Injectable antiretrovirals including non-nucleoside reverse transcriptase inhibitors are being evaluated for pre-exposure prophylaxis for HIV prevention. Mathematical modeling suggests that injectable pre-exposure prophylaxis among KwaZulu-Natal's at-risk populations could have substantial preventive impact but may increase drug resistance unless highly effective.
Keywords: drug resistance, HIV prevention, mathematical model, pre-exposure prophylaxis, South Africa
Abstract
Background. A long-acting injectable formulation of rilpivirine (RPV), under investigation as antiretroviral pre-exposure prophylaxis (PrEP), may facilitate PrEP adherence. In contrast, cross-resistance between RPV and nonnucleoside reverse-transcriptase inhibitors comprising first-line antiretroviral therapy (ART) could promote human immunodeficiency virus (HIV) drug resistance and reduce PrEP's effectiveness.
Methods. We use novel mathematical modeling of different RPV PrEP scale-up strategies in KwaZulu-Natal, South Africa, to investigate their effects on HIV prevention and drug resistance, compared with a reference scenario without PrEP.
Results. Pre-exposure prophylaxis scale-up modestly increases the proportion of prevalent drug-resistant infections, from 33% to ≤37%. The change in the number of prevalent drug-resistant infections depends on the interplay between PrEP factors (coverage, efficacy, delivery reliability, and scale-up strategy) and the level of cross-resistance between PrEP and ART. An optimistic scenario of 70% effective RPV PrEP (90% efficacious and 80% reliable delivery), among women aged 20–29 years, prevents 17% of cumulative infections over 10 years while decreasing prevalent resistance; however, prevention decreases and resistance increases with more conservative assumptions. Uncertainty analysis assuming 40%–70% cross-resistance prevalence predicts an increase in prevalent resistance unless PrEP's effectiveness exceeds 90%.
Conclusions. Prioritized scale-up of injectable PrEP among women in KwaZulu-Natal could reduce HIV infections, but suboptimal effectiveness could promote the spread of drug resistance.
In 2014 globally, there were 2 million new human immunodeficiency virus (HIV) infections and 1.2 million acquired immune deficiency syndrome-related deaths [1]. South Africa is the epicenter of the HIV pandemic, and its province KwaZulu-Natal has the leading prevalence, reaching 17% overall, 28% among adults aged 15–49, and 40% among women attending antenatal clinics [2, 3].
Antiretroviral therapy (ART) is a potent intervention for HIV treatment and prevention [4]. Antiretroviral therapy access is increasing globally, with ambitious targets set for 2020 [5]. However, in South Africa in 2012, only approximately one third of HIV-infected persons had access to ART and over half were unaware of their HIV status [2]. Male medical circumcision (MMC) and condom use are efficacious for HIV prevention [6, 7], but their utility maybe limited by modest demand [2]. Moreover, women do not directly benefit from MMC, nor can they or other vulnerable populations reliably negotiate condom use [8]. Thus, there is a crucial need for novel, effective methods of HIV prevention.
Oral antiretroviral (ARV) pre-exposure prophylaxis (PrEP) for HIV prevention is safe and efficacious when used consistently [9–11], and it is recommended for persons at substantial risk of acquiring HIV [12]. However, PrEP implementation in sub-Saharan Africa has not yet occurred for several reasons including unease about the following: adherence observed in clinical trials of women, 2 of which were stopped for futility [13, 14]; drug resistance and sexual risk compensation; long-term toxicity; potential competition with ART for resources; uncertain optimal scale-up strategies; and the fiscal impact of additional costs. Concerns about PrEP adherence and HIV drug resistance are paramount.
Injectable ARVs that require infrequent dosing, such as the second-generation nonnucleoside reverse-transcriptase inhibitor (NNRTI) rilpivirine (RPV), are being investigated as long-acting PrEP agents [15]. Rilpivirine is a promising PrEP candidate because of its potency, safety profile, long-acting preparation, and anti-HIV activity against both wild-type and variants harboring the K103N mutation that renders the first-generation NNRTIs ineffective [15–17]. Nevertheless, in our study [16] of 102 HIV isolates from patients failing first-line ART in South Africa with NNRTI resistance-associated mutations, 71 (70%) had ≥3 fold-increase in RPV inhibitory concentration IC90 (RPV concentration required to reduce HIV replication by 90%) compared with treatment-naive viruses from the same region, and 40 (39%) had ≥10 fold-increase in RPV IC90. The protein-adjusted RPV IC90 exceeded the median plasma drug concentrations achieved 28 and 56 days after a single 1200 mg RPV intramuscular dose [18] for approximately 30% and 39% of the overall (and 80% and 100% of the highly resistant) isolates, respectively. Thus, cross-resistance between RPV and other NNRTIs used for ART is common [16, 19, 20] and raises questions (1) about the potential population-level effects of long-acting RPV PrEP scale-up on the efficacy of ARVs for prevention and treatment and (2) on the spread of drug resistance. In addition, the principal factors that could maximize HIV prevention and minimize drug resistance from RPV PrEP scale-up are undefined.
To address these knowledge gaps, we construct and analyze a novel mathematical model that is calibrated to longitudinal HIV prevalence and incidence estimates from KwaZulu-Natal using a Bayesian framework and is informed by cross-resistance data [16]. We simulate optimistic and conservative scenarios of RPV PrEP scale-up, using unprioritized and prioritized population-level strategies. We compare simulation results with a reference HIV prevention scenario without PrEP, and we explore the extent of our prediction uncertainty and the sensitivity of findings to modeling assumptions. Our results herein provide critical insight into the dynamics and determinants of HIV prevention and drug resistance from RPV PrEP scale-up in KwaZulu-Natal. An economic evaluation is reported elsewhere [21].
METHODS
Model Structure and Calibration
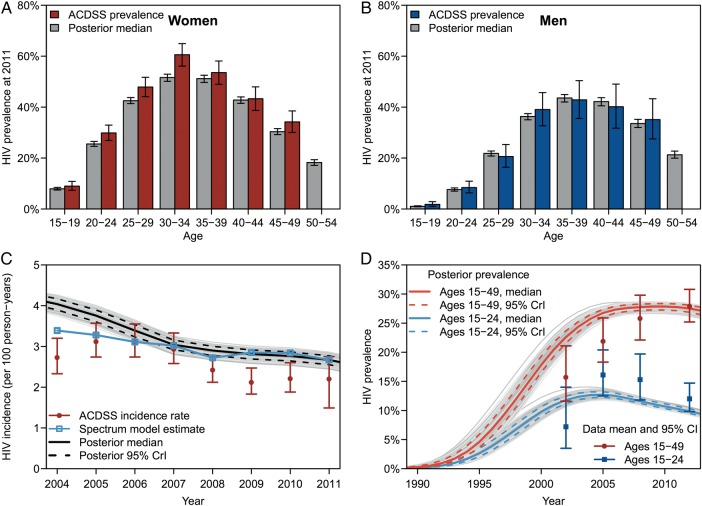
We constructed a mathematical model using coupled ordinary differential equations. The model population was stratified by gender, age (15–54 years), sexual behavior, HIV infection status and disease progression, MMC status, and ARV use. We used Bayesian melding [22] to calibrate the model to (1) longitudinal gender- and age-stratified HIV prevalence data and aggregate HIV incidence data from KwaZulu-Natal (Figure 1, Supplementary Figures 2–4) [24, 25] and (2) cross-sectional behavioral risk-stratified HIV prevalence data from South Africa [26]. Prior probability distributions for model inputs were informed by literature from KwaZulu-Natal, South Africa, and sub-Saharan Africa. After model calibration, we used the posterior mode input estimates. Pre-exposure prophylaxis-related assumptions are shown in Table 1, and model details are provided in the Supplementary Data.
Figure 1.
Model outputs for calibration and validation. Model calibration to human immunodeficiency virus (HIV) prevalence among (A) women and (B) men by age. Error bars show 95% confidence intervals for data and 95% credible intervals for model posterior estimates. (C) Model calibration to HIV incidence in the Africa Centre Demographic Surveillance Site (ACDSS) and comparison to the UNAIDS' Spectrum model [23]. (D) Model validation against HIV prevalence in KwaZulu-Natal among adults aged 15–24 and 15–49 from the 4 South African national household surveys [2]. Abbreviation: CrI, credible interval.
Table 1.
Model PrEP-Related Input Parameters
| Base-Case Scenarios |
Uncertainty Range | Source | |||
|---|---|---|---|---|---|
| Parameter | Symbol | Optimistic | Conservative | ||
| Initial year of PrEP scale-up, year | 2015 | 2015 | 2015–2020 | Assumed | |
| Time to reach target PrEP coverage, years | 5 | 5 | 2.5–7.5 | Assumed | |
| Intended duration of PrEP use, years | 5 | 5 | 2.5–7.5 | Assumed | |
| Proportion of PrEP users who drop out early, % | 40 | 40 | 5–60 | [27] | |
| Average duration of PrEP use, years | 1/η | 3 | 3 | Calculateda | |
| PrEP injection frequency, per year | ψ | 6 | 6 | 6 | [18] |
| HIV testing frequency in the PrEP program, per year | σψ | 2 | 2 | 1–6 | Assumed |
| PrEP reliability (% of injections that are efficacious), % | χ | 80 | 70 | 50–99 | [18] |
| PrEP efficacy against wild-type HIV, % | 90 | 70 | 50–99 | [9, 10] | |
| PrEP efficacy against ART-resistant HIV without PrEP cross-resistance (<3-fold change in RPV IC90), % | 90 | 70 | 50–99 | [9, 10, 16] | |
| PrEP efficacy against ART-resistant HIV with PrEP cross-resistance (3–9-fold change in RPV IC90), % | 45 | 35 | 0–50 | [9, 10, 16] | |
| PrEP efficacy against ART-resistant HIV with PrEP cross-resistance (≥10-fold change in RPV IC90), %b | 0 | 0 | 0 | [9, 10, 16] | |
| PrEP efficacy against PrEP-resistant HIV, % | 22.5 | 0 | 0–50 | Assumed | |
| Time to acquisition of PrEP resistance with wild-type HIV in entire cohort, years | ZW | 0.08 | 0.08 | 0.04–0.12 | [28] |
| Time to acquisition of PrEP resistance with reverted PrEP-resistant or cross-resistant HIV variant v in entire cohort, years | 0.5ZW | 0.5ZW | 0.5ZW | Assumed | |
| Rate of PrEP resistance emergence after a successful injection, per year | –ln(1–0.99)/Zv | –ln(1–0.99)/Zv | –ln(1–0.99)/Zv | Calculated | |
| Rate of PrEP resistance emergence after an unsuccessful injection, per year | 0 | Assumed | |||
| Persistence time of PrEP drug levels that may select drug resistance, monthsc | 3 | 6 | Approximately 2–6 | [18, 28] | |
| Prevalence of PrEP cross-resistance among persons with ART-resistant HIV, % | 40 | 70 | 0–100 | [16, 19, 20] | |
| Proportion of cross-resistant variants that have ≥10-fold change in RPV IC90, % | 100 | 50 | 50 | [16] | |
| Proportion of cross-resistant variants with ≥10-fold change in RPV IC90 that PrEP has 0% efficacy against, %b | 80 | 100 | 80–100 | [16] | |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IC, inhibitory concentration; PrEP, pre-exposure prophylaxis; RPV, rilpivirine.
a The average duration of PrEP use is (1 – p)x when x is the intended duration of use and p is the proportion who drop out of PrEP early for noncompliance.
b We assume that PrEP has 0% efficacy against a fraction of HIV variants with ≥10-fold change in RPV IC90 and partial efficacy against the remainder (ie, same efficacy as against HIV variants with 3- to 9-fold change in RPV IC90) [16].
c In primary base-case analyses, PrEP efficacy and drug levels disappear simultaneously after 2 months. In sensitivity analysis and secondary base-case analyses, PrEP levels may persist after efficacy disappears.
Interventions
We compared scenarios of combined ART, MMC, and PrEP scale-up to a reference scenario of just ART and MMC scale-up reflecting South Africa's National Strategic Plan [29] and HIV guidelines [30], ie, 80% coverage of MMC by 2017 and 80% coverage of ART initiated at CD4 cell count ≤500 cells/μL by 2020.
We simulated 3 PrEP strategies: (1) unprioritized PrEP covered 2.5%–15% of uninfected adults regardless of age, gender, or sexual behavior; (2) age-prioritized PrEP covered 15%–85% of women aged 20–29 years, while also reaching 2.5%–15% population-level coverage; (3) risk-prioritized PrEP covered 80% (range, 50%–90%) of uninfected female sex workers and their clients, but it reached only 0.8% in overall coverage due to the group's small size (0.4% of women and 2.1% of men) and high HIV prevalence (57% at 2015). Pre-exposure prophylaxis scale-up began at 2015, reached its coverage target over 5 years, and was then maintained until 2025.
Human Immunodeficiency Virus Drug Resistance
The model characterizes HIV variants as drug-sensitive or drug-resistant. Drug-resistant variants are either acquired from selection pressure from PrEP or ART or transmitted from a donor with drug-resistant HIV. Drug-resistant HIV may revert to drug-sensitive wild-type off of ARVs or in a new host, but archived resistance may re-emerge with subsequent ARV exposure. The model does not represent specific drug resistance mutations. Instead, it assumes the presence or absence of resistance to the first-generation NNRTIs (commonest drug-resistance pattern observed in subjects failing first-line ART in sub-Saharan Africa [31]), resistance to RPV, or cross-resistance between the two. We assume drug-resistant infection reduces the efficacy of ARVs for treatment and prevention.
Model Analyses
Base-Case Analyses
We simulated optimistic and conservative base-case scenarios with PrEP (Table 1). In the optimistic scenario, PrEP had 90% efficacy against wild-type HIV; 80% of PrEP injections successfully yielded efficacious drug levels (reliability), whereas 20% did not; and only successful injections caused PrEP resistance to emerge after breakthrough infection. The conservative scenario assumed the following: 70% PrEP efficacy against wild-type HIV; 70% PrEP reliability; and all injections promoted drug resistance emergence after breakthrough infection. Model assumptions related to cross-resistance were primarily informed by our in vitro work [16]. The prevalence of cross-resistance was 40% in the optimistic and 70% in the conservative scenario, whereas PrEP's relative (to wild-type) efficacy against RPV-resistant virus was 0%–50%. Persons could receive 6 PrEP injections per year for 5 years with HIV testing biannually; however, 40% dropped out early. In primary analysis, we assumed that both PrEP efficacy and resistance selection upon breakthrough infection occurred over the 2-month interval between scheduled injections. In secondary analysis, we assumed that although HIV preventive efficacy disappeared after 2 months, plasma RPV drug concentrations and risk for resistance selection persisted for 3 (optimistic) to 6 (conservative) months after an injection [18, 28].
Sensitivity and Uncertainty Analyses
We performed sensitivity and uncertainty analyses using 20 000 simulations of the reference scenario and each PrEP strategy, with intervention-related inputs drawn via Latin hypercube sampling (Table 1, Supplementary Tables 2–5). We quantified uncertainty in projections using medians and interquartile ranges (IQRs), and we computed standardized regression coefficients (SRCs) to measure the influence of model inputs on outcomes. We fitted response hypersurfaces using multivariate regression to illustrate the influence of key inputs.
RESULTS
Base-Case Analyses
Human Immunodeficiency Virus Prevention
Our reference scenario predicted approximately 0.7 million new HIV infections during 2015–2025. Figure 2 shows the cumulative new HIV infections prevented over 10 years in PrEP scenarios using different strategies in comparison with the reference scenario. Human immunodeficiency virus prevention was greater for the optimistic versus conservative PrEP scenario; for higher versus lower PrEP coverage; and for prioritized versus unprioritized PrEP scale-up. At 15% (uppermost) population-level coverage, 9.1% of infections were prevented in the optimistic unprioritized PrEP scenario, increasing to 17.2% when PrEP was age-prioritized (covering 85% of women aged 20–29 years). Risk prioritization reduced infections (8.1%) to a similar extent as unprioritized PrEP, but at a fractional population-level coverage (<1%). Human immunodeficiency virus prevention by all strategies was considerably reduced in the conservative PrEP scenario; to 5.5% when unprioritized, 10.3% when age prioritized, and 4.4% when risk prioritized. Supplementary Tables 6 and 7 give data for all coverage levels.
Figure 2.
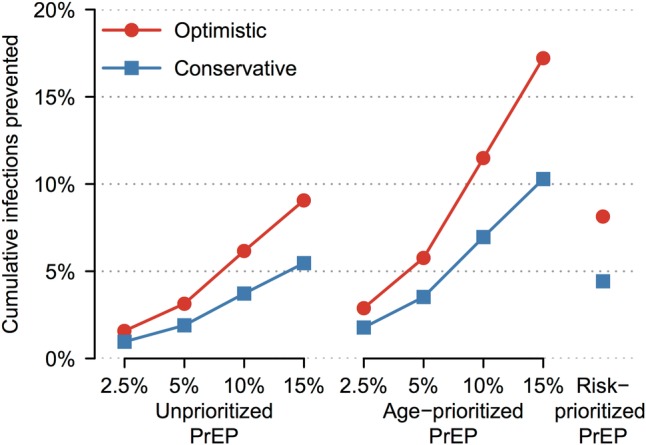
New human immunodeficiency virus (HIV) infections prevented over 10 years of pre-exposure prophylaxis (PrEP) scale-up in primary base-case analysis. The PrEP coverage levels of 2.5%–15% are shown for unprioritized and age-prioritized PrEP. Optimistic (conservative) scenario assumptions were as follows: 90% (70%) PrEP efficacy vs wild-type HIV, 0%–50% relative efficacy vs rilpivirine-resistant HIV, 80% (70%) PrEP reliability, 40% (70%) cross-resistance between antiretroviral treatment and PrEP, and successful (all) PrEP injections select drug-resistant HIV after breakthrough infection.
Human Immunodeficiency Virus Drug Resistance
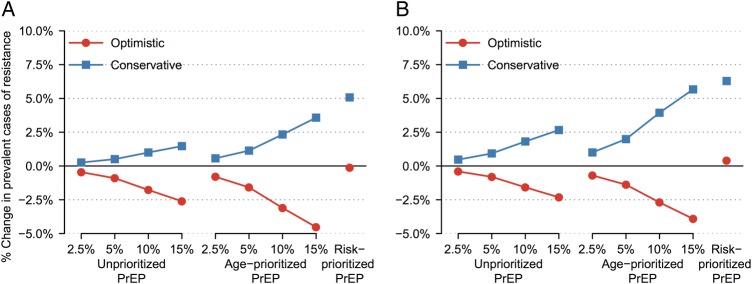
Without PrEP scale-up, drug-resistance prevalence (the proportion of HIV-infected persons with drug resistance) increased from 11.7% at 2015 to 33.1% (0.44 million cases) at 2025, due to scale-up of first-line ART. Compared with the reference scenario, all simulations with PrEP scale-up increased drug resistance prevalence; however, the bulk of resistance was from ART, whereas PrEP contributed <7% of the 0.42–0.46 million cases (Supplementary Tables 6 and 7). In contrast, PrEP scale-up could increase or decrease the number of prevalent drug-resistant cases (total resistance) after 10 years compared with the reference, depending on the PrEP scenario and strategy (Figure 3A). At 15% coverage, both unprioritized and age-prioritized PrEP strategies decreased total resistance in the optimistic scenario (by 2.6% and 4.5%, respectively), which conversely increased (by 1.5% and 3.6%) in the conservative scenario having less-optimistic PrEP- and resistance-related assumptions. In contrast, resistance cases were almost unchanged in the optimistic scenario with risk prioritization (0.1% decrease) and increased the most (by 5.1%) in the conservative scenario, reflecting this group's high HIV transmission risk. Supplementary Tables 6 and 7 give data for all coverage levels.
Figure 3.
Percentage change in prevalent drug-resistant cases after 10 years of pre-exposure prophylaxis (PrEP) scale-up in base-case analyses. Figures show results of (A) primary analysis, in which PrEP efficacy and drug levels persisted for 2 months after an injection, and (B) secondary analyses, in which PrEP efficacy disappeared after 2 months but drug levels persisted for 3 (optimistic) or 6 (conservative) months. Optimistic (conservative) scenario assumptions were as follows: 90% (70%) PrEP efficacy vs wild-type human immunodeficiency virus (HIV), 0%–50% relative efficacy vs rilpivirine-resistant HIV, 80% (70%) PrEP reliability, 40% (70%) cross-resistance between antiretroviral treatment and PrEP, and successful (all) PrEP injections select drug-resistant HIV after breakthrough infection.
Secondary Analysis
The primary analysis reported above assumed that RPV PrEP's efficacy and drug levels persisted for an average of 2 months. In secondary analysis, we assumed that although PrEP's efficacy disappeared after 2 months, drug levels persisted for 3 to 6 months with continued opportunity for resistance selection [18, 28]. Ten years after PrEP scale-up began (2025), total resistance modestly increased by 0.05%–2% in the secondary relative to primary analysis, mainly from an increase in resistance attributable to PrEP (Supplementary Tables 6 and 7). Although the resistance trends observed in the PrEP scenarios were qualitatively similar in the primary and secondary analyses, the decreases in total resistance were attenuated and, conversely, the increases were exacerbated by prolonged drug persistence in the latter (Figure 3B). In the secondary optimistic scenario at 2025, cases of PrEP resistance increased up to 25% with both unprioritized and age-prioritized PrEP strategies and by 21% with risk-prioritized PrEP (Supplementary Tables 6 and 7). Increases were comparable in the secondary conservative PrEP scenario: up to 35% when unprioritized, 37% when age prioritized, and 18% when risk prioritized. Despite the increases in total resistance seen in the secondary analysis, PrEP's contribution to total resistance remained small (<9%) compared with ART (Figure 4A and B), and there was minimal attenuation of HIV prevention from PrEP scale-up. Supplementary Tables 6 and 7 give data for all coverage levels.
Figure 4.
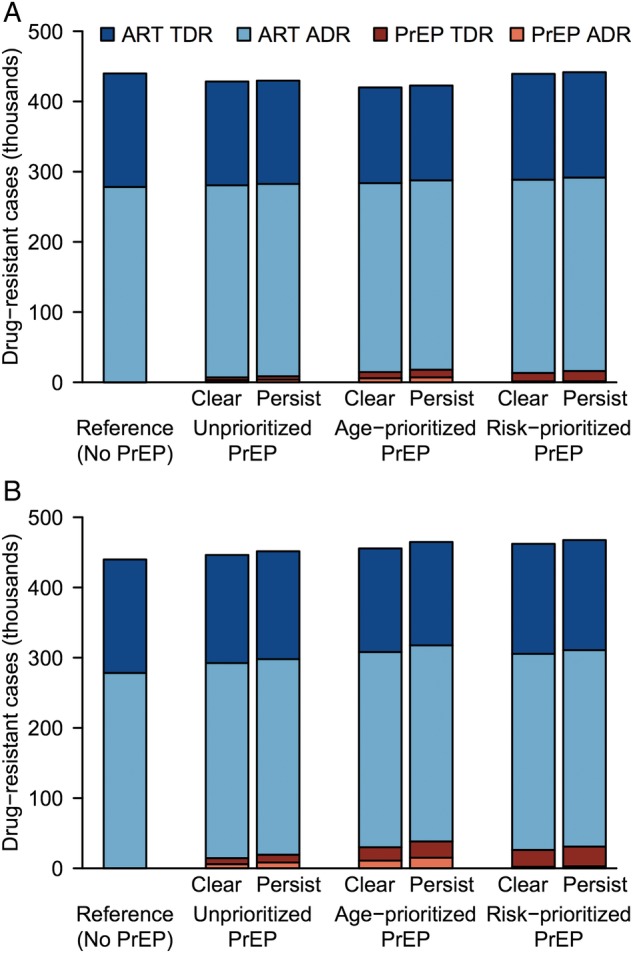
Prevalent drug-resistant cases after 10 years of pre-exposure prophylaxis (PrEP) scale-up. Panels show results of (A) optimistic and (B) conservative base-case scenarios. Optimistic (conservative) scenario assumptions were as follows: 90% (70%) PrEP efficacy, 80% (70%) PrEP reliability vs wild-type human immunodeficiency virus (HIV), 0%–50% relative efficacy vs rilpivirine-resistant HIV, 40% (70%) cross-resistance between antiretroviral treatment (ART) and PrEP, and successful (all) PrEP injections select drug-resistant HIV after breakthrough infection. In primary analysis, PrEP drug levels cleared when PrEP efficacy disappeared after 2 months (“Clear”); in secondary analysis, drug levels persisted for 3 (optimistic) or 6 (conservative) months, whereas efficacy disappeared after 2 months (“Persist”). Data are shown for 15% unprioritized and age-prioritized PrEP coverage. Abbreviations: ADR, acquired drug resistance; TDR, transmitted drug resistance.
Prediction Uncertainty
At coverage ranging between 2.5% and 15%, a median 3.9% (IQR, 2.5%–5.6%) of cumulative new infections were prevented by the unprioritized PrEP strategy; the preventive effect nearly doubled to 7.1% (IQR, 4.5%–10.1%) with age-prioritized PrEP and was comparable at 4.6% (IQR, 3.5%–6.1%) with risk-prioritized PrEP despite much lower (range, 0.4%–1.0%) coverage (Supplementary Table 8).
Although PrEP decreased cumulative new infections overall, it increased cumulative new infections having transmitted drug resistance (Supplementary Table 8), by 2.0% (IQR, 0.2%–4.3%) with unprioritized PrEP, which rose intermediately to 4.5% (IQR, 1.1%–9.2%) with age-prioritized PrEP and maximally to 14.8% (IQR, 9.4%–21.9%) with risk-prioritized PrEP. Likewise, PrEP increased total resistance (prevalent drug-resistant infections) in a majority of these simulations, given inclusion of conservative PrEP assumptions including prolonged drug persistence; unprioritized PrEP increased total resistance by a median of 1.9% (IQR, 0.5%–3.9%), age-prioritized PrEP increased total resistance by a median of 3.9% (IQR, 1.3%–7.7%), whereas risk-prioritized PrEP maximally increased drug-resistant infections by 7.3% (IQR, 4.4%–10.8%).
Sensitivity Analysis
We were not surprised to find that higher coverage with more effective PrEP prevented more infections: PrEP coverage, efficacy against wild-type virus, and reliability together explained 82% of the variance in infections prevented by unprioritized and age-prioritized PrEP (Supplementary Table 9). Results were qualitatively similar for risk-prioritized PrEP.
The extent of cross-resistance between ART and PrEP was the strongest driver of increases in cumulative transmitted resistance from unprioritized and age-prioritized PrEP (SRCs 0.38 and 0.36, respectively), because breakthrough infection was more likely among PrEP users whose partners harbored cross-resistant infection. The increase in total resistance from unprioritized PrEP also grew with increasing cross-resistance (SRC 0.30), as well as rising PrEP coverage (SRC 0.31) and longer persistence of acquired PrEP resistance (SRC 0.28), whereas higher PrEP efficacy and reliability reduced total resistance (SRCs −0.25 and −0.32). Results for prioritized PrEP were qualitatively similar (Supplementary Table 9).
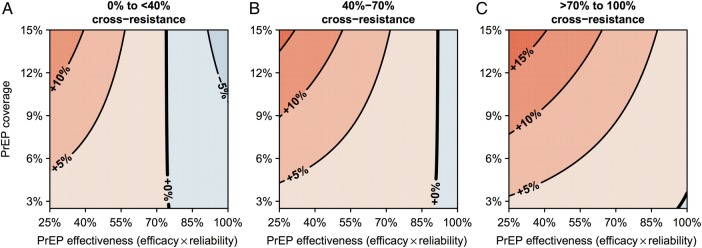
Age-prioritized PrEP tended to increase total resistance unless PrEP's efficacy (against wild-type virus) and reliability were both high; at 40%–70% cross-resistance, if effectiveness (the product of efficacy and reliability) was above approximately 90%, age-prioritized PrEP tended to decrease drug resistance (Figure 5B). The effectiveness threshold below which PrEP increased total resistance was proportional to the extent of cross-resistance between ART and PrEP (Figure 5A–C). Results were similar for unprioritized PrEP (Supplementary Figure 5A). Risk-prioritized PrEP increased total resistance regardless of PrEP's effectiveness (Supplementary Figure 5B), but the increase was less with more effective PrEP.
Figure 5.
Response surfaces showing the percentage change in prevalent drug-resistant cases after age-prioritized pre-exposure prophylaxis (PrEP) scale-up. Response surfaces were calculated as a function of PrEP effectiveness and coverage from sensitivity analysis simulations with (A) <40%, (B) 40%–70%, or (C) >70% cross-resistance between antiretroviral treatment and PrEP. Resistance decreases are shown in blue, increases are shown in red.
DISCUSSION
Pre-exposure prophylaxis is an efficacious intervention against HIV [9–11]. However, the optimal role of PrEP for HIV prevention in resource-limited settings is undefined. Pre-exposure prophylaxis scale-up is impeded by concerns about suboptimal adherence and the deleterious effects of more frequent drug resistance. Several injectable long-acting ARVs for PrEP, such as RPV, are currently under study [15]. Although there is optimism that injectable ARVs could provide a desirable and adherence-friendly delivery platform for PrEP, this is tempered by the knowledge that HIV drug resistance is increasing due to ART scale-up in sub-Saharan Africa [31], and that there is cross-resistance between the second-generation NNRTI PrEP candidates and the first-generation NNRTIs used for ART [16, 19, 20]. In this study, we constructed and analyzed a detailed mathematical model, calibrated using a Bayesian framework and informed by regional cross-resistance data [16], to explore scenarios of RPV PrEP scale-up in combination with ART and MMC, for HIV prevention in the KwaZulu-Natal province of South Africa. We determined outcomes over 10 years, relative to a reference scenario of ART and MMC without PrEP, reflecting South Africa's National Strategic Plan and HIV management guidelines [29, 30].
Our study highlights several important findings. First, the effects of RPV PrEP on HIV prevention and drug resistance are determined by PrEP's scale-up strategy (HIV prevention and total resistance increase with prioritization and higher coverage); PrEP efficacy against wild-type virus and PrEP reliability (both factors increase infections prevented and decrease total resistance); and the prevalence of cross-resistance between RPV PrEP and ART (increases transmitted drug resistance). Second, for both unprioritized and age-prioritized strategies, the extent of cross-resistance between PrEP and ART defines the minimum threshold for PrEP's effectiveness (the product of efficacy and reliability) that is required to mitigate an increase in total resistance. Third, persistence of RPV drug levels with opportunity for resistance selection, beyond the duration of efficacy, increases PrEP's contribution to total drug resistance (the number of prevalent infections with drug resistance); however, this effect remains modest compared with ART's contribution to resistance. Fourth, the prevalence of drug resistance (proportion of prevalent infections with drug resistance) increases (although modestly; from 33% to at most 37%) with the combined scale-up of ART with RPV PrEP compared with without PrEP. Finally, an optimistic scenario of 80% reliable RPV PrEP prioritized to women aged 20–29 years, assuming 90% efficacy against wild-type HIV and 40% cross-resistance with first-line ART, achieves almost double the HIV prevention at 10 years, compared with unprioritized PrEP, and decreases the total drug resistance in contrast to risk-prioritized PrEP. However, the total resistance prevalence increases in most uncertainty simulations regardless of the scale-up strategy.
For simulations of injectable RPV PrEP scale-up in KwaZulu-Natal, we confirm that HIV prevention is proportionate to the examined levels of PrEP's coverage and effectiveness, and that prevention is amplified by strategies that focus PrEP to key at-risk populations; our data extend findings from previous modeling of oral and topical PrEP, performed by us [32–36] and others [37–39]. Risk-prioritized RPV PrEP having high efficacy, reliability, and coverage prevents close to 10% of cumulative infections over 10 years, similar to unprioritized PrEP but at a fraction of population-level coverage (<1% vs 15%). On the other hand, at the same population-level coverage (ranging from 2.5% to 15%), age-prioritized PrEP focusing on 20- to 29-year-old women prevents almost twice as many infections as unprioritized PrEP. Commercial sex workers, their clients, and young women have high HIV incidence and prevalence in South Africa [2, 24, 26, 40]. Although the former 2 groups may be difficult to identify and reach [40], prioritization of PrEP to 20- to 29-year-old women may be logistically more feasible. Prioritization could be considered for women of other ages in KwaZulu-Natal; however, HIV prevention would be less due to lower HIV incidence compared with women aged 20–29 years [24].
We identify and interpret complex drug resistance patterns from different RPV PrEP scale-up strategies. As we and others have demonstrated previously for oral PrEP, the prevalence of drug resistance always increases when PrEP is scaled-up in combination with ART [32, 35, 36]; however, most of the resistance is from ART and not PrEP. If RPV PrEP drug levels persist long beyond their efficacious effect with ongoing risk of resistance selection [18, 28], our projections of PrEP resistance increase by 18%–37%; however, the increase in total resistance is limited (≤2%) because PrEP's relative contribution to the total drug resistance remains modest (<9%), with little change in its impact on prevention. A key insight from our study (observed in base-case and sensitivity analyses) is that the level of cross-resistance between RPV PrEP and ART plays a critical role in determining the direction and magnitude of the change in total drug resistance at a given level of PrEP effectiveness, as well as the extent of increase in transmitted drug resistance: in base-case analyses, scale-up of highly effective PrEP having 40% cross-resistance decreased total resistance, whereas less-effective PrEP having 70% cross-resistance increased resistance; sensitivity analysis identified cross-resistance as a key factor driving increases in incident and prevalent resistance. Raising PrEP coverage magnifies these increases or decreases in drug resistance. Risk-prioritization increases total resistance proportionate to the level of cross-resistance, demonstrating that high behavioral-risk groups not only make a disproportionate contribution to HIV transmission in the absence of interventions, but also to the spread of drug resistance, and would require more intensive resistance monitoring with PrEP scale-up. The thresholds for PrEP efficacy and reliability, below which total drug resistance rises from the scale-up of unprioritized and age-prioritized PrEP, are proportional to the level of cross-resistance. Our modeling suggests that at the current level of cross-resistance between RPV and first-generation NNRTIs in South Africa (40%–70%), scale-up of PrEP will likely increase total drug resistance unless PrEP is highly efficacious against wild-type virus and reliable (approximately 90% effective).
This study has several caveats. Precise details of our model's projections will be affected by variations in the structural and parameter assumptions embedded within it, especially those regarding sexual behavior [35]. Nevertheless, we used rigorous model construction, calibration, parameterization, and analysis. Because injectable PrEP agents are currently in development, their efficacy and drug resistance potential are unknown. To account for this knowledge gap, we consider different scenarios and strategies in our analyses. Our base-case PrEP efficacy assumptions are informed by oral PrEP studies [9, 10], which may not be representative of injectable PrEP agents from other ARV classes. Thus, we explore a wider efficacy range in sensitivity analysis. Our assumptions regarding cross-resistance between ART and PrEP and the potential efficacy of PrEP and ART against cross-resistant HIV are primarily informed by laboratory studies involving a limited set of 102 HIV isolates from patients failing first-line ART in South Africa [16]; nevertheless, we examine a wide estimate range in uncertainty and sensitivity analyses. We assume scale-up of only first-line ART because of limited (approximately 5%) current access to second-line ART in resource-constrained settings [41]; however, this may change in the future. We represent PrEP adherence by assuming delivery of regular injections with programmatic dropout but with maintenance of target coverage once achieved. We also represent PrEP reliability as the proportion of injections that successfully yield efficacious drug levels [18, 28]. In real-world use, if PrEP injections are irregular and PrEP coverage drops or conversely PrEP injections are 100% reliable, we may have respectively overestimated or underestimated RPV PrEP's impact on HIV prevention. Because HIV is predominantly transmitted heterosexually in South Africa [26], we did not model men who have sex with men or injection drug users; however, these at-risk populations may also benefit from PrEP [39]. Finally, our modeling context is the mature, generalized, high-prevalence HIV epidemic in KwaZulu-Natal, South Africa. Thus, our quantitative findings may not be directly generalizable to other contexts. Nonetheless, the qualitative insights from our modeling are likely to be robust.
CONCLUSIONS
Prioritized RPV PrEP scale-up could have considerable public health benefit. However, prevalent drug resistance will likely increase if PrEP efficacy and reliability are modest, given current levels of cross-resistance between first- and second-generation NNRTIs.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
Financial support. This work was supported by the Bill and Melinda Gates Foundation (grant OPP1005974; to U. L. A.).
Potential conflicts of interest. R. L. G., U. M. P., G. H., K. J. P., and U. L. A. report grants from the Bill and Melinda Gates Foundation during the conduct of the study. J. W. M. reports grants from the Bill and Melinda Gates Foundation during the conduct of the study; personal fees from University of Pittsburgh, personal fees from Gilead Sciences, and has stock options in Cocrystal Pharma, Inc., outside the submitted work, and has been issued a US patent 8,815,829 on 26 August 2014. E. B. reports grant support from the National Institute on Drug Abuse of the National Institutes of Health (R01-DA15612). All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Joint United Nations Programme on HIV/AIDS. Fact Sheet 2015 Global Statistics. Geneva: Joint United Nations Programme on HIV/AIDS, 2015. [Google Scholar]
- 2.Shisana O, Rehle T, Simbayi L et al. . South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town: HSRC Press, 2014. [Google Scholar]
- 3.South Africa National Department of Health. The 2013 National Antenatal Sentinel HIV Prevalence Survey South Africa. Pretoria: South Africa National Department of Health, 2015. [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M et al. . Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joint United Nations Programme on HIV/AIDS. Ambitious Treatment Targets: Writing the Final Chapter of the AIDS Epidemic. Geneva: Joint United Nations Programme on HIV/AIDS, 2014. [Google Scholar]
- 6.Mehta SD, Moses S, Agot K et al. . The long-term efficacy of medical male circumcision against HIV acquisition. AIDS 2013; 27:2709–899. [DOI] [PubMed] [Google Scholar]
- 7.Weller SC, Davis-Beaty K.. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev 2002:CD003255. [DOI] [PubMed] [Google Scholar]
- 8.Ramjee G, Daniels B. Women and HIV in sub-Saharan Africa. AIDS Res Ther 2013; 10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baeten JM, Donnell D, Ndase P et al. . Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant RM, Lama JR, Anderson PL et al. . Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thigpen MC, Kebaabetswe PM, Paxton LA et al. . Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. WHO Expands Recommendation on Oral Pre-Exposure Prophylaxis of HIV Infection (PrEP). Geneva: World Health Organization, 2015. [Google Scholar]
- 13.Van Damme L, Corneli A, Ahmed K et al. . Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marrazzo JM, Ramjee G, Richardson BA et al. . Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spreen WR, Margolis DA, Pottage JC Jr. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS 2013; 8:565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penrose KJ, Wallis CL, Scoulos-Hanson M et al. . High prevalence of cross-resistance to rilpivirine in subtype C HIV-1 isolates from first-line ART failures in South Africa. AIDS Res Hum Retroviruses 2014; 30:A166. [Google Scholar]
- 17.Azijn H, Tirry I, Vingerhoets J et al. . TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother 2010; 54:718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson AG, Else LJ, Mesquita PM et al. . A compartmental pharmacokinetic evaluation of long-acting rilpivirine in HIV-negative volunteers for pre-exposure prophylaxis. Clin Pharmacol Ther 2014; 96:314–23. [DOI] [PubMed] [Google Scholar]
- 19.Fofana DB, Soulie C, Balde A et al. . High level of HIV-1 resistance in patients failing long-term first-line antiretroviral therapy in Mali. J Antimicrob Chemother 2014; 69:2531–5. [DOI] [PubMed] [Google Scholar]
- 20.Wallis CL, Aga E, Ribaudo H et al. . Drug susceptibility and resistance mutations after first-line failure in resource limited settings. Clin Infect Dis 2014; 59:706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glaubius RL, Hood G, Penrose KJ et al. . Cost-effectiveness of injectable preexposure prophylaxis for HIV prevention in South Africa. Clin Infect Dis 2016; doi:10.1093/cid/ciw321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raftery AE, Bao L. Estimating and projecting trends in HIV/AIDS generalized epidemics using incremental mixture importance sampling. Biometrics 2010; 66:1162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahy M, Lewden C, Brinkhof MW et al. . Derivation of parameters used in Spectrum for eligibility for antiretroviral therapy and survival on antiretroviral therapy. Sex Transm Infect 2010; 86(Suppl 2):ii28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mossong J, Grapsa E, Tanser F et al. . Modelling HIV incidence and survival from age-specific seroprevalence after antiretroviral treatment scale-up in rural South Africa. AIDS 2013; 27:2471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanser F, Bärnighausen T, Grapsa E et al. . High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science 2013; 339:966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.South African Centre for Epidemiological Modelling and Analysis. The Modes of Transmission of HIV in South Africa. Stellenbosch: South African Centre for Epidemiological Modelling and Analysis, 2009. [Google Scholar]
- 27.The HIV Prevention Trials Network. HPTN 076: Phase II safety and acceptability of an investigational injectable product, TMC278 LA, for pre-exposure prophylaxis (PrEP). Available at: https://www.hptn.org/research/studies/152. Accessed 29 June 2016. [Google Scholar]
- 28.Penrose KJ, Parikh UM, Hamanishi KA et al. . Selection of rilpivirine-resistant HIV-1 in a seroconverter from the SSAT 040 trial who received the 300-mg dose of long-acting rilpivirine (TMC278LA). J Infect Dis 2016; 213:1013–7. [DOI] [PubMed] [Google Scholar]
- 29.South Africa National Department of Health. National Strategic Plan on HIV, STIs and TB, 2012–2016. Pretoria: Department of Health, 2012. [Google Scholar]
- 30.South Africa National Department of Health. National Consolidated Guidelines for the Prevention of Mother-to-Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents and Adults. Pretoria: Department of Health, 2014. [Google Scholar]
- 31.Gupta RK, Jordan MR, Sultan BJ et al. . Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet 2012; 380:1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbas UL, Glaubius R, Mubayi A et al. . Antiretroviral therapy and pre-exposure prophylaxis: combined impact on HIV transmission and drug resistance in South Africa. J Infect Dis 2013; 208:224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbas UL, Anderson RM, Mellors JW. Potential impact of antiretroviral chemoprophylaxis on HIV-1 transmission in resource-limited settings. PLoS One 2007; 2:e875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alistar S, Grant P, Bendavid E. Comparative effectiveness and cost-effectiveness of antiretroviral therapy and pre-exposure prophylaxis for HIV prevention in South Africa. BMC Med 2014; 12:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbas UL, Glaubius R, Hood G, Mellors JW. Antiretroviral treatment, preexposure prophylaxis, and drug resistance in sub-Saharan Africa: a consensus among mathematical models. J Infect Dis 2014; 209:164–6. [DOI] [PubMed] [Google Scholar]
- 36.van de Vijver DA, Nichols BE, Abbas UL et al. . Preexposure prophylaxis will have a limited impact on HIV-1 drug resistance in sub-Saharan Africa: a comparison of mathematical models. AIDS 2013; 27:2943–51. [DOI] [PubMed] [Google Scholar]
- 37.Supervie V, Barrett M, Kahn JS et al. . Modeling dynamic interactions between pre-exposure prophylaxis interventions & treatment programs: predicting HIV transmission & resistance. Sci Rep 2011; 1:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cremin I, Alsallaq R, Dybul M et al. . The new role of antiretrovirals in combination HIV prevention: a mathematical modelling analysis. AIDS 2013; 27:447–58. [DOI] [PubMed] [Google Scholar]
- 39.Gomez GB, Borquez A, Case KK et al. . The cost and impact of scaling up pre-exposure prophylaxis for HIV prevention: a systematic review of cost-effectiveness modelling studies. PLoS Med 2013; 10:e1001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Loggerenberg F, Mlisana K, Williamson C et al. . Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 Acute Infection Study. PLoS One 2008; 3:e1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Organization. Antiretroviral Medicines in Low- and Middle-Income Countries: Forecasts of Global and Regional Demand for 2013–2016. Geneva: World Health Organization, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.