This analysis evaluated the association of public versus private insurance with other risk factors and RSV disease in the REPORT study of preterm infants born at 32 to 35 weeks' gestational age who did not receive RSV immunoprophylaxis.
Keywords: ED visits, insurance status, preterm infants, respiratory syncytial virus
Abstract
Background. Database studies have identified that public health insurance status is associated with an increased risk of severe respiratory syncytial virus (RSV) disease in US infants. However, these studies did not adjust for the presence of other risk factors and did not evaluate the risk in preterm infants.
Methods. In this study, we evaluate the independent association between public insurance and severe RSV disease outcomes adjusting for other risk factors. The prospective, observational RSV Respiratory Events among Preterm Infants Outcomes and Risk Tracking (REPORT) study was conducted over 2 consecutive RSV seasons at 188 US clinical sites that enrolled preterm infants born at 32–35 wGA who had not received RSV immunoprophylaxis with palivizumab. Adjusted incidence rates per 100 infant-seasons of the RSV-associated endpoints of outpatient lower respiratory tract infection (LRI), emergency department (ED) visits, RSV hospitalizations (RSVHs), and intensive care unit admissions during peak RSV season (November–March) were compared for infants with private and public insurance.
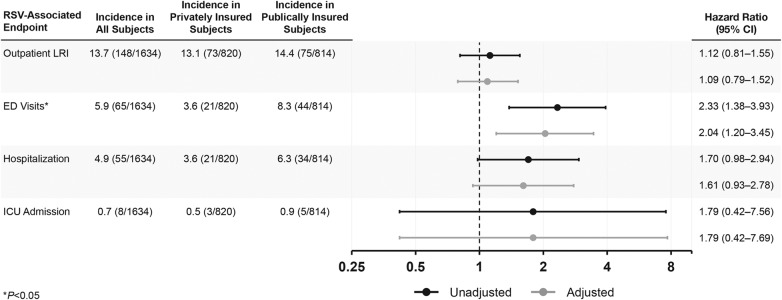
Results. Of 1642 evaluable infants enrolled in the REPORT study, 50.1% had private insurance and 49.9% had public health insurance. Adjusted rates of RSV outpatient LRIs were similar; however, rates of ED visits (hazard ratio [HR], 2.04; 95% confidence interval [CI], 1.20–3.45) were higher for subjects with public insurance, with a similar but nonsignificant trend observed for hospitalization (HR, 1.61; 95% CI, .93–2.78).
Conclusions. Socioeconomic status, as evaluated by public versus private healthcare insurance, is a significant independent risk factor for ED use in US preterm infants and may contribute to increased RSVHs in this population.
Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract infections (LRIs) and pneumonia in infants and young children [1, 2], and it is the leading cause of hospital admission in children during the first year of life [3]. Although RSV infects virtually all children at least once in the first 24 months of life [4], certain medical conditions increase the risk of severe RSV disease, including hemodynamically significant congenital heart disease, chronic lung disease of prematurity/bronchopulmonary dysplasia, and preterm birth ≤35 weeks' gestational age (wGA) [5, 6]. The most consistent demographic risk factors for severe RSV disease include young chronological age and exposure to young children through the presence of young siblings or daycare attendance [7]. In the United States, infants with public health insurance have been shown to have approximately 2-fold higher risk of hospitalization for an RSV LRI [8, 9] compared with infants with private health insurance. However, these studies were database analyses, presented univariate results, did not correct for other RSV risk factors at an individual level, and did not examine the effect on preterm infants [9, 10].
The RSV Respiratory Events among Preterm Infants Outcomes and Risk Tracking (REPORT) study was a large, prospective, multicenter study that evaluated the burden of medically attended, laboratory-confirmed RSV disease in preterm infants born at 32–35 wGA in the United States [11]. Risk factors identified for severe RSV LRI included young chronological age, daycare attendance, living with nonmultiple-birth siblings, and prior history of LRI [11]. In this study, we investigate the association of insurance status with other risk factors for severe RSV LRI in this population, correcting for those risk factors.
MATERIALS AND METHODS
Study Design and Statistical Analysis
The REPORT study methodology has been detailed previously [11]. In brief, REPORT was a prospective, observational study conducted over 2 consecutive RSV seasons (2009–2011) at 188 US clinical sites that enrolled preterm infants born at 32–35 wGA who had not received RSV immunoprophylaxis with palivizumab. Infants who presented with medically attended acute respiratory illness in the outpatient setting had nasal and pharyngeal swabs collected according to the study protocol for RSV detection using a quantitative real-time reverse transcriptase polymerase chain reaction-based assay [11]. Results of RSV tests conducted as a part of routine clinical care were also recorded. For this analysis, baseline demographics and risk factors for RSV were compared in infants with private versus public health insurance. Statistical significance was evaluated using χ2 tests.
Incidence rates per 100 infant-seasons of the RSV-associated endpoints of RSV outpatient LRI, emergency department (ED) visits, RSV hospitalizations (RSVHs), and intensive care unit (ICU) admissions during peak RSV season (November–March) were calculated for infants with private and public insurance. To examine the impact of any potential difference in RSV testing between the 2 cohorts, the proportion of illness events that were tested for RSV were examined for children with public and private insurance.
The Cox proportional hazard model was used for both univariate and multivariable analyses with calendar time input to adjust for seasonal changes in background rates. For the univariate analysis, only insurance (private vs public) was included as the independent variable in the model. The multivariable analysis included independent variables of insurance (private vs public) and significant non-socioeconomic risk factors at the significance level of 0.1 for each outcome of interest (RSV outpatient LRI visits, ED visits, RSVHs, and ICU admissions). Significant non-socioeconomic risk factors were selected using stepwise selection at 0.2 level for entry and 0.1 level for stay from 8 non-socioeconomic risk factors, chronological age at time of event, gestational age, sex, family history of asthma, family history of eczema, requirement for neonatal ICU or other specialized care facility at time of birth, requirement for mechanical ventilation at time of birth, and history of LRI. Impact of multiple births on outcomes adjusting for insurance status was also examined by including it as an independent variable in the final multivariable model.
RESULTS
Of 1642 evaluable infants enrolled in the REPORT study, 50.1% had private health insurance and 49.9% had public health insurance. Baseline demographic characteristics and risk factors differed significantly for those with public and private insurance. Relative to infants with private insurance, more infants with public insurance were African American (36% vs 6%) or Hispanic (18% vs 4%), had siblings <6 years (41% vs 33%) and 6–18 years (43% vs 22%), had more than 5 people living in the home (30% vs 14%), were exposed to tobacco smoke (24% vs 8%), were younger (73% vs 66% ≤3 months as of November 1), and had a history of LRI (6% vs 3%) (P < .05 for all comparisons based on χ2 test). Infants with public insurance were also less likely to be breastfed (32% vs 55%), attend daycare (5% vs 9%), be exposed to a wood or coal-burning device (5% vs 8%), and be a multiple birth (22% vs 38%) (P < .05 for all comparisons).
Rates of RSV disease outcomes by insurance type are presented in the Figure 1. Rates of RSV outpatient LRI were similar in subjects with private and public insurance. The more severe RSV-related outcomes of ED visits, hospitalizations, and ICU admission were approximately 2-fold higher for subjects with public insurance, although only the difference in ED visit rates reached statistical significance. The hazard ratios associated with public insurance were similar after adjusting for differences in the prevalence of non-socioeconomic factors between the 2 cohorts (Figure 1). Among the enrolled infants, 28 had a RSV-associated “Urgent Care/Other Outpatient” visit: 21 among infants covered with private insurance and 7 among infants with public insurance. Data on RSV testing frequency was generally similar in publicly and privately insured infants. The proportions of infants tested for RSV among those with public vs private insurance were 83.3% vs 90.6% for outpatient LRI, 75.6% vs 87.1% for LRI ED visits, and 97.9% vs 91.2% for LRI hospitalizations. Adjusting for insurance status, multiple births were not a statistically significant risk factor for RSV-associated endpoints.
Figure 1.
Incidence of respiratory syncytial virus (RSV)-associated endpoints by insurance status in infants 32–35 weeks' gestational age. *Comparisons between private and public insurances based on Cox model with calendar time input to adjust for seasonal changes in background rates. Abbreviations: CI, confidence interval; ED, emergency department; ICU, intensive care unit; LRI, lower respiratory tract infection.
DISCUSSION
We found that public insurance was independently associated with an approximate 2-fold higher risk for RSV ED visits and a nonstatistically significant increase in RSVHs, with no association with outpatient LRI events. The stronger association with ED visit risk could be due to the higher probability of publicly insured children seeking primary care in the ED setting [8]. The higher prevalence of other related risk factors in the publicly insured population, such as more siblings in the household, tobacco smoke exposure, and potentially less breastfeeding may have contributed to their increased incidence of severe RSV disease.
The current results also may explain the counterintuitive finding of lower RSVH risk in multiple-birth infants in the original multivariate analysis of the REPORT study. Multiple-birth infants were more likely to have private insurance, perhaps related to greater parental access to assisted reproductive technologies [8]. The lower risk of RSVH observed in multiple-birth infants may have been related to their socioeconomic status rather than a true independent decrease in risk due to multiple births.
Because the REPORT study enrolled infants not receiving RSV immunoprophylaxis, the study cohort underrepresented infants who were eligible for RSV immunoprophylaxis at the time the study was conducted, namely 32–34 wGA infants <3 months of age with additional risk factors (preschool-aged siblings or daycare attendance). As a result, the observed RSV disease rates likely underestimate the true risk in all 32–34 wGA infants in the absence of RSV immunoprophylaxis.
The strengths of this study include the large, prospectively recruited patient cohort that was generally similar to the US birth cohort of infants born at 32–35 wGA, the inclusion of data from 2 RSV seasons from 188 clinical sites across the United States, and the active surveillance and laboratory confirmation of RSV infection in symptomatic infants. The primary limitation of this study was that the patient cohort included only infants born at 32–35 wGA who did not receive RSV immunoprophylaxis, and thus the cohort is not representative of all infants born at 32–35 wGA in the United States. Infants ≤3 months of age at RSV season start were underrepresented among enrolled 32–34 wGA infants, particularly among those with private insurance. This effect of RSV immunoprophylaxis use was not observed for infants born at 35 wGA, consistent with the fact that they were not recommended for RSV immunoprophylaxis during the time when the REPORT study was conducted [12]. In addition, not all events were tested for RSV, which would lead to an underestimation of RSV ED and RSVH rates; however, the proportion of these events were similar between infants with private and public insurance and are unlikely to have significantly biased comparisons by insurance status. Likewise, many events were not tested for the presence of other respiratory viruses. Of those with multiplex testing for respiratory viruses, coinfections with RSV were rare (data not shown).
CONCLUSIONS
The association of insurance status as an independent risk factor for severe RSV LRI in preterm infants is important for several reasons. In epidemiological studies that evaluate the independence of sociodemographic and medical risk factors, it will be important to include the role of public insurance, especially when evaluating severe outcomes. For clinical trials, the association between public insurance and increased ED use questions the utility of RSV ED visits as a clinical trial endpoint in the United States, as suggested earlier [13]. Although including ED visits as a trial endpoint may decrease the sample size required for therapeutic and prophylactic endpoints, the lower implied acuity of illness among publicly insured children who use the ED for primary care might negate that advantage if the treatment or preventive agent deters more severe disease and not more mild disease. Future studies that include ED visits as a study endpoint should standardize these visits using severity scoring to ensure comparability of disease across various populations. In addition, an imbalance in the proportion of children with public insurance between study arms could lead to confounded results. Finally, although our study strongly supports a role for socioeconomic status as measured by public insurance as an independent risk factor for severe RSV disease resulting in ED visits and hospitalization, the wide confidence intervals point to the need for further critical appraisal of the role of insurance status as an independent risk for severe RSV disease in preterm and term infants.
Acknowledgments
Editorial assistance was provided by Complete Healthcare Communications, LLC (Chadds Ford, PA) and by Dr. Greg Tardie, The Lockwood Group (Stamford, CT) (funded by AstraZeneca LP).
Financial support. This study was funded by AstraZeneca LP.
Potential conflicts of interest. J. A. F. is a former employee of MedImmune. E. J. A. has received research funding to his institution on his behalf from MedImmune; he has received writing assistance from MedImmune; he has also served as a consultant to AbbVie Inc. X. W. was an employee of MedImmune at the time of study conduct and analysis. C. S. A. is an employee of AstraZeneca and has stock and stock options in the company. E. A. F. S. has received grant support to his institution from MedImmune and AbbVie Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Simoes EA. Respiratory syncytial virus infection. Lancet 1999; 354:847–52. [DOI] [PubMed] [Google Scholar]
- 2.Jain S, Williams DJ, Arnold SR et al. . Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall CB, Simoes EA, Anderson LJ. Clinical and epidemiologic features of respiratory syncytial virus. Curr Top Microbiol Immunol 2013; 372:39–57. [DOI] [PubMed] [Google Scholar]
- 4.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140:543–6. [DOI] [PubMed] [Google Scholar]
- 5.Boyce TG, Mellen BG, Mitchel EF Jr et al. . Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr 2000; 137:865–70. [DOI] [PubMed] [Google Scholar]
- 6.Moler FW, Khan AS, Meliones JN et al. . Respiratory syncytial virus morbidity and mortality estimates in congenital heart disease patients: a recent experience. Crit Care Med 1992; 20:1406–13. [DOI] [PubMed] [Google Scholar]
- 7.Langley GF, Anderson LJ. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr Infect Dis J 2011; 30:510–7. [DOI] [PubMed] [Google Scholar]
- 8.Frogel M, Nerwen C, Cohen A et al. . Prevention of hospitalization due to respiratory syncytial virus: results from the Palivizumab Outcomes Registry. J Perinatol 2008; 28:511–7. [DOI] [PubMed] [Google Scholar]
- 9.Sangaré L, Curtis MP, Ahmad S. Hospitalization for respiratory syncytial virus among California infants: disparities related to race, insurance, and geography. J Pediatr 2006; 149:373–7. [DOI] [PubMed] [Google Scholar]
- 10.Greenbaum AH, Chen J, Reed C et al. . Hospitalizations for severe lower respiratory tract infections. Pediatrics 2014; 134:546–54. [DOI] [PubMed] [Google Scholar]
- 11.Ambrose CS, Anderson EJ, Simoes EA et al. . Respiratory syncytial virus disease in preterm infants in the United States born at 32–35 weeks gestation not receiving immunoprophylaxis. Pediatr Infect Dis J 2014; 33:576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Committee on Infectious Diseases. From the American Academy of Pediatrics: policy statements--modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics 2009; 124:1694–701. [DOI] [PubMed] [Google Scholar]
- 13.Simoes EA, Carbonell-Estrany X, Guilbert T et al. . Clinical endpoints for respiratory syncytial virus prophylaxis trials in infants and children in high-income and middle-income countries. Pediatr Infect Dis J 2015; 34:1086–92. [DOI] [PubMed] [Google Scholar]