Abstract
Point-of-care viral load tests are being developed to monitor patients on antiretroviral therapy (ART) in sub-Saharan Africa. Test design involves trade-offs between test attributes, including accuracy, complexity, robustness, and cost. We used a model of the human immunodeficiency virus epidemic and ART program in Zimbabwe and found that the attributes of a viral load testing approach that are most influential for cost effectiveness are avoidance of a high proportion of failed tests or results not received, use of an approach that best facilitates retention on ART, and the ability to facilitate greater use of differentiated care, including through expanding coverage of testing availability.
Keywords: antiretroviral therapy, cost-effectiveness, model, point-of-care, viral load
Since 2013, the World Health Organization (WHO) recommended viral load as the preferred approach for monitoring patients on antiretroviral therapy (ART) [1]. Although in high-income countries tests are done on plasma samples obtained by venipuncture, in low-income settings a more realistic option, particularly for more rural areas, is the use of dried blood spots (DBS), which are relatively easy to collect and can be transported to regional laboratories in stable condition [1, 2]. The intent is to receive the results at the clinic within a few weeks and then provide appropriate treatment at the patient's next visit to the clinic. Although this approach is increasingly being adopted [3], there remains interest in development of tests that require minimal user training and can be performed in the clinic while a patient is present (ie, at point of care [POC]) [4–7]. Several attributes need to be considered when designing such tests, including accuracy, whether the format allows easy use by lower cadres of staff, and in locations that do not have a reliable electricity supply. Developers must balance trade-offs between these attributes and with the cost at which a test can be produced. In this study, we use a modeling approach to explore the impacts of different test attributes on cost effectiveness and target test costs. This builds upon previous work on POC viral load tests in relation to the use of DBS [8]).
METHODS
We use an existing individual-based stochastic model of heterosexual transmission, progression, and treatment of human immunodeficiency virus (HIV) infection, which incorporates treatment adherence and acquisition and transmission of drug resistance mutations [8] (Supplementary Material). The epidemic and ART program data simulated relate to Zimbabwe (Supplementary Table 1, Figure 1). We assumed that CD4 count monitoring was used before 2017 and that viral load monitoring using the WHO-recommended 1000 cps/mL threshold for switching is then introduced. Measurement of viral load ≥1000 cps/mL prompts enhanced adherence support with a follow-up measure taken after 3 months. A measured viral load <1000 cps/mL in the past year is assumed to lead to a reduction in non-ART programmatic costs due to lowered frequency of clinic visits in people on first-line ART (ie, viral load-informed differentiated care [8]).
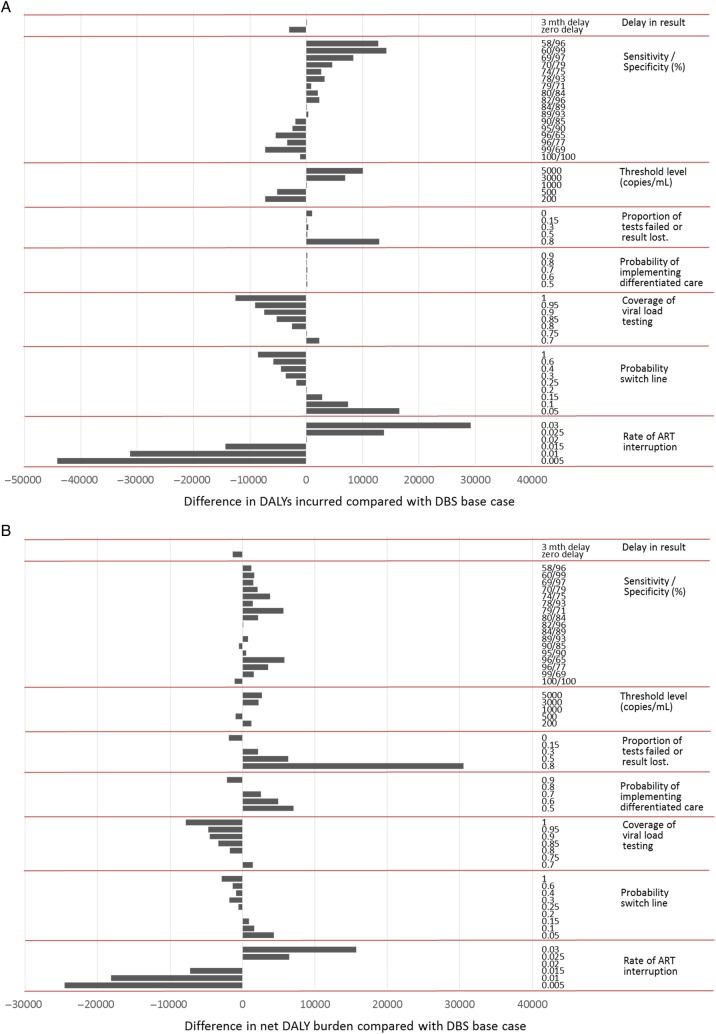
Figure 1.
(A) Comparison of health outcomes according to individual attributes of the viral load testing approach, shown as the difference in disability-adjusted life years (DALYs) incurred compared with dried blood spots (DBS) base scenario. The influence of the attribute is indicated by the range in difference in DALYs incurred for plausible values of the attribute. The DALYS are per 3 months over 20 years from 2017, discounted at 3% per year. (B) Comparison of cost effectiveness according to individual attributes of the viral load testing approach, shown as the difference in net DALYs compared with DBS base scenario. The influence of the attribute is indicated by the range in difference in net DALYs for plausible values of the attribute. ART, antiretroviral therapy.
A range of attributes relating to the viral load testing approach were considered, and these are outlined in Table 1. We first simulated outcomes over the years 2017–2036 based on a set of viral load testing attributes that were considered to approximate the use of a DBS approach and the current situation in Zimbabwe (“base DBS scenario”) (Table 1). We then explored the effects of changes in the attribute values, one-by-one, on population health. Population health is measured using disability-adjusted life years (DALYs) incurred; a generic measure of the burden of disease in the population that captures both premature mortality and morbidity as a result of ill health.
Table 1.
Key Attributes of a Viral Load Testing Approach and Values for Attributes Considered
| Attribute of Viral Load Testing Approach | Value in Base Scenario (Using DBS) | Other Values Considered |
Comment | |
|---|---|---|---|---|
| (1) Delay in result | 3 mo delay | No delay | By definition, any delay will be avoided with a POC test, and therefore the result will be available at the same visit. | |
| (2) Sensitivity/specificity relative to plasma for 1000 copies/mL cutoff | 84%/89% | 58/96 | 84/89 | If a plasma sample is used for a POC test, then the accuracy of the test could be higher than for DBS. In calibrating any test, it is useful to know whether to prioritize sensitivity or specificity. |
| 60/99 | 89/93 | |||
| 69/97 | 90/85 | |||
| 70/79 | 95/90 | |||
| 74/75 | 96/65 | |||
| 78/93 | 96/77 | |||
| 79/71 | 99/69 | |||
| 80/84 | 100/100 | |||
| 82/96 | ||||
| (3) VL threshold used to define first-line ART failure (cutoff for qualitative or semiquantitative assays) | 1000 copies/mL | 200, 500, 3000, 5000 | WHO failure threshold is 1000 copies/mL. This choice of threshold is important if a qualitative test is being developed that reads as positive or negative rather than providing a value. | |
| (4) Proportion of tests failed or result lost | 0.15 | 0.8, 0.5, 0.3, 0 | It is likely that most tests that are done using POC will actually be used because the patient is still present when the result is received. For DBS, a higher proportion of tests will not get used due to communication/linkage failures, leading to delay in informed decision making and wasted costs. We assume that if a test fails, then it is attempted again after 3 mo. | |
| (5) Probability of differentiated care if VL < 1000 being implemented | 0.8 | 0.5, 0.6, 0.7, 0.9 | POC test should enable VL-informed differentiated care because the date of a person's next clinic appointment can be set with the person present, avoiding the need to call people later to adjust the timing of their next visit. | |
| (6) Coverage of population with VL testing | 75% of population | 70%, 80%, 85%, 90%, 95%, 100% | POC tests should enable greater access to (coverage of) VL testing in a country, because it is an additional option to DBS testing. | |
| (7) Probability of switch to second-line ART per 3 mo once failure definition met | 0.2 per 3 mo | 0.05, 0.10, 0.15, 0.25, 0.30, 0.35, 0.40, 0.60, 1.00 | It is theoretically possible that a POC test will result in a more rapid switch, due to the ability to act on VL result while the patient is present. | |
| (8) Probability of ART interruption/loss to follow up | 0.020 per 3 mo | 0.030, 0.025, 0.015, 0.010, 0.005 | Given the enabling of VL-informed differentiated care, POC tests could result in lower rates of disengagement from care, although this is uncertain and needs to be assessed in studies. | |
Abbreviations: ART, antiretroviral therapy; DBS, dried blood spots; HIV, human immunodeficiency virus; POC, point of care; VL, viral load; WHO, World Health Organization.
(1) Delay may be up to 3 months. (2) The sensitivity and specificity are not input parameters: they are outputs that depend on the assumed standard deviation for the measurement variability and any offset (see Supplementary Material, section 9). Values Informed by overview of various studies comparing VL values on DBS/plasma (eg, [1, 9, 10]). (3) [11]. (4) This is failure due to technical reasons in the laboratory or failure for results to be successfully returned: the value is likely to vary by setting within countries as well as between countries—the value of 0.15 is a conservative estimate of what is achievable with a DBS transport network (eg, [12]; F Cowan (oral personal communication, CESHHAR, Zimbabwe, 10 December 2015)). It is not yet certain whether use of DBS for viral load testing can achieve similar results, because the number of tests to be done will be much higher. (5) Assumption: 0.80 may be rather high for DBS, and data are required to inform this. (6) Coverage assumed to be 75% in base case: this is closely linked to (4) above, and 75% is conservative for what is feasible for EID; an assumption is made, even for base scenario DBS, that the probability of switch is higher with viral load monitoring than with CD4 count monitoring (0.001 per 3 months). (7) Differs by setting (eg, [13, 14]). (8) Consistent with [15] (see details in Supplementary Material). This is base rate of interruption - it is increased within first 2 years of ART and in those with suboptimal lifetime tendency to adhere (see details in Supplementary Material).
In the DBS base scenario, it is assumed that 75% of the population have access to viral load testing, whereas the remainder would continue to be monitored using CD4 count testing. In previous modeling results, DALY outcomes from monitoring the CD4 count using a switch criteria based on a threshold of 200 cells/mm3 were not appreciably different from monitoring with viral load [8]. In this study, to capture the intended effects of viral load testing, we assume a relatively high rate of switch to second-line ART when the failure criteria have been met with viral load monitoring (0.2 per 3 months), which is greater than the 0.001 per 3 months with CD4 count monitoring (based on the low number of people on second-line ART).
Economic Considerations
Program costs resulting from different viral load testing attributes were also considered, enabling assessment of cost-effectiveness on the basis of net DALYs. This is a measure analogous to net health benefit [16] and compares the health gains from an intervention with the health losses associated with displaced or forgone intervention that can no longer be provided due to the commitment of limited healthcare resources to the evaluated intervention (ie, it compares health gains to health opportunity costs). The cost-effectiveness threshold (CET) is central to the estimation of net DALYs—it is an estimate of the cost per DALY averted of interventions displaced/foregone at the margin when a new intervention in introduced [17]. Net DALYs is then calculated as DALYs + costs/CET. For most healthcare systems, the CET is generally not readily apparent, but a value of $500 per DALY averted is thought to be realistic for low-income settings in sub-Saharan Africa because alternative policy interventions (eg, expanded ART provision) offer health gains at about this level [18]. The testing policy with the lowest net DALYs is deemed the cost-effective policy from amongst those evaluated because it leads to the greatest reduction in population burden of disease (ie, lowest DALYs).
The opportunity cost of interventions displaced at the margin, represented by the CET, also informs the price within which interventions must be delivered to offer population health improvement and be deemed cost effective. For the value of each viral load testing attribute, the upper-bound cost that the viral load test would need to be delivered is provided. A health system perspective was adopted, and costs incurred by patients were not included. Both costs and DALYs averted were discounted to present value using a 3% per annum discount rate.
Unit costs (in US dollars at 2015 prices) are detailed in Supplementary Material. Costs of viral load assays were set at a base value of $22, based on cost estimates using the DBS approach and counting all components of delivery (reagents, equipment, human resources, buildings, set-up costs, etc [referred to as a “fully-loaded” cost]) (see details in Supplementary Material). Annual program costs for clinic visits (not including drug or viral load/CD4 count tests) are $80 per year [19] with an assumed reduction to $40 per year after measurement of viral suppression because of reduced clinical visit frequency within a differentiated care model [8]. Health utilities and disability weights to calculate DALYs averted were derived from a recent comprehensive study [20].
RESULTS
The status of the simulated Zimbabwean adult population in 2014 is shown in Supplementary Table 1. Figure 1A shows the effect on DALYs incurred over 20 years of differences in attribute values associated with viral load testing. The delay associated with DBS testing had a relatively modest influence on the number of DALYs incurred. The sensitivity of the test for detecting a value above 1000 cps/mL was more influential, with generally higher sensitivity leading to a lower number of DALYs incurred, regardless of specificity and due to enhanced adherence support and switching to second-line ART in those who require this. When the sensitivity was approximately 90% or above, then a lower specificity was associated with a lower number of DALYs incurred, reflecting that those with viral loads between 50 and 1000 copies/mL may also benefit from a switch to second-line ART. The viral load threshold used is also moderately influential, with more DALYs averted the lower the threshold. The proportion of tests that fail or the results lost was also influential, due to the inability to act on viral load failure in a timely way or at all. The probability that viral load-informed differentiated care was implemented did not influence the DALYs, because we did not assume any positive or negative health effects of such a policy, only an impact on costs. The rate of switch to second-line ART in people with first-line failure is also influential on DALYs incurred. It is uncertain whether the viral load testing format (laboratory based or POC) will influence this parameter, although it is plausible that if the result is available while the patient is present, then a switch is more likely to be instigated compared with a laboratory result that is only available on a return visit. The extent to which a test can result in increased coverage of viral load positively influenced the DALY outcome, mostly through the assumed greater rate of switch to second-line ART where viral load testing is used. The rate of ART interruption or loss to follow-up is highly influential, although, again, it is uncertain whether the form of viral load testing will influence this parameter.
In Figure 1B, we considered the influence of each factor on the net DALYs, which takes into account the opportunity costs of differences in cost in addition to the DALYs incurred. The relative influence of the various factors was similar to Figure 1A, with a few exceptions. Lower specificity was associated with higher net DALYs, due to the additional cost of second-line drugs, and a sensitivity and specificity of approximately 90% was close to the that with 100% sensitivity and specificity. The viral load threshold to define ART failure was less influential on net DALYs than it was on DALYs, because use of higher thresholds reduced costs. The proportion of tests that fail or are lost was even more influential on net DALYs than on DALYs, due to the cost incurred with repeated attempts to successfully measure viral load. The probability of implementing viral load-informed differentiated care was also influential, due to the cost savings for clinic. The probability of switching to second-line ART once the failure criteria were met was less influential on net DALYs than on DALYs, again due to the higher costs of second-line ART.
In Table 2, for each level of each viral load testing attribute, we provided the upper-bound cost for a POC test to be considered as cost effective compared with the DBS base scenario. For example, if a POC test demonstrated an increase in the probability of viral load-informed differentiated care from 0.8 to 0.9, then, with no other difference compared with the DBS base scenario, it could cost $24 and still be cost effective compared with the DBS base scenario with the $22 test cost. Alternatively, if a POC test demonstrated a halving of the rate of ART interruption or loss to follow-up compared with the DBS approach, with no other difference compared with the DBS base scenario, then it would be cost effective up to a cost of $41 per test. Furthermore, if the approach resulted in 100% coverage, then a test could cost up to $28 and remain cost effective; that is, if the test replaced the $22 test in all. If the tests were used to fill the coverage “gap”, then it could cost much more, while preserving the same overall cost of viral load testing, and remain cost effective.
Table 2.
Maximum Cost of Viral Load Test for Net Health Benefit in the Context of Changes in Values of Each Attribute of the Viral Load Testing Approach, Changing the Value of Only One Attribute at a Time
| Attribute of Viral Load Testing Approach | Values for Factor Considered |
Maximum Fully Loaded Cost of Viral Load Test for Reduced Net DALYs Compared With Base Scenario |
||
|---|---|---|---|---|
| (1) Delay in result | No delay | $23 | ||
| 3 mo delaya | $22 | |||
| (2) Sensitivity/specificity relative to plasma for 1000 copies/mL cutoff | Sensitivity/Specificity | |||
| 58/96 | 84/89a | $21 | $22 | |
| 60/99 | 89/93 | $20 | $21 | |
| 69/97 | 90/85 | $20 | $22 | |
| 70/79 | 95/90 | $19 | $21 | |
| 74/75 | 96/65 | $17 | $15 | |
| 78/93 | 96/77 | $20 | $17 | |
| 79/71 | 99/69 | $15 | $20 | |
| 80/84 | 100/100 | $19 | $23 | |
| 82/96 | $21 | |||
| (3) VL threshold used to define first-line ART failure (copies/mL) (cutoff for qualitative assay) | 200 | $20 | ||
| 500 | $23 | |||
| 1000a | $22 | |||
| 3000 | $19 | |||
| 5000 | $19 | |||
| (4) Proportion of tests failed or result lostb | 0.80 | $5 | ||
| 0.50 | $16 | |||
| 0.30 | $19 | |||
| 0.15a | $22 | |||
| 0.00 | $24 | |||
| (5) Probability of differentiated care if VL < 1000 being implemented | 0.5 | $14 | ||
| 0.6 | $16 | |||
| 0.7 | $19 | |||
| 0.8a | $22 | |||
| 0.9 | $24 | |||
| (6) Difference in coverage of population with VL testing | 70% | $20 | ||
| 75%a | $22 | |||
| 80% | $23 | |||
| 85% | $25 | |||
| 90% | $26 | |||
| 95% | $26 | |||
| 100% | $28 | |||
| (7) Probability of switch to second-line ART per 3 mo once failure definition met | 0.05 | $17 | ||
| 0.10 | $20 | |||
| 0.15 | $20 | |||
| 0.20a | $22 | |||
| 0.25 | $22 | |||
| 0.30 | $23 | |||
| 0.40 | $23 | |||
| 0.60 | $23 | |||
| 1.00 | $25 | |||
| (8) Probability of ART interruption/loss to follow up (per 3 mo) | 0.030 | $3 | ||
| 0.025 | $14 | |||
| 0.020a | $22 | |||
| 0.015 | $29 | |||
| 0.010 | $41 | |||
| 0.005 | $48 | |||
Abbreviations: ART, antiretroviral therapy; DALYs, disability adjusted life years; VL, viral load.
a Base scenario (VL cost $22).
b Assumed that there is a 90% chance that the cost of the test is nevertheless incurred.
DISCUSSION
Our results indicate that the attributes of a viral load testing approach most influential for cost effectiveness are avoidance of a high proportion of failed tests or results not being received and, so far as it can be influenced by the viral load monitoring strategy, use of an approach that best facilitates retention on ART. Also of particular importance was the ability to facilitate greater use of differentiated care through expanding coverage of availability of viral load testing. These attributes are most likely to be found with a robust POC viral load test that facilitates decision making while the patient is present. It is noteworthy that a 3-month delay in receiving a result does not result in a significant negative impact, and also that sensitivity and specificity both in the region of 90% appear to be acceptable. Due to the higher cost of second-line drugs, the influence of the switching rate in those with first-line ART failure was not one of the most important attributes for cost effectiveness. Our results emphasize the need for POC viral load tests and may inform the development of tests as well as the design of future implementation studies and trials on viral load testing approaches. Our results suggest that it is important for studies to consider how POC viral load testing impacts the patient care model, such as earlier switch to second-line drugs or better retention in care. Whether these factors are indeed improved with POC testing will have to be evaluated in implementation studies, and data could then be used to inform our cost-effectiveness modeling. It may prove to be important, for example, that the waiting time to receive a result from a POC assay is as low as possible.
Several POC viral load assays have already been developed (eg, by Cepheid, Liat, and Alere), and some pilot studies have been completed. Many of these assays already meet high levels of accuracy, but our results indicate that perhaps other parameters (such as potential coverage and assay costs) still need to be prioritized.
The WHO has established the ASSURED (affordable, sensitive, specific, user-friendly, rapid/robust, equipment-free and deliverable to end-users) criteria for POC diagnostics [21]. It has been suggested that the WHO preference for equipment-free POC tests presents a potential conflict with the need to deliver high sensitivity and specificity [22]. Our results suggest that the population health effects of reduced sensitivity and specificity, of an equivalent level to the DBS base scenario, for example, are modest compared with the potential benefits of increased access and ease of patient management. Weidemaier et al [16] argue that ASSURED criteria imply that resource capacities for where interventions are ultimately to be used should be taken into consideration throughout the technology development process. This study attempts to facilitate this for POC viral load tests.
We assigned a set of attribute values to the DBS base scenario, but there remains uncertainty whether these can be achieved in practice. We considered compiling 1 or 2 plausible sets of attributes that might reflect a particular POC test. We elected not to do this because any given choice of parameter sets seems extremely arbitrary. There are no data available on real-life implementation of true POC assays to inform the values. As data become available on attributes of different testing approaches, our model can be used to compare cost effectiveness both between POC tests and with the DBS alternative.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
Author Contributions. All authors contributed to defining the analysis concept and design, providing critical input to the conduct of the modeling analysis, and writing the manuscript. A. P., V. C., and F. N. developed the model and conducted the modeling analysis.
Financial support. This work was funded by the Bill & Melinda Gates Foundation grant number OPP1064862. We thank colleagues supporting the Legion computing cluster (Legion@UCL) for critical computing support.
Potential conflicts of interest. A. P. reports grants from the Bill & Melinda Gates Foundation, during the conduct of the study; and personal fees from Gilead Sciences, Abbvie, GSK Biologicals, and Ashfield Communications, outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Technical and operational considerations for implementing HIV viral load testing. Available at: http://www.who.int/hiv/pub/arv/viral-load-testing-technical-update/en/ Accessed 14 August 2016.
- 2.Smit PW, Sollis KA, Fiscus S et al. . Systematic review of the use of dried blood spots for monitoring HIV viral load and for early infant diagnosis. PLoS One 2014; 9:e86461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ministry of Health & Child Care. Operational and Service Delivery Manual for the Prevention, Care and Treatment of HIV in Zimbabwe AIDS & TB Programme. Zimbabwe, 2015. http://www.differentiatedcare.org/Portals/0/adam/Content/hc5QvbYtZUSXdE-1M-pxcQ/File/Operational%20And%20Service%20Delivery%20Manual%20(6).pdf. [Google Scholar]
- 4.Rowley CF. Developments in CD4 and viral load monitoring in resource-limited settings. Clin Infect Dis 2014; 58:407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shafiee H, Wang SQ, Inci F et al. . Emerging technologies for point-of-care management of HIV infection. Annu Rev Med 2015; 66:387–405. [DOI] [PubMed] [Google Scholar]
- 6.Stevens W, Gous N, Ford N, Scott LE. Feasibility of HIV point-of-care tests for resource-limited settings: challenges and solutions. BMC Med 2014; 12:173 doi:10.1186/s12916-014-0173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UNITAID. HIV/AIDS Diagnostics Technology Landscape. Semi-annual update. 2015. Available at: http://www.aidsdatahub.org/hivaids-diagnostics-technology-landscape-semi-annual-update-murtagh-mm-2015 Accessed 14 August 2016.
- 8.Working Group on Modelling of Antiretroviral Therapy Monitoring Strategies in Sub-Saharan Africa. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature 2015; 528:S68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavedzenge SN, Davey C, Chirenje T et al. . Finger prick dried blood spots for HIV viral load measurement in field conditions in Zimbabwe. PloS ONE 2015; 10:e0126878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smit PW, Sollis KA, Fiscus S et al. . Systematic review of the use of dried blood spots for monitoring HIV viral load and for early infant diagnosis. PLoS ONE 2014; 9:e86461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva:World Health Organization, 2013. Available at: http://www.who.int/hiv/pub/guidelines/arv2013/en/. Accessed 1 september 2016. [PubMed] [Google Scholar]
- 12.Chatterjee A, Tripathi S, Gass R, et al. Implementing services for Early Infant Diagnosis (EID) of HIV: a comparative descriptive analysis of national programs in four countries. BMC Public Health 2011; 11:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston V, Fielding K, Charalambous S, Churchyard G, Phillips AN, Grant A. Outcomes following virological failure and predictors of switching to second-line antiretroviral therapy in a South African treatment program JAIDS 2012;61:370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox MP, van Cutsem G, Giddy J et al. . Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. JAIDS 2012; 60:428–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kranzer K, Ford N. Unstructured treatment interruption of antiretroviral therapy in clinical practice: a systematic review. Tropical Medicine and International Health 2011; 16: 1297–313. [DOI] [PubMed] [Google Scholar]
- 16.Stinnett AA, Mullahy J. Net health benefits a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998; 18(2 Suppl):S68–80. [DOI] [PubMed] [Google Scholar]
- 17.Claxton K, Walker S, Palmer S, Sculpher M. Appropriate Perspectives for Health Care Decisions, Centre for Health Economics. Research Paper 54, University of York; 2010. [Google Scholar]
- 18.Woods E, Revill P, Sculpher M, Claxton K. Country-Level Cost-Effectiveness Thresholds: Initial Estimates and the Need for Further Research, March 2015. Available at: https://www.york.ac.uk/media/che/documents/papers/researchpapers/CHERP109_cost-effectiveness_threshold_LMICs.pdf Accessed 14 August 2016. [DOI] [PMC free article] [PubMed]
- 19.Tagar E, Sundaram M, Condliffe K et al. . Multi-country analysis of treatment costs for HIV/AIDS (MATCH): facility-level ART unit cost analysis in Ethiopia, Malawi, Rwanda, South Africa and Zambia. PLoS One 2014; 9:e108304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salomon JA, Vos T, Hogan DR et al. . Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet 2012; 380:2129–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kettler H, White K, Hawkes S. Mapping the landscape of diagnostics for sexually transmitted infections, 2004. Available at: http://www.who.int/tdr/publications/documents/mapping-landscape-sti.pdf Accessed 15 August 2016.
- 22.Weidemaier K, Carrino J, Curry A et al. . Advancing rapid point-of-care viral diagnostics to a clinical setting. Future Virology 2015; 10:313–28. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.