Key findings include a high prevalence of APRI score indicating significant fibrosis/cirrhosis in ART-naïve individuals particularly among HIV/HBV-co-infected individuals and a regression of APRI to <1.5 after 12-24 months of ART in the majority of participants with APRI score indicating significant fibrosis, irrespective of HBV status.
Keywords: APRI, hepatitis B virus, HIV, liver fibrosis, Tanzania
Abstract
Background. We evaluated the prevalence of chronic hepatitis B virus (HBV) infection and liver fibrosis/cirrhosis in human immunodeficiency virus (HIV)-infected individuals enrolled in a rural Tanzanian prospective cohort and assessed hepatic fibrosis progression 12–24 months after antiretroviral treatment (ART) initiation.
Methods. All ART-naive HIV-infected adults ≥15-year-old enrolled in the Kilombero and Ulanga Antiretroviral Cohort who started ART between 2005 and 2015 were included. Pre-ART factors associated with significant liver fibrosis (aspartate aminotransferase-to-platelet ratio index [APRI] >1.5) and cirrhosis (APRI > 2.0) were identified using logistic regression.
Results. Of 3097 individuals screened, 227 (7.3%; 95% CI, 6.4–8.2) were hepatitis B surface antigen (HBsAg) positive. Before ART initiation, 9.1% individuals had significant liver fibrosis and 5.3% had cirrhosis. Human immunodeficiency virus/HBV-coinfected individuals were more likely to have an APRI score indicating significant fibrosis (14.2% vs 8.7%, P = .03) and cirrhosis (9.2% vs 4.9%, P = .03) than HBV-uninfected patients. CD4 cell count <200 cell/μL and alcohol consumption were independently associated with pre-ART APRI score, indicating significant fibrosis and cirrhosis in multivariable analyses. Among individuals with elevated APRI measurements pre- and 12–24 months post-ART initiation, 53 of 57 (93.0%) of HIV-monoinfected and 4 of 5 (80.0%) of HIV/HBV-coinfected had a regression to APRI < 1.5.
Conclusions. Hepatic fibrosis and cirrhosis were common in our cohort, especially among HIV/HBV-coinfected individuals. The APRI improved in most patients. Pre-ART HBsAg screening and early onset of tenofovir-based ART for HIV/HBV-coinfection should be prioritized in sub-Saharan Africa.
Antiretroviral therapy (ART) has improved survival rates among persons living with human immunodeficiency virus (PLWHIV) in sub-Saharan Africa (SSA). As survival increases, other comorbidities such as infection by hepatitis B virus (HBV) are emerging as important causes of morbidity and mortality in this population. Up to 15% of PLWHIV are infected with HBV in SSA [1]. In Tanzania, serum hepatitis B surface antigen (HBsAg) prevalence has been reported to be up to 17.3% [2, 3] and in rural areas up to 9.2% among HIV-infected individuals [4]. Although impact of HBV on HIV progression is less clear, several non-African and African cohort studies strongly suggest that HIV-individuals are at increased mortality when coinfected with HBV [5–7]. Hepatitis B virus coinfection could impair immunological recovery and increase risk of hepatotoxicity [8] and has been identified as the main aetiological agent for hepatocellular carcinoma (HCC) in small African cohorts [9, 10].
Current World Health Organization (WHO) guidelines for the prevention, care, and treatment of persons with chronic hepatitis B infection recommend aspartate aminotransferase (AST)-to-platelet ratio index (APRI) as the preferred noninvasive test to assess for the presence of cirrhosis in resource-limited settings, as an alternative to more expensive and often not available options such as transient elastography (TE) and liver biopsy [11]. Noninvasive tests of liver fibrosis have been poorly studied in chronic hepatitis B in SSA [12, 13], and very few have assessed the progression of hepatic fibrosis after ART initiation. Prior data from cross-sectional studies in SSA using TE have reported a high prevalence of liver disease in both HIV-monoinfected and HIV/HBV-coinfected [14, 15]. However, the regression of liver fibrosis and cirrhosis assessed by liver biopsy has only been demonstrated in HBV-monoinfected to date [16, 17].
In this study conducted in a rural Tanzanian cohort of PLWHIV initiating ART, we investigated the prevalence of HBV coinfection and hepatic fibrosis/cirrhosis using APRI, and we evaluated the progression of liver fibrosis after ART initiation.
METHODS
Study Setting and Population
The Kilombero and Ulanga Antiretroviral Cohort (KIULARCO) is an ongoing, open, prospective cohort that comprises all patients who visit the Chronic Diseases Clinic of Ifakara (CDCI) within the Saint Francis Referral Hospital (SFRH) and provide their written informed consent to participate. More details about the KIULARCO are given elsewhere [18–20]. The SFRH is the largest healthcare facility in the Kilombero district of the Morogoro region in southern Tanzania, providing treatment and care for a population of approximately 600 000 inhabitants and an estimated 30 000 patients living with HIV/acquired immune deficiency syndrome (AIDS). Established in 2004, this was the first rural clinic accredited to be a Care and Treatment Centre of the National AIDS Control Program in Tanzania, and more than 9000 patients have been enrolled into care. Participants are followed-up as per national standard of care guidelines. Medical histories, physical examination, laboratory tests, and blood samples are obtained at the time of registration in the clinic, before ART initiation, 2 weeks and 3 months after ART initiation, and every 6 months thereafter. Plasma from blood samples is collected at every clinical visit and cryopreserved at −80°C.
For this study, we included all adult (>15 years) ART-naive PLWHIV enrolled in KIULARCO since January 2005, under active follow-up in November 2015 and with an available HBsAg result. Participants on ART before enrollment, those who did not provide informed consent, and those without an HBsAg test were excluded for the analysis. Among the eligible individuals, we excluded those with missing platelet (PLT) values and/or AST for regression analysis. Subsequently, we excluded HIV-individuals that remained ART-naive by November 2015, those with <12 months of follow-up since enrollment, those with a missing post-ART APRI, and those on ART for <12 months for analysis of APRI score change.
Definitions
Hepatitis B Virus Status
The HBsAg was detected by Determine HBsAg (Alere Inc., Waltham, MA). Analysis were performed on cryopreserved plasma samples from patients enrolled before January 2013 and from 1 blood draw per subject after January 2013. Because seroconversion of HBsAg (complete clearance of HBsAg) is exceptional even in the presence of HBV-suppressive agents, we assumed that all patients with a positive HBsAg test were already HBV-infected at enrollment in the cohort [21]. Among patients enrolled before January 2013, ART had been previously initiated following current Tanzanian National ART Guidelines in the absence of HBsAg data. Those who tested positive were consequently switched to an ART regimen including tenofovir disoproxil fumarate (TDF) combined with either lamivudine (3TC) or emtricitabine (FTC).
Alcohol Consumption
Participants were asked to answer “yes” or “no” to the questions “Do you regularly drink alcohol?” and to give the “number of standard drinks per day”. Alcohol consumption was defined as a positive answer to the first question and a value ≥1 to the second question. In addition, we quantified the number of standard drinks per day and considered “Heavy alcohol drinking” as the consumption of 4 or more standard drinks for men and 3 or more for women.
Liver Fibrosis
We assessed liver fibrosis using APRI score, and values thresholds used were >1.5 for significant hepatic fibrosis and >2.0 for cirrhosis, as recommended by the WHO [11]. The upper limit of the norm for AST was 40 IU/L. The APRI was calculated pre-ART (before or within 3 months of ART initiation) in patients who started ART and at the most recent clinical visit for those who did not start ART during the follow up. Thereafter, APRI was calculated at 12 months (post-ART) after ART initiation (window from 12 to 24 months). We defined “Regression to APRI ;< 1.5” as a change from APRI ≥ 1.5 at baseline to <1.5 at 12–24 months after ART initiation, and we defined “Progression to APRI ;> 1.5” as a change from APRI ;≤ 1.5 at baseline to >1.5 at 12–24 months after ART initiation.
Statistical Analysis
Baseline characteristics between HIV-monoinfected and HIV/HBV-coinfected were compared using the χ2 and/or Fisher exact tests for categorical variables and the Wilcoxon non-parametric test for continuous variables. Univariate and multivariate logistic regression analysis were used to estimate the association between covariates and APRI score indicating significant fibrosis and cirrhosis. We adjusted the multivariate model for age (continuous) and found it to be nonlinear, so we included the squared term. We could not use tuberculosis (TB) drugs in the model, because there was no patient on TB drugs included in the final model. Results were presented with odds ratios (OR) and 95% confidence intervals (CIs). We compared APRI score change between HIV-monoinfected and HIV/HBV-coinfected in a subset of individuals with both pre- and post-ART APRI measurements and APRI score >1.5 at baseline using the χ2 and/or Fisher exact test. Statistical analyses were performed using Stata 14 software (StataCorp, Houston, TX).
Ethical Considerations
Written informed consent was sought from all participants at registration at the CDCI, and all data were deidentified. Samples were collected during routine clinical visits, and ethical approval for its cryopreservation and retrospective analysis was obtained from the Ifakara Health Institute institutional review board, the National Health Research Ethics Review Committee of the National Institute for Medical Research of Tanzania, the Tanzanian Commission of Science and Technology, and the Ethics Committee of the University and State of Basel, Switzerland.
RESULTS
From January 2005 to November 2015, a total of 3097 HIV-infected individuals were eligible for the study analysis (see Supplementary Figure 1). Of those, 227 (7.3%) were HBsAg positive. The APRI at baseline was available for 1707 of 3097 (55.1%) individuals, 1566 of 2870 (56.6%) in the HIV-monoinfected group and 141 of 227 (62.1%) in the HIV/HBV-coinfected group. Among them, 500 of 1707 (29.3%) had both pre-ART and post-ART APRI after excluding participants who remained ART-naive after 24 months of follow up and those on ART for <12 months. Of these, 44 of 500 (8.8%) were HIV/HBV-coinfected. Main characteristics of excluded individuals did not differ remarkably from eligible individuals except for the fact that more than 70% of them started with 3TC without TDF (see Supplementary Table 1). The majority of them were enrolled before 2012, when TDF was not yet included as first-line ART regimen in the Tanzanian National Guidelines.
Baseline Characteristics
Baseline characteristics of the study population are summarized in Table 1. Approximately three quarters of participants were female, in greater percentage in the HIV-monoinfected group compared with the HIV/HBV-coinfected group (68.3% vs 52.0%, respectively). Median CD4 cell count and percentage of all individuals at ART initiation was low: 185 cell/μL (interquartile range [IQR], 84–302) and 10% (IQR, 4–18) with no differences between groups. Overall, 10.5% of HIV-individuals had PLT < 150 × 109/L, although this proportion was higher in HIV/HBV-coinfected compared with HIV-monoinfected (17.9% vs 9.9%). Alcohol consumption was self-reported in 25.2% of all participants, and 7.4% of them were classified as heavy drinkers. Eleven percent of all individuals did not initiate ART during the follow-up period, with a higher proportion remaining ART-naive in the HIV-monoinfected group compared with the HIV/HBV-coinfected group (11.4% vs 7.1%, respectively). Among the 2753 individuals who initiated ART, 1463 (53.1%) received y as the sole HBV-active drug and 1099 (39.9%) received TDF in combination with either FTC or 3TC as initial ART.
Table 1.
Pre-ART Characteristics of Individuals by Infection Status
| Characteristic | Infection Status |
||
|---|---|---|---|
| All (n = 3097) | HIV Monoinfected (n = 2870) | HIV/HBV Coinfected (n = 227) | |
| Female gender (n [%]) | 2078 (77.1) | 1960 (68.3) | 118 (52.0) |
| Pregnanta (n [%]) | 122 (7.1) | 118 (7.3) | 4 (4.0) |
| Age (median [IQR]), years | 38.0 (31.0–45.6) | 38.1 (31.8–45.6) | 37.0 (31.7–45.4) |
| Alcohol useb (n [%]) | 730 (25.2) | 671 (24.9) | 60 (28.2) |
| Heavy drinkersc (n [%]) | 14 (7.4) | 13 (7.6) | 1 (5.6) |
| BMId (median [IQR]), kg/m2 | 21.0 (18.9–23.4) | 21.0 (18.9–23.5) | 20.5 (18.3–23.9) |
| ART naive (n [%]) | 344 (11.1) | 328 (11.4) | 16 (7.1) |
| ART regimen at initiatione (n [%]) | |||
| 3TC monotherapy | 1463 (53.1) | 1355 (53.3) | 108 (51.2) |
| TDF + 3TC | 645 (23.4) | 584 (23.0) | 61 (28.9) |
| TDF + FTC | 454 (16.5) | 422 (16.6) | 32 (15.2) |
| Other | 191 (6.9) | 181 (7.1) | 10 (4.7) |
| WHO stage at ART startf (n [%]) | |||
| 1 or 2 | 1415 (56.6) | 1310 (56.8) | 105 (54.4) |
| 3 or 4 | 1084 (43.4) | 996 (43.2) | 88 (45.6) |
| CD4 count baselineg (median [IQR]), cells/µL | 215 (89–404) | 218 (90–405) | 185 (65–365) |
| CD4 count baselineh (median [IQR]), % | 12 (5–21) | 12 (5–21) | 12 (4–19) |
| CD4 count pre-ARTi (median [IQR]), cells/µL | 185 (84–302) | 185 (85–303) | 183 (63–296) |
| CD4 count pre-ARTj (median [IQR]), % | 10 (4–18) | 10 (4–18) | 10 (4–18) |
| Use of antituberculous drugsk (n [%]) | 86 (2.8) | 77 (2.7) | 9 (4.0) |
| PLT < 150 × 109/Ll (n [%]) | 179 (10.5) | 154 (9.9) | 25 (17.9) |
| PLT (median [IQR]), 109/L | 250 (183–322) | 252 (186–323) | 220 (159–307) |
| ASTm (median [IQR]), U/L | 53 (29–92) | 53 (29–93) | 56 (32–86) |
Abbreviations: APRI, aspartate aminotransferase-to-platelet ratio; ART, antiretroviral therapy; BMI, body mass index; FTC, emtricitabine; HBV, hepatitis B infection; HIV, human immunodeficiency virus; IQR, interquartile range; PLT, platelet; TDF, tenofovir disoproxil fumarate; WHO, World Health Organization; 3TC, lamivudine.
a Pregnancy status was available for 1728 individuals.
b Information on alcohol consumption at baseline was available for 2903 individuals.
c Alcohol amounts were available for 190 current drinkers individuals.
d BMI was available for 2353 individuals.
e ART regimen at initiation was available for 2753 individuals.
f WHO stage at ART initiation was available for 2499 individuals.
g CD4 cell count at baseline was available for 2189 individuals.
h CD4% at baseline was available for 2119 individuals.
i CD4 cell count at ART initiation was available for 2127 individuals.
j CD4% at ART initiation was available for 2074 individuals.
k Use of antituberculous drugs was available for 3097 individuals.
l Platelet count was available for 2993 individuals.
m AST was available for 2993 individuals.
Prevalence of Aspartate Aminotransferase-to-Platelet Ratio Index Score Indicating Significant Fibrosis and Cirrhosis
Among individuals with available pre-ART APRI, 156 of 1707 (9.1%) had APRI score indicating significant fibrosis and 90 of 1707 (5.3%) had APRI score indicating cirrhosis at baseline. Human immunodeficiency virus/HBV-coinfected individuals exhibited higher median APRI score than HIV-monoinfected (0.57 [IQR, 0.32–1.08] vs 0.37 [IQR, 0.22–0.77], P < .001). The prevalence of APRI score indicating significant fibrosis (APRI > 1.5) and cirrhosis (APRI > 2) among HIV/HBV-coinfected was 14.2% and 9.2%, and it was significantly higher than in HIV-monoinfected (8.7% [P = .03] and 4.9% [P = .03], respectively) (Table 2).
Table 2.
APRI Score at Baseline (n = 1707) and at 12–24 Months (n = 1106)
| APRI Score | Baseline |
12–24 Months Post-ART |
||||||
|---|---|---|---|---|---|---|---|---|
| All (n = 1707) | HIV Monoinfected (n = 1566) | HBV/HIV Coinfected (n = 141) | P Value | All (n = 1106) | HIV Monoinfected (n = 1050) | HBV/HIV Coinfected (n = 56) | P Value | |
| APRI, median (IQR) | 0.38 (0.22–0.79) | 0.37 (0.22–0.77) | 0.57 (0.32–1.08) | <.001 | 0.41 (0.23–0.77) | 0.40 (0.22–0.77) | 0.50 (0.28–0.93) | .05 |
| APRI > 1.5, n (%) | 156 (9.1) | 136 (8.7) | 20 (14.2) | .03 | 56 (5.1) | 51 (5.0) | 5 (5.8) | .74 |
| APRI > 2, n (%) | 90 (5.3) | 77 (4.9) | 13 (9.2) | .03 | 26 (2.4) | 22 (2.2) | 4 (4.7) | .14 |
Abbreviations: APRI, AST-to-platelet ratio index; ART, antiretroviral therapy; HBV, hepatitis B infection; HIV, human immunodeficiency virus; IQR, interquartile range.
Risk Factors for Aspartate Aminotransferase-to-Platelet Ratio Index Score Indicating Significant Fibrosis
Baseline variables that were associated with significant liver fibrosis in the univariable analyses were HBsAg positivity (OR = 1.73; 95% CI, 1.05–2.88; P = .03), CD4 cell count <200 cells/µL (OR = 1.82; 95% CI, 1.25–2.68; P = .002), and alcohol consumption (OR = 1.63; 95% CI, 1.13–2.36; P = .01). In the multivariable analysis, only CD4 < 200 cells/µL (OR = 1.72; 95% CI, 1.16–2.54; P = .007) and alcohol consumption (OR = 1.60; 95% CI, 1.06–2.40; P = .03) remained independently associated with APRI score >1.5 at baseline (Table 3). All models were adjusted for age, which exhibited a significant nonlinear effect with both younger and older individuals exhibiting higher risk of fibrosis and cirrhosis.
Table 3.
Correlates of Pre-ART Liver Fibrosis and Cirrhosis (n = 1707)
| Characteristic | Significant Fibrosis (APRI > 1.5) |
Cirrhosis (APRI > 2) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n/Na | Univariate |
Multivariable |
n/N | Univariate |
Multivariable |
|||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |||
| HBV test | .03 | .22 | .03 | .13 | ||||||
| Negative | 136/1566 | 1 | 1 | 77/1566 | 1 | 1 | ||||
| Positive | 20/141 | 1.73 (1.05–2.88) | 1.44 (.81–2.59) | 13/141 | 1.96 (1.06–3.63) | 1.71 (.85–3.46) | ||||
| CD4 cell count | .002 | .01 | .04 | .05 | ||||||
| CD4 > 200 cells/μL | 42/597 | 1 | 1 | 24/597 | 1 | 1 | ||||
| CD4 < 200 cells/μL | 92/757 | 1.82 (1.25–2.68) | 1.72 (1.16–2.54) | 50/757 | 1.69 (1.03–2.78) | 1.64 (.99–2.72) | ||||
| Alcohol consumption | .01 | .03 | .01 | .04 | ||||||
| No | 101/1257 | 1 | 1 | 58/1257 | 1 | 1 | ||||
| Yes | 46/369 | 1.63 (1.13–2.36) | 1.60 (1.06–2.40) | 29/367 | 1.76 (1.11–2.78) | 1.72 (1.03–2.87) | ||||
Abbreviations: APRI, AST-to-platelet ratio index; CI, confidence intervals; HBV, hepatitis B infection; HIV, human immunodeficiency virus.
a n/N is the number of HIV individuals with significant fibrosis-cirrhosis/total number of HIV individuals for a specific category.
Aspartate Aminotransferase-to-Platelet Ratio Index Score Changes in Patients on Antiretroviral Therapy
Of the 500 individuals with pre- and post-ART APRI, 62 of 500 (12.4%) had APRI > 1.5 at baseline and 34 of 500 (6.8%) had APRI > 2 (see Supplementary Table 2). Overall, 426 of 500 (85.2%) did not experience an APRI score change at 12–24 months on ART. Otherwise, the proportion of individuals with APRI > 1.5 decreased after ART initiation in both HIV- and HIV/HBV-infected groups. There was a decline in median APRI from baseline to the 12–24 months measurement for all individuals (P < .001) including HIV-monoinfected (P < .001) and HIV/HBV-coinfected (P = .45). Among 62 of 500 (12.4%) individuals with APRI score indicating significant fibrosis at baseline, we observed a regression to APRI < 1.5 in 57 of 62 (92.0%) of them, 53 of 62 (84.5%) were HIV-monoinfected and 4 of 62 (6.5%) were HIV/HBV-coinfected (Figure 1). Only 4 of 62 (6.5%) individuals with APRI > 1.5 at baseline progressed to APRI > 2 post-ART, and 3 of 62 (4.8%) remained with APRI > 1.5.
Figure 1.
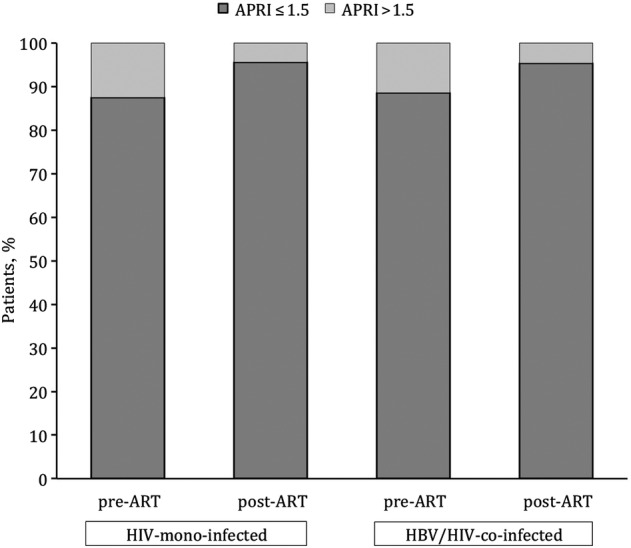
Aspartate aminotransferase-to-platelet ratio index (APRI) score change at 12–24 months after antiretroviral therapy (ART) initiation for all individuals with both pre- and post-ART APRI (n = 500).
Regression to APRI < 1.5 was observed in 53 of 57 (93.0%) of HIV-monoinfected and in 4 of 5 (80%) HIV/HBV-coinfected individuals. Furthermore, of 34 of 500 (6.8%) with cirrhosis at baseline, regression to APRI < 2 was observed in 2 of 2 (100%) of HIV/HBV-coinfected and in 29 of 32 (90.6%) of HIV-monoinfected. In contrast, 17 of 438 (3.9%) of HIV-infected individuals progressed to APRI > 1.5, 16 of 438 (3.7%) HIV-monoinfected and 1 of 438 (0.7%) HIV/HBV-coinfected.
Aspartate Aminotransferase-to-Platelet Ratio Index Variation by Treatment Regimen
Among the 62 individuals with APRI > 1.5 at baseline, 44 of 62 (71.0%) had initiated ART with 3TC as the sole drug with dual activity against HIV and HBV, and 16 of 62 (25.8%) initiated ART with TDF either in combination with FTC or 3TC. Among HIV/HBV-coinfected individuals with pre-ART APRI > 1.5 who initiated ART, 1 of 1 (100%) on TDF and 3 of 4 (75%) without TDF regressed to APRI < 1.5 after 12–24 months of ART initiation.
DISCUSSION
Few studies have evaluated liver fibrosis using APRI in ART-naive individuals in SSA and its variation after ART initiation. Key findings of our study include a high prevalence of APRI score indicating significant fibrosis and cirrhosis among ART-naive HIV-infected individuals in rural Tanzania, particularly among HBsAg-positive individuals, and a regression of APRI to <1.5 after 12–24 months of ART, irrespective of HBV-infection status in the majority of participants with elevated APRI scores.
The prevalence of HBsAg positivity was 7.3% (95% CI, 6.4–8.2), consistent with previous studies from sub-Saharan countries [2] and particularly from Tanzanian studies where the prevalence of HBV infection among PLWHIV ranged from 3.2% to 17.3% [1]. Similar to the results from a large Zambian cohort [22], the overall baseline prevalence of pre-ART APRI score indicating significant fibrosis (APRI > 1.5) and cirrhosis (APRI > 2) was 9.1% and 5.3%, respectively. In the Multicentre AIDS Cohort Study, baseline hepatic fibrosis score assessed by APRI was significantly higher in HIV-positive individuals with either HBV or HCV coinfection compared with those with HIV-monoinfection [23]. In our cohort, both APRI score indicating significant fibrosis and cirrhosis (14.2% and 9.2%, respectively) were more prevalent among HIV/HBV-coinfected individuals. As also seen in studies from high-income countries [23, 24] and sub-Saharan countries [14, 15], we found a significant association between HBV infection and liver fibrosis. However, after adjusting for CD4, age, gender, and alcohol consumption, the strength and precision of this association decreased.
A majority of HIV-individuals from our cohort did not exhibit APRI score variation after 12–24 months on ART. It is remarkable that among HIV-individuals with APRI score indicating significant fibrosis, more than 90% presented a regression to APRI < 1.5 12–24 months after ART initiation. The regression of APRI score was particularly evident among HIV-monoinfected individuals. However, whether ART-induced regression of liver fibrosis is more likely in HIV-monoinfected compared with HIV/HBV-coinfected remains unclear. Although treatment for chronic HBV infection with 3TC and TDF has shown its efficacy in reducing and even regressing liver histological fibrosis [16, 25, 26], more data are needed to determine whether HBV treatment will have similar effects in sub-Saharan African populations.
The APRI has been endorsed by the WHO using a single high cutoff >2 for cirrhosis as key criteria for prioritizing initiation of ART in resource-constrained settings [11]. The diagnostic accuracy for hepatic fibrosis using APRI has been widely investigated in HBV-monoinfected [27] and less frequently in ART-naive HIV-monoinfected and HIV/HBV-coinfected individuals from high-income countries [22, 23, 28]. However, although APRI is cheap and accessible, it is not an optimal test for liver fibrosis assessment when compared with TE, which has exhibited better accuracy particularly in HIV/HBV-coinfected individuals [14, 29]. For instance, APRI has demonstrated a low sensitivity for detecting liver cirrhosis in some African studies when applying the WHO cutoff [30]. An important challenge with the use of APRI in HIV/HBV-coinfected individuals is the interpretation of fibrosis change over time: ART often reverses HIV-associated thrombocytopenia and liver transaminases values. Thus, it is impossible to know whether changes in APRI after ART start correspond to hepatic inflammation decrease or true histological regression of fibrosis. Very few studies have shown histological regression of HBV-related fibrosis on treatment, and these changes started to occur from after 24 to 48 months [31, 32]. In our cohort, only 10% of all participants had low PLT at baseline and AST elevations were rare, but we are confident that this was not a major issue in our study. In addition, APRI score values might be overestimated by thrombocytopenia and liver inflammation secondary to opportunistic infections, especially tuberculosis and its treatment. In our study, none of the patients included in the APRI regression analysis was on anti-TB drugs. In addition, in a previous study, we demonstrated the absence of hepatitis delta coinfection [20] among HIV/HBV-coinfected individuals from our cohort. Whereas the effect of these confounders when assessing liver fibrosis using APRI score in Africa are still unknown, a novel noninvasive test, the γ-glutamyl transpeptidase-to-platelet ratio, was recently shown to have better accuracy than APRI for identifying significant liver fibrosis in HBV-monoinfected patients [30]. Its performance in SSA has not yet been elucidated [33], but it did not show a good enough positive predictive value in other western cohorts to be considered as a recommended biomarker in HIV/HBV-coinfected patients [34].
CD4 cell count of <200 cells/µL before ART initiation was associated with an increased risk of APRI score indicating significant fibrosis and cirrhosis, as expected, because previous studies have shown that HIV-related immunosuppression could lead to a more rapid progression to liver fibrosis and cirrhosis in coinfected individuals [35, 36]. Consumption of alcohol was also a predictors in our cohort for having APRI score indicating significant fibrosis and cirrhosis. Alcohol consumption is indeed a well known risk factor for liver fibrosis, and it is common in Eastern Africa especially among young people [37]. However, the heterogeneity of instruments used for the screening and assessment of alcohol consumption in other studies and the fact that we did not use WHO-validated and WHO-recommended tests such as AUDIT make it difficult to evaluate whether our results on alcohol use prevalence are higher than [7] or similar [14] to others.
This study has some limitations. First, <20% of all eligible individuals were available for the APRI score variation analysis, resulting in a limited number of individuals included in the analysis and a reduced strength of our results. However, the exclusion of a large proportion of patients from the longitudinal analyses may not have biased our results, because the main demographic and clinical characteristics of these patients were similar to the ones of the analysis cohort. Second, we could not properly characterize HBV infection with additional HBV serological markers such as HBeAg and quantification of HBV deoxyribonucleic acid levels, 2 well known predictors of liver disease in HBsAg-positive patients. In addition, HCV-infection screening was not performed and thus could not be included in our logistic regression analysis. Nevertheless, several studies in SSA exhibited a low prevalence or absence of anti-HCV in HIV-infected individuals [7, 38], including a previous study conducted in our setting [4]. Finally, information on other well known risk factors for liver fibrosis such as aflatoxin levels, use of traditional herbal medicine, and microbiological tests to exclude endemic infections such as malaria or schistosomiasis were not assessed, and we cannot exclude residual confounding.
CONCLUSIONS
In conclusion, our study is among the first assessing APRI score variation after ART initiation in SSA. Our data showed a considerable proportion of HIV-individuals presenting with liver fibrosis in whom APRI score regressed after ART initiation. This study highlights the need for specific national guidelines on the management of HBV/HIV coinfection in areas of high HBV endemicity. Further research is required to better characterize HBV liver-related fibrosis, which remains a necessity to monitor and prevent evolution towards end-stage liver diseases. Efforts must be placed in comparing accuracy of different noninvasive tests in this population, in finding more reliable markers of hepatic fibrosis, and in implementing optimal screening methods for HCC, given that HBV the is leading causative agent in the sub-Saharan region.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank all patients and staff of the Chronic Diseases Clinic of Ifakara for their participation. We also give special thanks to the members of the KIULARCO Study Group: Aschola Asantiel, Manuel Battegay, Adolphina Chale, Diana Faini, Ingrid Felger, Gideon Francis, Hansjakob Furrer, Anna Gamell, Tracy Glass, Christoph Hatz, Speciosa Hwaya, Aneth Vedastus Kalinjuma, Bryson Kasuga, Namvua Kimera, Yassin Kisunga, Thomas Klimkait, Emilio Letang, Antonia Luhombero, Lameck B. Luwanda, Herry Mapesi, Leticia Mbwile, Mengi Mkulila, Julius Mkumbo, Margareth Mkusa, Dorcus K. Mnzava, Germana Mossad, Dolores Mpundunga, Athumani Mtandanguo, Kim Mwamelo, Selerine Myeya, Sanula Nahota, Regina Ndaki, Agatha Ngulukila, Alex John Ntamatungiro, Leila Samson, George Sikalengo, Marcel Tanner, Fiona Vanobberghen, and Maja Weisser.
Disclaimers. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned below.
Financial support. The Kilombero and Ulanga Antiretroviral Cohort and the Chronic Diseases Clinic of Ifakara receive financial support from the Government of the Canton of Basel, Switzerland, the Swiss Tropical and Public Health Institute, the Ifakara Health Institute, the Government of Tanzania, and USAID through TUNAJALI-Deloitte. A. R. received a grant from the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC 2014) and from the College of Physicians of the Balearic Islands (COMIB 2014). G. W. was supported by an Ambizione-PROSPER fellowship from the Swiss National Science Foundation (PZ00P3_154730).
Potential conflicts of interests. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Collaborators: The KIULARCO Study Group, Aschola Asantiel, Manuel Battegay, Adolphina Chale, Diana Faini, Ingrid Felger, Gideon Francis, Hansjakob Furrer, Anna Gamell, Tracy Glass, Christoph Hatz, Speciosa Hwaya, Aneth Vedastus Kalinjuma, Bryson Kasuga, Namvua Kimera, Yassin Kisunga, Thomas Klimkait, Emilio Letang, Antonia Luhombero, Lameck B Luwanda, Herry Mapesi, Leticia Mbwile, Mengi Mkulila, Julius Mkumbo, Margareth Mkusa, Dorcus K Mnzava, Germana Mossad, Dolores Mpundunga, Athumani Mtandanguo, Kim Mwamelo, Selerine Myeya, Sanula Nahota, Regina Ndaki, Agatha Ngulukila, Alex John Ntamatungiro, Leila Samson, George Sikalengo, Marcel Tanner, Fiona Vanobberghen, and Maja Weisser
References
- 1.Barth RE, Huijgen Q, Taljaard J, Hoepelman AI. Hepatitis B/C and HIV in sub-Saharan Africa: an association between highly prevalent infectious diseases. A systematic review and meta-analysis. Int J Infect Dis 2010; 14:e1024–1031. [DOI] [PubMed] [Google Scholar]
- 2.Matthews PC, Geretti AM, Goulder PJ, Klenerman P. Epidemiology and impact of HIV coinfection with hepatitis B and hepatitis C viruses in sub-Saharan Africa. J Clin Virol 2014; 61:20–33. [DOI] [PubMed] [Google Scholar]
- 3.Nagu TJ, Bakari M, Matee M. Hepatitis A, B and C viral co-infections among HIV-infected adults presenting for care and treatment at Muhimbili National Hospital in Dar es Salaam, Tanzania. BMC Public Health 2008; 8:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franzeck FC, Ngwale R, Msongole B et al. Viral hepatitis and rapid diagnostic test based screening for HBsAg in HIV-infected patients in rural Tanzania. PLoS One 2013; 8:e58468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann CJ, Seaberg EC, Young S et al. Hepatitis B and long-term HIV outcomes in coinfected HAART recipients. AIDS 2009; 23:1881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikolopoulos GK, Paraskevis D, Hatzitheodorou E et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis 2009; 48:1763–71. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins C, Christian B, Ye J et al. Prevalence of hepatitis B co-infection and response to antiretroviral therapy among HIV-infected patients in Tanzania. AIDS 2013; 27:919–27. [DOI] [PubMed] [Google Scholar]
- 8.Wandeler G, Gsponer T, Bihl F et al. Hepatitis B virus infection is associated with impaired immunological recovery during antiretroviral therapy in the Swiss HIV cohort study. J Infect Dis 2013; 208:1454–8. [DOI] [PubMed] [Google Scholar]
- 9.Ocama P, Opio KC, Kagimu M et al. Hepatitis B virus and HIV infection among patients with primary hepatocellular carcinoma in Kampala, Uganda. Afr Health Sci 2011; 11(Suppl 1):S20–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umoh NJ, Lesi OA, Mendy M et al. Aetiological differences in demographical, clinical and pathological characteristics of hepatocellular carcinoma in The Gambia. Liver Int 2011; 31:215–21. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 12.Bonnard P, Sombie R, Lescure FX et al. Comparison of elastography, serum marker scores, and histology for the assessment of liver fibrosis in hepatitis B virus (HBV)-infected patients in Burkina Faso. Am J Trop Med Hyg 2010; 82:454–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mbaye PS, Sarr A, Sire JM et al. Liver stiffness measurement and biochemical markers in Senegalese chronic hepatitis B patients with normal ALT and high viral load. PLoS One 2011; 6:e22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawkins C, Agbaji O, Ugoagwu P et al. Assessment of liver fibrosis by transient elastography in patients with HIV and hepatitis B virus coinfection in Nigeria. Clin Infect Dis 2013; 57:e189–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stabinski L, Reynolds SJ, Ocama P et al. High prevalence of liver fibrosis associated with HIV infection: a study in rural Rakai, Uganda. Antivir Ther 2011; 16:405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcellin P, Gane E, Buti M et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013; 381:468–75. [DOI] [PubMed] [Google Scholar]
- 17.Xu B, Lin L, Xu G et al. Long-term lamivudine treatment achieves regression of advanced liver fibrosis/cirrhosis in patients with chronic hepatitis B. J Gastroenterol Hepatol 2015; 30:372–8. [DOI] [PubMed] [Google Scholar]
- 18.Haraka F, Glass TR, Sikalengo G et al. A bundle of services increased ascertainment of tuberculosis among HIV-infected individuals enrolled in a HIV cohort in rural sub-Saharan Africa. PLoS One 2015; 10:e0123275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letang E, Muller MC, Ntamatungiro AJ et al. Cryptococcal antigenemia in immunocompromised human immunodeficiency virus patients in rural Tanzania: a preventable cause of early mortality. Open Forum Infect Dis 2015; 2:ofv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winter A, Letang E, Kalinjuma AV et al. Absence of hepatitis delta infection in a large rural HIV cohort in Tanzania. Intern J Infect Dis 2016; 46:8–10. [DOI] [PubMed] [Google Scholar]
- 21.Wang HS, Han SH. Management of hepatitis B in special patient populations. Clin Liver Dis 2010; 14:505–20. [DOI] [PubMed] [Google Scholar]
- 22.Vinikoor MJ, Sinkala E, Mweemba A et al. Elevated AST-to-platelet ratio index is associated with increased all-cause mortality among HIV-infected adults in Zambia. Liver Int 2015; 35:1886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price JC, Seaberg EC, Badri S et al. HIV monoinfection is associated with increased aspartate aminotransferase-to-platelet ratio index, a surrogate marker for hepatic fibrosis. J Infect Dis 2012; 205:1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thio CL, Seaberg EC, Skolasky R et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360:1921–6. [DOI] [PubMed] [Google Scholar]
- 25.Liaw YF, Sung JJ, Chow WC et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351:1521–31. [DOI] [PubMed] [Google Scholar]
- 26.Marcellin P, Heathcote EJ, Buti M et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008; 359:2442–55. [DOI] [PubMed] [Google Scholar]
- 27.Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology 2015; 61:292–302. [DOI] [PubMed] [Google Scholar]
- 28.DallaPiazza M, Amorosa VK, Localio R et al. Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC Infect Dis 2010; 10:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miailhes P, Pradat P, Chevallier M et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J Viral Hepat 2011; 18:61–9. [DOI] [PubMed] [Google Scholar]
- 30.Lemoine M, Shimakawa Y, Nayagam S et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut 2016; 65:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hui CK, Leung N, Shek TW et al. Sustained disease remission after spontaneous HBeAg seroconversion is associated with reduction in fibrosis progression in chronic hepatitis B Chinese patients. Hepatology 2007; 46:690–8. [DOI] [PubMed] [Google Scholar]
- 32.Ray Kim W, Berg T, Asselah T et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol 2016; 64:773–80. [DOI] [PubMed] [Google Scholar]
- 33.Stockdale AJ, Geretti AM; Hepatitis B Infection in Kumasi Study Group. Reply to Boyd and Lacombe. Clin Infect Dis 2016; 62:130–1. [DOI] [PubMed] [Google Scholar]
- 34.Stockdale AJ, Phillips RO, Beloukas A et al. Liver fibrosis by transient elastography and virologic outcomes after introduction of tenofovir in lamivudine-Experienced adults with HIV and hepatitis B virus coinfection in Ghana. Clin Infect Dis 2015; 61:883–91. [DOI] [PubMed] [Google Scholar]
- 35.Puoti M, Torti C, Bruno R et al. Natural history of chronic hepatitis B in co-infected patients. J Hepatol 2006; 44(1 Suppl):S65–70. [DOI] [PubMed] [Google Scholar]
- 36.Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology 2009; 49(5 Suppl):S138–45. [DOI] [PubMed] [Google Scholar]
- 37.Francis JM, Grosskurth H, Changalucha J et al. Systematic review and meta-analysis: prevalence of alcohol use among young people in eastern Africa. Trop Med Int Health 2014; 19:476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann CJ, Dayal D, Cheyip M et al. Prevalence and associations with hepatitis B and hepatitis C infection among HIV-infected adults in South Africa. Int J STD AIDS 2012; 23:e10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


