.15 ng/mL; Q2, 0.16 to ≤0.52 ng/mL; Q3, 0.53 to ≤1.17 ng/mL; Q4, 1.18 ng/mL. sFlt-1: Q1, ≤0.79 ng/mL; Q2, 0.80 to ≤2.01 ng/mL; Q3, 2.02 to ≤4.07 ng/mL; Q4, >4.08 ng/mL. sICAM-1: Q1, ≤596.76 ng/mL; Q2, 596.77 to ≤737.82 ng/mL; Q3, 737.83 to ≤927.21 ng/mL; Q4, >927.22 ng/mL. sTREM-1: Q1, ≤0.13 ng/mL; Q2, 0.14 to ≤0.27 ng/mL; Q3, 0.28 to ≤0.52 ng/mL; Q4, >0.53 ng/mL.
Clinical and Laboratory Findings Associated With In-hospital and Postdischarge Mortality
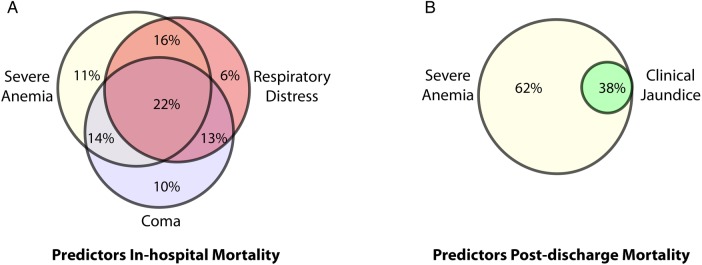
A description of the study cohort and an evaluation of factors associated with in-hospital and postdischarge mortality are shown in Table 1. The majority of deaths occurred within 24 hours of admission (9 of 15 in-hospital deaths, 60%). Only 1 child who died in-hospital survived beyond day 2. Acidosis was common with 34.3% of all children having lactate >5 mmol/L at admission; however, acidosis was more common in children who died (66.7%) and was associated with an increased risk of death (relative risk [RR], 1.2; 95% confidence interval [CI], 1.0–1.3). Presenting signs associated with in-hospital mortality were severe anemia, coma, features of respiratory distress, and shock (P < .05, χ2 test). The interrelationships between the most common clinical prognostic signs are shown in Figure 3. Overall, children who died in-hospital had more severe malaria criteria at presentation than children who survived (P < .0001; Table 1).
Figure 3.
Venn diagram depicting the frequency of clinical prognostic signs associated with in-hospital and postdischarge mortality. Venn diagram illustrating the most frequent presenting signs associated with (A) in-hospital mortality, and (B) postdischarge mortality assessed at 6 month follow-up. The percentages indicate the proportion of the population with the combination of signs.
All children who died after hospital discharge initially presented with severe anemia, were more likely to have jaundice (P = .051), and had lower parasitemia at presentation than survivors (Table 1, Figure 3). Elevated plasma LDH levels were associated with both in-hospital and postdischarge mortality. There was a trend towards an increased number of transfusions during hospitalization in children who died postdischarge (median number of transfusions: in survivors, 1; in postdischarge deaths, 2; P = .079). Repeat hemoglobin testing was performed in 71 children at day 14 based on clinical evidence of anemia/pallor at the time of follow up and/or anemia at discharge. There was no significant difference in hemoglobin levels at day 14 between children who survived to 6-month follow up and children who subsequently died (P = .248). There was no difference between the number of severe malaria criteria at presentation between children who survived until 6-month follow up and those who died after hospital discharge (P = .591; Table 1).
Association Between Biomarker Levels and Morbidity and Mortality
We explored the association between biomarkers and disease severity across common manifestations of severe malaria. Elevated levels of Ang-2, sFlt-1, and sTREM-1 were associated with derangements in multiple organ systems at presentation including respiratory distress, severe anemia, and hemoglobinuria (P < .05). After adjustment for lactate levels, the association between Ang-2, sFlt-1, and sTREM-1 and respiratory distress was lost, but the association between Ang-2 and sFlt-1 and severe anemia remained significant. Angiopoietin-2 and sTREM-1 were associated with disease severity, with biomarker levels increasing with increased LOD score (P < .05, test for trend). The CXCL10 and sICAM-1 were not associated with individual or composite measures of disease severity.
Levels of Ang-2, CXCL10, sFlt-1, sTREM-1, and lactate at presentation were higher in children who subsequently died in-hospital (n = 15 of 179; P < .001, P = .027, P = .004, P = .065, and P = .008, respectively) (Figure 2). We examined the kinetics of the biomarkers in children who died where a day 2 sample was available for testing (n = 6). The Ang-2 and CXCL10 levels remained elevated on day 2 in children who subsequently died (P = .004 and P = .03 , respectively). Although sICAM-1 levels were not higher at presentation among children who died in-hospital, sICAM-1 levels were elevated on day 2 of hospitalization in children who subsequently died compared with those who survived (P = .014).
Finally, we examined whether biomarker levels over hospitalization were associated with all-cause mortality at 6-month follow up (n = 8 deaths postdischarge). We compared biomarker levels on each day of hospitalization in children discharged alive who survived until 6-month follow up versus those who subsequently died. Angiopoietin-2 was the only biomarker associated with postdischarge mortality (Figure 4). Using an LME model of log-transformed Ang-2 concentration over time showed that longitudinal Ang-2 levels were significantly higher among patients who died postdischarge compared with known survivors (P < .0001). At admission, Ang-2 levels in the highest quartile were associated with a 1.2-fold increased risk of death postdischarge (RR, 1.2; 95% CI, 1.0–1.4). The Ang-2 levels at admission had an AUC of 0.82 (95% CI, .71–.93) to predict postdischarge death (P = .004). Because a total of 16 children were lost to follow up, we performed a sensitivity analysis assuming first that all children lost to follow up survived and second that all children lost to follow up died (including children over 5 years of age that were not followed). Using both approaches, Ang-2 remained significantly associated with postdischarge mortality (P = .002 assuming all children with unknown outcome at 6 months survived; P = .026 assuming all children with unknown outcome at 6 months died). None of the other biomarkers were associated with postdischarge mortality in the sensitivity analysis. There were no differences in levels of biomarkers and neurodisability at discharge (P > .05 for all markers).
Figure 4.
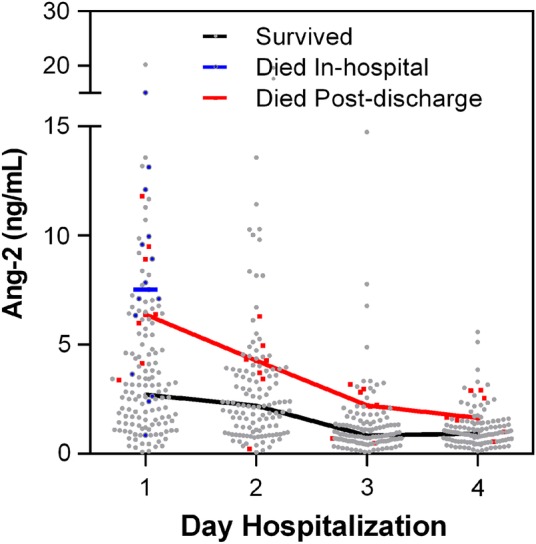
Angiopoietin-2 (Ang-2) levels over hospitalization in children who died after hospital discharge compared with known survivors. Scatter plot with line connecting the median levels of Ang-2 during hospitalization in children who died postdischarge, or those who survived until 6 months follow up, with the in-hospital deaths included as a reference.
Biomarkers to Improve Clinical Prediction
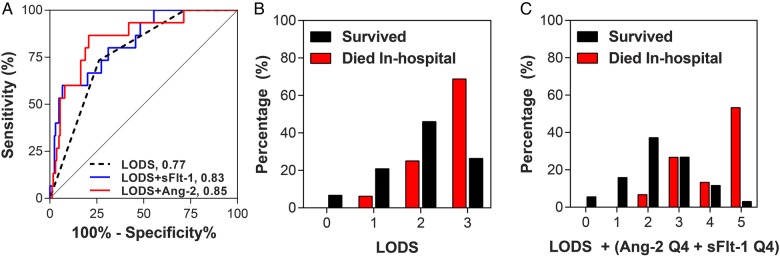
To assess whether integrating biomarker data into pediatric disease severity scores could improve prediction, we compared the AUC curves generated using the predictive probabilities derived from logistic regression models including the LOD score with or without the individual biomarkers. The model including LODS alone had an AUC of 0.77 (95% CI, .67–.88). Models including Ang-2 or sFlt-1 were significantly better than LODS alone at predicting in-hospital mortality with AUCs of 0.85 (95% CI, .79–.90; P = .03) and 0.83 (95% CI, .77–.88, P = .03), respectively (Figure 5A). Lactate, CXCL10, and sTREM-1 did not add value to LODS (P > .05).
Figure 5.
The predictive ability of the Lambaréné Organ Dysfunction Score (LODS) to predict in-hospital mortality with or without host biomarkers. (A) Receiver operating characteristic curves generated from the predictive probabilities of logistic regression models with in-hospital mortality as the dependent variable and LODS (black line), LODS and angiopoietin-2 (Ang-2) (red line), or LODS and soluble FMS-like tyrosine kinase-1 (sFlt-1) (blue line) as independent variables. (B) Histogram showing the distribution of LOD scores as a percentage of children who were discharged alive (black bars) vs those who died in-hospital (red bars). (C) Histogram showing the distribution of combined biomarker-LOD score incorporating as a percentage of children who were discharged alive (black bars) vs those who died in-hospital (red bars).
Using a complementary approach, we asked whether biomarkers could be added to LODS to generate a simple predictive clinical/biomarker model of malaria outcome [14, 17]. Children with a biomarker level in the highest quartile were at increased risk of death compared with children in the bottom 3 quartiles. Children with high Ang-2 (>5.75 ng/mL) and sFlt-1 (>4.08 ng/mL) levels had an increased risk of in-hospital mortality with RR of 3.0 (95% CI, 1.3–7.0) and 2.4 (95% CI, 1.2–4.9), respectively. We assigned a value of “1” if children had Ang-2 or sFlt-1 levels in the highest quartile or a value of “0” for levels in the bottom 3 quartiles, and we incorporated these data into a revised biomarker-LOD (bLOD) score. The LODS alone (score: 0–3) had an AUC of 0.77. A score with Ang-2 (score: 0–4) had a significantly higher AUC of 0.84 (P = .02). Adding sFlt-1 to the LOD score (score: 0–4) resulted in a trend towards a higher AUC of 0.83 (P = .06), whereas bLOD score including both Ang-2 and sFlt-1 had the highest AUC of 0.86 (95% CI, .81–.91). A LODS score >2 had a sensitivity to predict in-hospital death of 73.3% with a specificity of 73.8% and a positive and negative predictive value of 20.4% and 96.8%, respectively (Figure 5B). A bLOD score >2 had a sensitivity and specificity to predict death of 93.3% and 58.5% with corresponding positive and negative predictive values of 17.1% and 99.0%, respectively (Figure 5C).
DISCUSSION
In this study, we examined clinical and biomarker predictors of short- and long-term mortality in Ugandan children with severe malaria treated with intravenous artesunate. We validated a panel of biomarkers previously associated with malaria mortality in an independent population [14]. The Ang-2, CXCL10, and sFlt-1 were significantly elevated at admission in children who died in-hospital. Levels of the biomarkers decreased as children recovered, with most biomarkers normalizing to day 14 levels by day 3 of hospitalization. Elevated levels of Ang-2, sTREM-1, CXCL10, and sICAM-1 at admission were associated with prolonged clinical recovery times. Furthermore, elevated levels of Ang-2 over hospitalization were associated with postdischarge mortality. Using 2 complementary approaches, Ang-2 and sFlt-1 were shown to improve prognostic accuracy above LODS alone, whereas lactate did not. Together, these data validate these biomarkers as quantitative measures of disease severity that are associated with clinical recovery, and they may have utility in assessing both short- and long-term risk of death in children with severe malaria.
In this study, we confirm and extend reports from both adults and children describing Ang-2 as a marker of disease severity and mortality in severe malaria [12–17, 23, 24]. The Ang-2 levels were elevated in children who presented with respiratory distress, severe anemia, and hemoglobinuria and were associated with the LOD score. Children with higher Ang-2 levels at admission took longer to regain full consciousness, sit unsupported, or feed (Table 2). In addition, high Ang-2 levels were associated with increased risk of death in-hospital and within 6-month follow up. Children who died postdischarge showed an initial decrease in Ang-2 but had persistently higher Ang-2 levels on day 4 of hospitalization (Figure 4). Together, these data support the idea that endothelial activation is an important determinant of clinical outcome and recovery in severe malaria. More importantly, these data also suggest that persistent endothelial activation and dysfunction may predict and impact future survival.
Children who died postdischarge had clinical features suggestive of hemolysis (severe anemia, elevated plasma LDH, clinical jaundice), which may be associated with persistent endothelial activation [25]. Despite a limited number of deaths in this study, we show that Ang-2 improved prediction over LODS alone using logistic regression models or a simple scoring system to predict outcome. These data are supported by a previous report from Malawi, where Ang-2 added value to clinical predictors of mortality, but lactate did not [17]. Angiopoietin-2 is a robust biomarker that is readily detectable in serum, plasma, and lysed whole blood [13, 14, 16, 17, 24, 26], making it one of the most promising prognostic biomarkers identified to date.
Soluble FMS-like tyrosine kinase-1 is a splice variant of the vascular endothelial growth factor receptor (VEGFR)-1 (Flt-1) receptor. Soluble FMS-like tyrosine kinase-1 binds VEGF preventing it from signaling. Soluble FMS-like tyrosine kinase-1 is expressed by monocytes and endothelium and is induced by hypoxia and VEGF [27, 28]. The role of sFlt-1 in critical illness is unclear; however, it may be involved in maintaining vascular integrity by sequestering VEGF and preventing it from inducing vascular permeability. Soluble FMS-like tyrosine kinase-1 levels correlated with lactate and were associated with the presence of respiratory distress, severe anemia, and hemoglobinuria at presentation. Furthermore, sFlt-1was associated with increased risk of in-hospital mortality. Our findings are consistent with a report of elevated sFlt-1 in severe malaria and in children with fatal severe malarial anemia [14]. Soluble FMS-like tyrosine kinase-1 is synergistically induced in mononuclear cells treated with complement C5a and malaria bioactive product pfGPI [29] and has been implicated in the pathogenesis of placental malaria [30]. The FLT1 genotype is under natural selection in malaria-endemic areas [31]. Together, these findings support an important role for sFlt-1 in malaria responses. Increased sFlt-1 levels have also been associated with disease severity [32] and mortality in sepsis [33], consistent with increased sFlt-1 expression in conditions with systemic inflammation and vascular leak. Mechanistic studies will be required to elucidate whether increased sFlt-1 levels in malaria are contributing to disease severity or are a consequence of tissue hypoxia and/or increased VEGF expression [15, 34].
Intercellular adhesion molecule-1 binds parasitized erythrocytes contributing to sequestration, microvascular occlusion, tissue hypoxia, and endothelial dysfunction [35, 36]. Intercellular adhesion molecule-1 is expressed on endothelium, monocytes, and lymphocytes and can be cleaved through ectodomain shedding by matrix metalloproteinase-9 [37] in response to inflammatory stimuli, including TNF [38]. Several groups have reported elevated sICAM-1 levels in severe [15, 39–42] and fatal malaria [14, 39]. In this cohort, sICAM-1 was elevated at admission and decreased over the course of hospitalization but remained elevated at day 4, which is consistent with reports that sICAM-1 remains elevated for at least 1 month after severe malaria [40, 43]. Furthermore, elevated sICAM-1 was associated with prolonged clinical recovery times, including time to fever resolution, time to first sit, and duration of hospitalization. Although sICAM-1 was not higher at admission in the children who died on the first day of hospitalization, there was a trend of increasing sICAM-1 levels on day 2 of hospitalization in children who subsequently died in-hospital. These data are consistent with sICAM-1 representing a marker of disease severity in malaria.
The CXCL10/IP-10 is a proinflammatory chemokine that is a prognostic marker in malaria, with studies reporting elevated CXCL10 in both children and adults with fatal malaria [14, 44, 45]. Recent studies suggest CXCL10 may be involved in the depletion of endothelial progenitor cells (EPCs) in malaria [46]. In vitro studies implicated heme-induced apoptosis of EPCs through TLR4-mediated CXCL10 expression [46]. Endothelial progenitor cells are important for microvascular repair, and low levels of circulating EPCs have been reported in Ghanaian children with cerebral malaria [47]. Furthermore, CXCL10 has also been shown to exert an apoptotic effect on human brain microvascular endothelial cells and neuroglia cells in vitro [48]. In this cohort, CXCL10 levels were elevated in children who died in-hospital, were associated with a prolonged time to fever resolution, and returned to baseline by day 3 in survivors. We did not observe an association between CXCL10 levels and specific manifestations of severe malaria (eg, cerebral malaria), or neurologic outcomes, suggesting that elevated CXCL10 levels are not specific to neurological manifestations of malaria but are associated with disease severity.
Soluble triggering receptor expressed on myeloid cells-1 is an inflammatory protein recently described as a novel prognostic marker in Ugandan children with severe malaria [14]. In this study, we confirmed that sTREM-1 is associated with disease severity in pediatric severe malaria. The sTREM-1 levels at admission were associated with time to first sit, time to regain consciousness, and time to first feed. Although sTREM-1 was not significantly associated with mortality in this study, we had limited power given the small sample size. The sTREM-1 levels were increased in children with respiratory distress, severe anemia, and hemoglobinuria at presentation and were associated with the LOD score.
This study prospectively validated a panel of mortality biomarkers in the context of a clinical trial. There were several strengths to this study including longitudinal sampling, which enabled us to examine the kinetics of biomarker recovery in survivors. The collection of detailed clinical information enabled us to evaluate the association between biomarkers and recovery times in survivors. We were able to evaluate clinical and biochemical predictors associated with late death in pediatric malaria. Although mortality in-hospital was characterized by multiorgan dysfunction, acidosis, endothelial activation, and inflammatory biomarkers suggestive of a progressive and overwhelming systemic inflammatory response syndrome, children who died postdischarge were all admitted with severe anemia, and they were more likely to have jaundice, elevated LDH, lower parasitemia, and persistent elevations in Ang-2 over hospitalization. We speculate that children who died postdischarge had chronic hemolysis, perhaps mediated through repeated malaria infections, because children with known hemoglobinopathies were excluded from the trial. Although we cannot confirm the cause of death in these children, rebound anemia and/or infection are likely causes.
This study was not powered to detect differences in mortality between study arms and included a relatively small number of fatal cases, so we were limited in our ability to perform multivariate analyses to examine independent predictors of in-hospital and postdischarge mortality. In addition, we only followed children less than 5 years out to 6 months for cognitive testing (19 children were excluded from follow up according to the study protocol), and 16 children were lost to follow up. It is possible that some of the children lost to follow up represent additional study deaths. We performed a sensitivity analysis assuming that all of these children died and all survived, and the results remained significant. Because many health centers in resource-limited settings lack adequate diagnostics to investigate the etiology of illness in children presenting with fever, it will be important to validate this panel of biomarkers (1) in the context of both malarial and nonmalarial fever and (2) to evaluate whether these biomarkers can predict the development of complications to guide referral. Additional validation studies will be required to assess (1) whether biomarkers could be used before hospital discharge to identify children in need of more intensive follow up and (2) how information from fast recovery (eg, venous lactate) versus slower recovery (eg, Ang-2, sICAM-1) biomarkers could be integrated for continual risk assessment over hospitalization.
CONCULSIONS
The results from this study confirm and extend previous reports that systemic endothelial activation is associated with morbidity and mortality in malaria. In this study, we demonstrate that host biomarkers represent objective measures to monitor clinical recovery over hospitalization. Furthermore, persistent endothelial activation predicted both short- and long-term survival in children with severe malaria. These findings suggest a need for more intensive follow up in children presenting with anemia and evidence of persistent endothelial activation. Further validation of these biomarkers across field-sites in malaria-endemic areas are required to evaluate whether host biomarkers have utility in identifying children most likely to benefit from targeted interventions. Finally, because some of these markers (for example, Ang-2) are critical regulators of endothelial activation and integrity, they provide a direct measure of the degree of underlying microvascular dysfunction and represent novel potential targets for directed intervention. Ultimately, strategies that reduce inflammation and improve endothelial function may reduce morbidity and mortality associated with severe malaria.
Supplementary Material
Acknowledgments
Disclaimer. The funders had no role in study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the article for publication.
Financial support. This work was supported by a kind donation from Kim Kertland; the Tesari Foundation; the Sandra Rotman Centre for Global Health; and the Canadian Institutes of Health Research (MOP-115160, -13721, -136813), Canada Research Chair in Molecular Parasitology (to K. C. K.), Canada Research Chair in Infectious Diseases and Inflammation (to W. C. L.), CIHR Clinician-Scientist Training Award (to M. H.) CIHR Post-PhD Fellowship (to A. L. C.).
Potential conflicts of interest. A. L. C., K. C. K., and W. C. L. are listed as inventors on a patent relating to biomarkers for malaria and for early determination of critical or life-threatening responses to illness. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. World Malaria Report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 2.Boivin MJ, Bangirana P, Byarugaba J et al. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics 2007; 119:e360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John CC, Bangirana P, Byarugaba J et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics 2008; 122:e92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter JA, Mung'ala-Odera V, Neville BG et al. Persistent neurocognitive impairments associated with severe falciparum malaria in Kenyan children. J Neurol Neurosurg Psychiatry 2005; 76:476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bangirana P, Opoka RO, Boivin MJ et al. Severe malarial anemia is associated with long-term neurocognitive impairment. Clin Infect Dis 2014; 59:336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helbok R, Kendjo E, Issifou S et al. The Lambaréné Organ Dysfunction Score (LODS) is a simple clinical predictor of fatal malaria in African children. J Infect Dis 2009; 200:1834–41. [DOI] [PubMed] [Google Scholar]
- 7.Kumar N, Thomas N, Singhal D et al. Triage score for severity of illness. Indian Pediatr 2003; 40:204–10. [PubMed] [Google Scholar]
- 8.Berkley JA, Ross A, Mwangi I et al. Prognostic indicators of early and late death in children admitted to district hospital in Kenya: cohort study. BMJ 2003; 326:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyke KE, Burges R, Cissoko Y et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun 2004; 72:5630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grau GE, Taylor TE, Molyneux ME et al. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med 1989; 320:1586–91. [DOI] [PubMed] [Google Scholar]
- 11.Othoro C, Lal AA, Nahlen B, Koech D et al. A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J Infect Dis 1999; 179:279–82. [DOI] [PubMed] [Google Scholar]
- 12.Yeo TW, Lampah DA, Gitawati R et al. Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci U S A 2008; 105:17097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovegrove FE, Tangpukdee N, Opoka RO et al. Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS One 2009; 4:e4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erdman LK, Dhabangi A, Musoke C et al. Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: a retrospective case-control study. PLoS One 2011; 6:e17440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conroy AL, Phiri H, Hawkes M et al. Endothelium-based biomarkers are associated with cerebral malaria in Malawian children: a retrospective case-control study. PLoS One 2010; 5:e15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conroy AL, Lafferty EI, Lovegrove FE et al. Whole blood angiopoietin-1 and -2 levels discriminate cerebral and severe (non-cerebral) malaria from uncomplicated malaria. Malar J 2009; 8:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conroy AL, Glover SJ, Hawkes M et al. Angiopoietin-2 levels are associated with retinopathy and predict mortality in Malawian children with cerebral malaria: a retrospective case-control study. Crit Care Med 2012; 40:952–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkes M, Opoka RO, Namasopo S et al. Inhaled nitric oxide for the adjunctive therapy of severe malaria: protocol for a randomized controlled trial. Trials 2011; 12:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawkes MT, Conroy AL, Opoka RO et al. Inhaled nitric oxide as adjunctive therapy for severe malaria: a randomized controlled trial. Malar J 2015; 14:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Idro R, Aloyo J, Mayende L et al. Severe malaria in children in areas with low, moderate and high transmission intensity in Uganda. Trop Med Int Health 2006; 11:115–24. [DOI] [PubMed] [Google Scholar]
- 21.Hawkes M, Conroy AL, Opoka RO et al. Use of a three-band HRP2/pLDH combination rapid diagnostic test increases diagnostic specificity for falciparum malaria in Ugandan children. Malar J 2014; 13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conroy AL, Hawkes M, Elphinstone RE et al. Acute kidney injury is common in pediatric severe malaria and is associated with increased mortality. Open Forum Infect Dis 2016; 3:ofw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prapansilp P, Medana I, Mai NT et al. A clinicopathological correlation of the expression of the angiopoietin-Tie-2 receptor pathway in the brain of adults with Plasmodium falciparum malaria. Malar J 2013; 12:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain V, Lucchi NW, Wilson NO et al. Plasma levels of angiopoietin-1 and -2 predict cerebral malaria outcome in Central India. Malar J 2011; 10:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elphinstone RE, Riley F, Lin T et al. Dysregulation of the haem-haemopexin axis is associated with severe malaria in a case-control study of Ugandan children. Malar J 2015; 14:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conroy AL, Liles WC, Molyneux ME et al. Performance characteristics of combinations of host biomarkers to identify women with occult placental malaria: a case-control study from Malawi. PLoS One 2011; 6:e28540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nevo O, Soleymanlou N, Wu Y et al. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol 2006; 291:R1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito T, Takeda N, Amiya E et al. VEGF-A induces its negative regulator, soluble form of VEGFR-1, by modulating its alternative splicing. FEBS Letters 2013; 587:2179–85. [DOI] [PubMed] [Google Scholar]
- 29.Conroy A, Serghides L, Finney C et al. C5a enhances dysregulated inflammatory and angiogenic responses to malaria in vitro: potential implications for placental malaria. PLoS One 2009; 4:e4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conroy Andrea L, Silver Karlee L, Zhong K et al. Complement activation and the resulting placental vascular insufficiency drives fetal growth restriction associated with placental malaria. Cell Host Microbe 2013; 13:215–26. [DOI] [PubMed] [Google Scholar]
- 31.Muehlenbachs A, Fried M, Lachowitzer J et al. Natural selection of FLT1 alleles and their association with malaria resistance in utero. Proc Natl Acad Sci U S A 2008; 105:14488–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skibsted S, Jones AE, Puskarich MA et al. Biomarkers of endothelial cell activation in early sepsis. Shock 2013; 39:427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro NI, Schuetz P, Yano K et al. The association of endothelial cell signaling, severity of illness, and organ dysfunction in sepsis. Critical Care 2010; 14:R182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casals-Pascual C, Idro R, Gicheru N et al. High levels of erythropoietin are associated with protection against neurological sequelae in African children with cerebral malaria. Proc Natl Acad Sci U S A 2008; 105:2634–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beare NA, Harding SP, Taylor TE et al. Perfusion abnormalities in children with cerebral malaria and malarial retinopathy. J Infect Dis 2009; 199:263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanson J, Lee SJ, Hossain MA et al. Microvascular obstruction and endothelial activation are independently associated with the clinical manifestations of severe falciparum malaria in adults: an observational study. BMC Med 2015; 13:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiore E, Fusco C, Romero P, Stamenkovic I. Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene 2002; 21:5213–23. [DOI] [PubMed] [Google Scholar]
- 38.Becker JC, Dummer R, Hartmann AA et al. Shedding of ICAM-1 from human melanoma cell lines induced by IFN-gamma and tumor necrosis factor-alpha. Functional consequences on cell-mediated cytotoxicity. J Immunol 1991; 147:4398–401. [PubMed] [Google Scholar]
- 39.Tchinda VH, Tadem AD, Tako EA et al. Severe malaria in Cameroonian children: correlation between plasma levels of three soluble inducible adhesion molecules and TNF-[alpha]. Acta Tropica 2007; 102:20–8. [DOI] [PubMed] [Google Scholar]
- 40.Jakobsen PH, Morris-Jones S, Ronn A et al. Increased plasma concentrations of sICAM-1, sVCAM-1 and sELAM-1 in patients with Plasmodium falciparum or P. vivax malaria and association with disease severity. Immunology 1994; 83:665–9. [PMC free article] [PubMed] [Google Scholar]
- 41.Turner GD, Ly VC, Nguyen TH et al. Systemic endothelial activation occurs in both mild and severe malaria. Correlating dermal microvascular endothelial cell phenotype and soluble cell adhesion molecules with disease severity. Am J Pathol 1998; 152:1477–87. [PMC free article] [PubMed] [Google Scholar]
- 42.Cserti-Gazdewich CM, Dzik WH, Erdman L et al. Combined measurement of soluble and cellular ICAM-1 among children with Plasmodium falciparum malaria in Uganda. Malar J 2010; 9:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moxon CA, Chisala NV, Wassmer SC et al. Persistent endothelial activation and inflammation after Plasmodium falciparum infection in Malawian children. J Infect Dis 2014; 209:610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain V, Armah H, Tongren J et al. Plasma IP-10, apoptotic and angiogenic factors associated with fatal cerebral malaria in India. Malar J 2008; 7:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Armah H, Wilson N, Sarfo B et al. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar J 2007; 6:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dickinson-Copeland CM, Wilson NO, Liu M et al. Heme-mediated induction of CXCL10 and depletion of CD34+ progenitor cells is Toll-like receptor 4 dependent. PLoS One 2015; 10:e0142328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gyan B, Goka BQ, Adjei GO et al. Cerebral malaria is associated with low levels of circulating endothelial progenitor cells in African children. Am J Trop Med Hyg 2009; 80:541–6. [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson NO, Solomon W, Anderson L et al. Pharmacologic inhibition of CXCL10 in combination with anti-malarial therapy eliminates mortality associated with murine model of cerebral malaria. PLoS One 2013; 8:e60898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.