During 2002–2011, fluoroquinolones were the most frequently prescribed antibiotic class for women aged ≥18 years with a diagnosis of uncomplicated urinary tract infection. Outpatient antibiotic stewardship initiatives should include efforts to reduce overuse of fluoroquinolones for uncomplicated UTI.
Keywords: adverse drug event, fluoroquinolone, outpatient antibiotic stewardship, uncomplicated urinary tract infection
Abstract
Background. Urinary tract infection (UTI) is one of the most common diagnoses leading to an antibiotic prescription for women seeking ambulatory care. Understanding current national outpatient antibiotic prescribing practices will help ongoing stewardship efforts to optimize antibiotic use; however, information on recent national outpatient antibiotic prescribing trends for UTI is limited.
Methods. We analyzed the National Ambulatory Medical Care and National Hospital Ambulatory Medical Care Survey datasets from 2002 to 2011. Outpatient visits of women aged ≥18 years with a diagnosis of uncomplicated UTI were included for analysis. We conducted weighted descriptive analyses, examined time trends in antibiotic prescribing, and used multivariable logistic regression to identify patient and provider factors associated with fluoroquinolone prescribing.
Results. A total of 7111 visits were identified. Eighty percent of visits resulted in an antibiotic prescription; fluoroquinolones were the most frequently prescribed antibiotics throughout the study period (49% overall). Older patients (adjusted odds ratio [AOR] for adults aged ≥70 years = 2.5; 95% confidence interval [CI], 1.6–3.8) and patients treated by internists (AOR = 2.0; 95% CI, 1.1–3.3) were more likely than younger patients and those treated by family practitioners to receive fluoroquinolones. Outpatient visits in the West US Census region were less likely to be associated with fluoroquinolone prescribing (AOR = 0.6; 95% CI, .4–1.0) compared with visits in the Northeast.
Conclusions. Fluoroquinolones were the most frequently selected antibiotic treatment for uncomplicated UTI in women during the study period. Outpatient antibiotic stewardship initiatives should include efforts to reduce overuse of fluoroquinolones for uncomplicated UTI.
Each year, approximately 97 million outpatient visits are associated with antibiotic prescriptions, and urinary tract infection (UTI) is one of the most common diagnoses leading to an antibiotic prescription for women seeking ambulatory care [1, 2]. Increasing fluoroquinolone use for UTI [3–6] has coincided with increases in the incidence of fluoroquinolone resistance among uropathogens [7], including ciprofloxacin-resistant Escherichia coli among US outpatients [8]. The Infectious Diseases Society of America (IDSA) guideline published in 1999 recommended the use of trimethoprim-sulfamethoxazole as first-line therapy in the treatment of acute uncomplicated bacterial cystitis [9]. Although trimethoprim-sulfamethoxazole is still a first-line treatment option for acute uncomplicated cystitis in the most recent guideline published in 2011, increases in antibiotic-resistant uropathogens since the 1999 guideline resulted in (1) the inclusion of nitrofurantoin among the first-line antibiotic options and (2) emphasis on using fluoroquinolones as an alternative, second-line option [10].
The increase in fluoroquinolone resistance has major implications for the treatment of more serious infections, such as community-acquired pneumonia, healthcare-associated pneumonia, and complicated UTIs [7]. An additional complication of fluoroquinolone use is subsequent infection with Clostridium difficile, manifesting as diarrhea that often recurs and can progress to sepsis and death; the Centers for Disease Control and Prevention (CDC) has estimated that there are approximately 450 000 C difficile infections in the United States each year [11]. Exposure to fluoroquinolone antibiotics has been well described as a risk factor for C difficile infection in the inpatient setting [12, 13]. A recent meta-analysis concluded that exposure to fluoroquinolones was associated with a more than 6-fold increased risk for developing community-acquired C difficile infections compared with those without fluoroquinolone exposure [14]. Comparatively, patients who received sulfonamides/trimethoprim had a 2-fold higher risk of C difficile infection [14].
Understanding current national outpatient antibiotic prescribing practices will help ongoing stewardship efforts to optimize antibiotic use. However, limited information on recent national outpatient antibiotic prescribing trends for UTI is available [4, 5]. The objectives of this study were (1) to describe changes in ambulatory care antibiotic prescribing for uncomplicated UTI visits in women aged ≥18 years and (2) to identify factors associated with fluoroquinolone prescribing for uncomplicated UTI.
METHODS
Data Sources
We used the National Ambulatory Medical Care Survey (NAMCS) and National Hospital Ambulatory Medical Care Survey (NHMACS) to conduct this study. National Ambulatory Medical Care Survey and NHAMCS are publicly available datasets from the National Center for Health Statistics at the CDC [15]. In brief, NAMCS is a survey that conducts samples of visits to nonfederally employed office-based physicians. Anesthesiologists, pathologists, and radiologists are excluded from the survey. National Ambulatory Medical Care Survey has a 3-stage probability sampling design; (1) a probability sample based on geographic regions, (2) then stratified samples of practicing physicians, and lastly (3) patient visits within practices [16]. Physicians participating in NAMCS participate for a 1-week reporting period. At each visit, data are collected regarding patient demographics, symptoms, diagnoses, and medications provided. National Hospital Ambulatory Medical Care Survey is a survey of emergency departments, outpatient departments, and ambulatory surgery centers of noninstitutional general and short-stay hospitals [15]. National Hospital Ambulatory Medical Care Survey uses a 4-stage probability sampling design: the first stage samples geographic regions, the second stage is hospitals within the regions, the third stage is clinics within hospitals, and the last stage is patient visits [16]. All emergency departments and ambulatory care surgery centers within a selected hospital are included in the survey. During a 4-week reporting period, data for sampled visits are collected regarding patient demographics, complaints, diagnoses, and medical therapy. Data are weighted in both NAMCS and NHAMCS to produce national estimates. Unweighted response rates during the study period ranged from 54% to 71% for NAMCS, 67% to 75% for outpatient departments in NHAMCS, and 80% to 91% for emergency departments in NHAMCS.
Study Population
Women aged ≥18 years who presented to a healthcare facility with an acute or new-onset (ie, patient reason for visit “acute” problem or episode was labeled as “initial” visit to the emergency department) uncomplicated UTI during 2002–2011 were included. This study period was selected as 2011 was the most recent year available before changes in the survey sampling design. A diagnosis of uncomplicated UTI was defined as any visit assigned an International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis code of UTI (599.0) or acute cystitis (595.0 or 595.9). Exclusion criteria included visits with admission to the hospital, pregnancy (V22, 630), or presence of indwelling urinary catheter (996.64). Visits with a history of urinary stones (592.x), urinary neoplasm (189.0, 198.0, 223.0, 236.91, 188.x, 223.3, 236.7, 239.4), kidney transplant (996.81, V42.0), with coexisting infection as defined by Kallen et al [4] (ie, upper respiratory tract infection [460–466 and 473], pneumonia [480–488], otitis media [382], or cellulitis and abscess [680–686]), or those with upper UTI (590) were also excluded. In addition, visits associated with any intravenous antibiotic use were also excluded from the analysis. The flow chart summarizing the selection of visits is shown in Figure 1.
Figure 1.
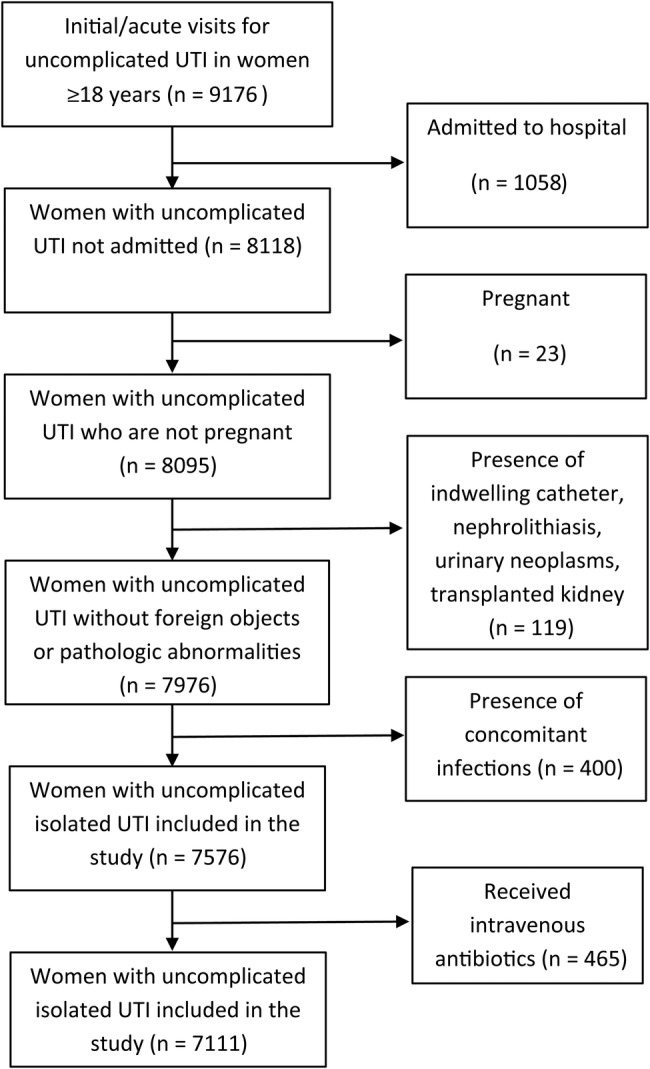
Flowchart describing the selection of outpatient visits, the National Ambulatory Medical Care Survey/National Hospital Ambulatory Medical Care Survey 2002–2011. Abbreviation: UTI, urinary tract infection.
Definition
Each visit was associated with up to 6 medications and their corresponding drug classes. We determined antibiotic classes (eg, fluoroquinolones) and individual medications (eg, nitrofurantoin) using the Multum Lexicon therapeutic classification system [17].
Analysis
Descriptive analyses of demographics, insurance status, visit setting, provider specialty (available for NAMCS only), presence of midlevel provider (ie, nurse practitioners physician assistants, and midwives), and geographic location were performed on all eligible visits. The χ2 tests were used to compare categorical variables. We performed logistic regression with 2-year time periods as a predictor variable to assess time trends in prescribing of each antibiotic or antibiotic class during the entire study period. Multivariable logistic regression was performed to determine factors associated with fluoroquinolone prescribing. All analyses were performed using Stata 12 software (StataCorp, College Station, TX) and were weighted to account for all components of the multistage probability survey design.
RESULTS
A total of 7111 uncomplicated UTI visits were included for analysis for the study period. Characteristics of the included visits are summarized in Table 1. The majority of visits were made by whites (82%), and approximately half of visits were covered by private insurance (53%) and were seen by a family or general practitioner (55%).
Table 1.
Characteristics of All Visits for Uncomplicated UTI in Women Aged ≥18 Years, NAMCS/NHAMCS 2002–2011 (N = 7111)
| Characteristic | Percentage (95% CI) |
|---|---|
| Age group (years) | |
| 18–29 | 26% (24%–29%) |
| 30–49 | 35% (33%–38%) |
| 50–69 | 22% (20%–24%) |
| ≥70 | 17% (15%–19%) |
| Race | |
| White | 82% (79%–84%) |
| Black | 15% (13%–18%) |
| Other | 3% (2%–4%) |
| Insurance status | |
| Private | 53% (50%–56%) |
| Medicare/Medicaid | 35% (32%–38%) |
| Other | 12% (11%–14%) |
| Visit setting | |
| Physician's office | 67% (64%–69%) |
| Hospital outpatient department | 8% (7%–10%) |
| Emergency department | 25% (23%–27%) |
| Provider specialtya | |
| Family/general practice | 55% (50%–59%) |
| Internal medicine | 24% (20%–29%) |
| Urology | 7% (5%–8%) |
| Other | 15% (11%–18%) |
| US Census region | |
| Northeast | 15% (12%–19%) |
| Midwest | 23% (18%–29%) |
| South | 44% (38%–50%) |
| West | 18% (14%–23%) |
| Metropolitan statistical area | |
| No | 17% (11%–26%) |
| Yes | 83% (74%–89%) |
| Midlevel provider presentb | |
| No | 90% (89%–92%) |
| Yes | 10% (8%–11%) |
| Time period | |
| 2002–2003 | 20% (17%–23%) |
| 2004–2005 | 20% (17%–22%) |
| 2006–2007 | 19% (17%–22%) |
| 2008–2009 | 21% (19%–24%) |
| 2010–2011 | 20% (18%–22%) |
Abbreviations: CI, confidence interval; NAMCS, National Ambulatory Medical Care Survey; NHAMCS, National Hospital Ambulatory Medical Care Survey; UTI, urinary tract infection.
a Data available for NAMCS only.
b Midlevel providers included nurse practitioners, physician assistants, and midwives. Midwives were not present in emergency department settings.
Table 2 (top) summarizes the proportion of visits that involved an antibiotic prescription during 2002–2011. Of all uncomplicated UTI visits, 80% (95% confidence interval [CI], 77%–82%) involved an antibiotic prescription, and there was no statistically significant change in this proportion during the study period. Among the antibiotics or antibiotic classes prescribed, fluoroquinolones were the most frequently prescribed (49% overall), followed by sulfonamides (27%), and nitrofurantoin (19%) (Table 2, bottom). The proportion of visits associated with sulfonamide prescribing decreased during the study period (35% in 2002–2003 to 23% in 2010–2011; P = .02), whereas an increase was observed for nitrofurantoin prescribing (14% in 2002–2003 to 24%; P < .01). There were no statistically significant changes in prescribing of fluoroquinolones and other antibiotic classes. Of all visits associated with a fluoroquinolone prescription, ciprofloxacin was prescribed in the greatest proportion of visits (78%; 95% CI, 73%–81%), followed by levofloxacin (21%; 95% CI, 17%–25%).
Table 2.
Antibiotica Prescribing for Uncomplicated UTI in Women Aged ≥18 Years, NAMCS/NHAMCS 2002–2011
| Antibiotic/Antibiotic Class | 2002–2003 | 2004–2005 | 2006–2007 | 2008–2009 | 2010–2011 | All Years | P Valueb |
|---|---|---|---|---|---|---|---|
| Percentage of Visits Resulting in Antibiotic Prescription by Year (N = 7111) | |||||||
| Any antibiotic | 77% | 83% | 82% | 81% | 77% | 80% | .76 |
| Fluoroquinolones | 36% | 46% | 37% | 38% | 37% | 39% | .47 |
| Sulfonamides | 27% | 19% | 26% | 19% | 18% | 22% | .02 |
| Nitrofurantoin | 11% | 15% | 14% | 17% | 18% | 15% | <.01 |
| Cephalosporins | 3% | 2% | 3% | 4% | 3% | 3% | .34 |
| Other | 2% | 2% | 3% | 3% | 2% | 3% | .41 |
| Percentage of Selected Antibiotic Classes Prescribed of All Prescribed Antibiotics (N = 5722) | |||||||
| Fluoroquinolones | 47% | 56% | 46% | 47% | 48% | 49% | .53 |
| Sulfonamides | 35% | 23% | 31% | 24% | 23% | 27% | .02 |
| Nitrofurantoin | 14% | 18% | 17% | 21% | 24% | 19% | <.01 |
| Cephalosporins | 4% | 3% | 4% | 5% | 4% | 4% | .32 |
| Other | 2% | 3% | 4% | 4% | 3% | 3% | .38 |
Abbreviations: NAMCS, National Ambulatory Medical Care Survey; NHAMCS, National Hospital Ambulatory Medical Care Survey; UTI, urinary tract infection.
a Antibiotics included penicillins, cephalosporins, macrolides, fluoroquinolones, lincomycin derivatives, tetracyclines, sulfonamides, and nitrofurantoin. Intravenous antibiotics were excluded. These were as follows: aminoglycosides, carbapenems, nafcillin, oxacillin, penicillin G, ticarcillin/clavulanic acid, piperacillin, piperacillin/tazobactam, vancomycin, and daptomycin.
b The P value for trend is based upon unadjusted logistic regression with time period as a predictor variable.
Results of the multivariable analysis assessing factors associated with fluoroquinolone prescribing are summarized in Table 3. Compared with the youngest age group (ages 18–29), older age groups were associated with increased odds of fluoroquinolone prescribing (adjusted odds ratio [AOR] for ages 30–49 = 1.7, 95% CI, 1.3–2.2; AOR for ages 50–69 = 1.8, 95% CI, 1.3–2.4; AOR for those aged ≥70 = 2.5, 95% CI, 1.6–3.8), and visits to an internist were associated with increased odds of prescribing fluoroquinolones compared with visits to family or general practitioners (AOR = 2.0; 95% CI, 1.1–3.3). Visits in the West US Census region were associated with lower odds of fluoroquinolone prescribing compared with the Northeast (AOR = 0.6; 95% CI, .4–1.0).
Table 3.
Characteristics Associated With Fluoroquinolone Prescribing for Uncomplicated UTI in Women Aged ≥18 Years Involving Antibiotic Prescription, NAMCS/NHAMCS 2002–2011 (N = 5686)
| Characteristic | Percentage of Visits That Involved Fluoroquinolone Prescription | P Value* | AOR (95% CI) for Fluoroquinolone Prescribing |
|---|---|---|---|
| Age group (years) | <.001 | ||
| 18–29 | 39% | 1.00 | |
| 30–49 | 51% | 1.67 (1.26–2.21) | |
| 50–69 | 52% | 1.75 (1.28–2.37) | |
| ≥70 | 58% | 2.47 (1.62–3.77) | |
| Race | .57 | ||
| White | 49% | 1.00 | |
| Black | 48% | 0.99 (.69–1.41) | |
| Other | 41% | 0.73 (.39–1.38) | |
| Insurance | .28 | ||
| Private | 50% | 1.00 | |
| Medicare/Medicaid | 49% | 0.83 (.63–1.10) | |
| Other | 43% | 0.83 (.58–1.21) | |
| Visit setting | .27 | ||
| Physician's office | 50% | 1.00 | |
| Hospital outpatient department | 45% | 0.91 (.68–1.22) | |
| Emergency department | 47% | 1.07 (.84–1.36) | |
| Provider specialtya | .01 | ||
| Family/general practice | 48% | 1.00 | |
| Internal medicine | 62% | 1.95 (1.14–3.31) | |
| Urology | 42% | 0.72 (.42–1.24) | |
| Other | 39% | 0.70 (.39–1.27) | |
| US Census region | .24 | ||
| Northeast | 54% | 1.00 | |
| Midwest | 47% | 0.73 (.45–1.18) | |
| South | 50% | 0.81 (.52–1.27) | |
| West | 43% | 0.61 (.38–1.00) |
The number in bold fonts indicate P < .05.
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; NAMCS, National Ambulatory Medical Care Survey; NHAMCS, National Hospital Ambulatory Medical Care Survey; UTI, urinary tract infection.
a Data available for NAMCS only. Accordingly, odds ratios are from a separate model that includes only data from NAMCS.
* χ2 test.
DISCUSSION
During 2002–2011, fluoroquinolones were the most frequently selected antibiotics for the management of uncomplicated UTI in women in the outpatient setting. National Ambulatory Medical Care Survey data from 1989 to 1998 showed that fluoroquinolone prescribing surpassed trimethoprim-sulfamethoxazole for uncomplicated UTI in women [18]. Therefore, fluoroquinolones have remained the most frequently prescribed antibiotic class for uncomplicated UTI in US outpatients [4, 5]. At the same time, a decrease in sulfonamide prescribing and an increase in nitrofurantoin prescribing was observed. Although some of this change may be attributed to the latest IDSA guideline encouraging use of nitrofurantoin as a first-line agent [10], the decrease in sulfonamide prescribing is likely due to the increase in the proportion of sulfonamide-resistant uropathogens [8], which, as a result, made nitrofurantoin a preferred treatment option [19].
Concerns for increases in fluoroquinolone resistance are not only a problem associated with uropathogens. Given the broad spectrum of activity, high potency, good oral bioavailability, and the ability to reach high drug concentrations in most target tissues [20], fluoroquinolones are one of the most frequently prescribed antibiotic classes in the outpatient setting [2]. Results from a national surveillance study conducted in Canada showed that ciprofloxacin-resistant Streptococcus pneumoniae rates increased significantly during the 9-year study period, in conjunction with increased ciprofloxacin consumption [21]. In 2013, the CDC published a report in which antibiotic resistance threats of each bacteria were categorized as urgent, serious, or concerning public health threats [22]. In the report, C difficile is categorized as an urgent public health threat given the burden in the United States and its association with antibiotic use [22].
Fluoroquinolones have also been associated with severe adverse events, such as rate-corrected electrocardiographic QT interval prolongation, tendinitis and tendon rupture, and seizures [23]. On May 12, 2016, the US Food and Drug Administration advised that serious side effects associated with fluoroquinolone use “generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options” [24].
Given these compelling reasons, nitrofurantoin and trimethoprim-sulfamethoxazole should be chosen for outpatient treatment of uncomplicated cystitis, whenever appropriate as determined by local resistance patterns; although resistance to trimethoprim-sulfamethoxazole is variable, the prevalence of nitrofurantoin-resistant uropathogens has remained low. In addition, due to its relatively narrow spectrum of activity and limited effect on fecal flora, nitrofurantoin is considered to have less potential for adverse events and collateral damage compared with other antibiotics used for UTI, such as C difficile colitis or selection of drug-resistant organisms [10, 25–27].
In our study, older patients were more likely than younger patients to receive fluoroquinolones, and internists were more likely to prescribe fluoroquinolones compared with family practitioners. Similar findings have been reported previously [4, 18]. Increased fluoroquinolone prescribing among older adults may be driven by higher frequency of trimethoprim-sulfamethoxazole resistance among older age groups [28]. In addition, nitrofurantoin is not recommended in individuals with decreased renal function (previously creatinine clearance ≤60 mL/minute, now revised to <30 mL/minute) [29], which is more likely to be present among older adults. Moreover, the Star Ratings system, introduced by the Centers for Medicare and Medicaid Services in 2007 for Medicare Advantage programs [30], listed nitrofurantoin as a high-risk medication for older adults and discouraged its use [31]. The rating system was revised and now discourages long-term nitrofurantoin use (>90 days) rather than short-term use [32]. The reasons for higher fluoroquinolone prescription among internal medicine providers may be due to differences in the patient population, such as higher prevalence of comorbidities or medication allergies that would preclude the use of antibiotics other than fluoroquinolones, although the reasons are unclear. At the same time, this finding suggests an opportunity to include internists in future outpatient antibiotic stewardship initiatives aimed at improving appropriate antibiotic use for the management of uncomplicated UTI. Additional studies are needed to understand determinants of fluoroquinolone prescribing for uncomplicated UTI in order to optimize its use.
The importance of antibiotic stewardship efforts has been increasingly recognized. In a policy statement developed jointly by the Society of Healthcare Epidemiology of America, the Pediatric Infectious Diseases Society, and IDSA, antibiotic stewardship is defined as “coordinated interventions designed to improve and measure the appropriate use of antimicrobial agents by promoting the selection of the optimal antimicrobial drug regimen including dosing, duration of therapy, and route of administration” [33]. Studies among general practitioners have shown that interventions such as audit and feedback [34, 35] and academic detailing [36, 37] have resulted in more appropriate use of antibiotics for UTI in the intervention group. Increased awareness of the importance of antibiotic stewardship has led to initiatives at the national level. For example, in September 2014, President Obama signed an executive order prioritizing federal efforts to combat the rise in antibiotic-resistant bacteria [38]. The following March, the White House issued the National Action Plan for Combating Antibiotic Resistant Bacteria, which calls for a 50% reduction in inappropriate antibiotic use among monitored conditions in outpatient settings by 2020.
Our study has several limitations. First, NAMCS and NHAMCS do not capture all outpatient antibiotic prescriptions. For example, we likely underestimated antibiotic prescribing for UTI because telephone consultations and prescribing were not included. Urinary tract infection is one of the most common conditions associated with such prescribing [39]. In addition, visits to retail clinics and urgent care centers, which have been expanding over the years [39], are also not captured by the 2 surveys. Second, we are not able to fully assess the appropriateness of antibiotic selection, because NAMCS and NHAMCS do not contain all information on factors that could influence providers' antibiotic selection, such as patients' medication allergy or antibiotic resistance in the region. Third, although we attempted to restrict the included visits to those linked with acute or new-onset UTIs, follow-up visits still might have been included, given that 20% of the visits were not associated with any antibiotic prescription. Finally, the study period ends in 2011, which may not allow enough time to assess the impact of the new IDSA guidelines on antibiotic prescribing patterns. However, fluoroquinolones have consistently been the most frequently selected antibiotic class despite the observed changes in trimethoprim-sulfamethoxazole and nitrofurantoin use, which suggests that fluoroquinolone overuse for uncomplicated UTI is likely to continue.
CONCLUSIONS
In conclusion, an additional decade of information showed that fluoroquinolones remain the most frequently prescribed antibiotics for uncomplicated UTI in women. Additional studies are needed to determine the most effective drivers to optimize prescribing for UTI in ambulatory care settings. Providers and practices should consider assessing antibiotic selection for UTI as potential target for antibiotic stewardship implementation.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Niska R, Bhuiya F, Xu J. National hospital ambulatory medical care survey: 2007 emergency department summary. Natl Health Stat Report 2010; 26:1–31. [PubMed] [Google Scholar]
- 2.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother 2014; 69:234–40. [DOI] [PubMed] [Google Scholar]
- 3.Caterino JM, Weed SG, Espinola JA, Camargo CA Jr. National trends in emergency department antibiotic prescribing for elders with urinary tract infection, 1996–2005. Acad Emerg Med 2009; 16:500–7. [DOI] [PubMed] [Google Scholar]
- 4.Kallen AJ, Welch HG, Sirovich BE. Current antibiotic therapy for isolated urinary tract infections in women. Arch Intern Med 2006; 166:635–9. [DOI] [PubMed] [Google Scholar]
- 5.May L, Mullins P, Pines J. Demographic and treatment patterns for infections in ambulatory settings in the United States, 2006–2010. Acad Emerg Med 2014; 21:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taur Y, Smith MA. Adherence to the Infectious Diseases Society of America guidelines in the treatment of uncomplicated urinary tract infection. Clin Infect Dis 2007; 44:769–74. [DOI] [PubMed] [Google Scholar]
- 7.Dalhoff A. Resistance surveillance studies: a multifaceted problem--the fluoroquinolone example. Infection 2012; 40:239–62. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez GV, Master RN, Karlowsky JA, Bordon JM. In vitro antimicrobial resistance of urinary Escherichia coli isolates among U.S. outpatients from 2000 to 2010. Antimicrob Agents Chemother 2012; 56:2181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren JW, Abrutyn E, Hebel JR et al. . Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis 1999; 29:745–58. [DOI] [PubMed] [Google Scholar]
- 10.Gupta K, Hooton TM, Naber KG et al. . International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52:e103–20. [DOI] [PubMed] [Google Scholar]
- 11.Lessa FC, Mu Y, Bamberg WM et al. . Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kallen AJ, Thompson A, Ristaino P et al. . Complete restriction of fluoroquinolone use to control an outbreak of Clostridium difficile infection at a community hospital. Infect Control Hosp Epidemiol 2009; 30:264–72. [DOI] [PubMed] [Google Scholar]
- 13.Pepin J, Saheb N, Coulombe MA et al. . Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis 2005; 41:1254–60. [DOI] [PubMed] [Google Scholar]
- 14.Deshpande A, Pasupuleti V, Thota P et al. . Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother 2013; 68:1951–61. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Ambulatory Health Care Data. Available at: http://www.cdc.gov/nchs/ahcd.htm Accessed 11 March 2015.
- 16.Centers for Disease Control and Prevention National Center for Health Statistics. Ambulatory Health Care Data. Scope and Sample Design. Available at: http://www.cdc.gov/nchs/ahcd/ahcd_scope.htm#namcs_scope Accessed 6 January 2016.
- 17.Centers for Disease Control and Prevention. Ambulatory Health Care Data. Trend Analysis Using NAMCS and NHAMCS Drug Data. Available at: http://www.cdc.gov/nchs/ahcd/trend_analysis.htm Accessed 15 March 2016.
- 18.Huang ES, Stafford RS. National patterns in the treatment of urinary tract infections in women by ambulatory care physicians. Arch Intern Med 2002; 162:41–7. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez GV, Baird AM, Karlowsky JA et al. . Nitrofurantoin retains antimicrobial activity against multidrug-resistant urinary Escherichia coli from US outpatients. J Antimicrob Chemother 2014; 69:3259–62. [DOI] [PubMed] [Google Scholar]
- 20.Lode HM. Preserving the efficacy of front-line fluoroquinolones through selective use to optimise clinical outcomes. Int J Antimicrob Agents 2014; 43:497–507. [DOI] [PubMed] [Google Scholar]
- 21.Adam HJ, Hoban DJ, Gin AS, Zhanel GG. Association between fluoroquinolone usage and a dramatic rise in ciprofloxacin-resistant Streptococcus pneumoniae in Canada, 1997–2006. Int J Antimicrob Agents 2009; 34:82–5. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/index.html. Accessed 5 February 2015.
- 23.Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis 2005; 41(Suppl 2):S144–57. [DOI] [PubMed] [Google Scholar]
- 24.FDA Drug Safety Communication . FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. In: US Food and Drug Administration, Press Release. May 12, 2016.
- 25.Paterson DL. “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis 2004; 38(Suppl 4):S341–5. [DOI] [PubMed] [Google Scholar]
- 26.Mavromanolakis E, Maraki S, Samonis G et al. . Effect of norfloxacin, trimethoprim-sulfamethoxazole and nitrofurantoin on fecal flora of women with recurrent urinary tract infections. J Chemother 1997; 9:203–7. [DOI] [PubMed] [Google Scholar]
- 27.Stewardson AJ, Gaia N, Francois P et al. . Collateral damage from oral ciprofloxacin versus nitrofurantoin in outpatients with urinary tract infections: a culture-free analysis of gut microbiota. Clin Microbiol Infect 2015; 21:344.e1–11. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez GV, Adams SJ, Baird AM et al. . Escherichia coli antimicrobial resistance increased faster among geriatric outpatients compared with adult outpatients in the USA, 2000–10. J Antimicrob Chemother 2013; 68:1838–41. [DOI] [PubMed] [Google Scholar]
- 29.The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015: updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–46. [DOI] [PubMed] [Google Scholar]
- 30.Sprague L. The Star Rating System and Medicare Advantage Plans. Issue Brief Natl Health Policy Forum 2015; 854. Available at: https://www.nhpf.org/library/details.cfm/2988. Accessed 1 July 2016. [PubMed] [Google Scholar]
- 31.Center for Medicare & Medicaid Services. Use of high risk medications in the Elderly (DAE). Available at: https://www.cms.gov/medicare/medicare-fee-for-service-payment/physicianfeedbackprogram/downloads/elderly-high-risk-medications-dae.pdf. Accessed 1 July 2016. [Google Scholar]
- 32.Center of Medicare & Medicaid Services. Drug lists for “use of high risk medications in the elderly": measure in the 2012 group practice quality and resource use reports. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeedbackProgram/Downloads/PY2012-High-Risk-Meds.pdf. Accessed 1 July 2016.
- 33.Society for Healthcare Epidemiology of America; Infectious Diseases Society of America; Pediatric Infectious Diseases Society Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol 2012; 33:322–7. [DOI] [PubMed] [Google Scholar]
- 34.Lagerlov P, Loeb M, Andrew M, Hjortdahl P. Improving doctors’ prescribing behaviour through reflection on guidelines and prescription feedback: a randomised controlled study. Qual Health Care 2000; 9:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundborg CS, Wahlstrom R, Oke T et al. . Influencing prescribing for urinary tract infection and asthma in primary care in Sweden: a randomized controlled trial of an interactive educational intervention. J Clin Epidemiol 1999; 52:801–12. [DOI] [PubMed] [Google Scholar]
- 36.Braybrook S, Walker R. Influencing prescribing in primary care: a comparison of two different prescribing feedback methods. J Clin Pharm Ther 1996; 21:247–54. [DOI] [PubMed] [Google Scholar]
- 37.Peterson GM, Stanton LA, Bergin JK, Chapman GA. Improving the prescribing of antibiotics for urinary tract infection. J Clin Pharm Ther 1997; 22:147–53. [DOI] [PubMed] [Google Scholar]
- 38.The White House. Combating antibiotic-resistant bacteria. Executive Order 13676 of September 18, 2014. 79 RF 56931. Available at: https://www.gpo.gov/fdsys/pkg/FR-2014-09-23/pdf/2014-22805.pdf. Accessed 6 January 2016.
- 39.Ewen E, Willey VJ, Kolm P et al. . Antibiotic prescribing by telephone in primary care. Pharmacoepidemiol Drug Saf 2015; 24:113–20. [DOI] [PubMed] [Google Scholar]


