Abstract
Background. Access to hepatitis C virus (HCV) treatment is limited in low- and middle-income countries (LMICs). Noninvasive biomarkers, such as fibrosis 4 (FIB-4) and aminotransferase to platelet ratio index (APRI), are low-cost alternatives to staging liver disease and identifying treatment need in people with chronic HCV infection, but their accuracy has not been evaluated in LMICs.
Methods. We tested the accuracy of FIB-4 and APRI at validated cutoffs (FIB-4 <1.45, >3.25; APRI <0.5, >1.5) in predicting severe liver stiffness by elastography among 281 persons chronically infected with HCV. Multivariable logistic and Cox regression were used to identify markers of improved prediction and mortality, respectively.
Results. Sensitivity and specificity of FIB-4 and APRI for predicting severe stiffness were 62% and 87% and 61% and 83%, respectively. Fibrosis 4 and APRI were less accurate in excluding significant stiffness; however, performance of models significantly improved with γ-glutamyl transpeptidase (GGT) and body mass index (BMI) (area under receiver operating characteristic curve, 0.81; 95% confidence interval, .76–.87). Severe liver stiffness predicted via FIB-4 >3.25, APRI >1.5, and a modified FIB-4 that included GGT and BMI were significantly associated with increased mortality.
Conclusions. Fibrosis 4 and APRI may be useful in identifying individuals with severe stiffness who need treatment and continued monitoring in LMICs. Exclusion of significant stiffness may be improved by including GGT and BMI to FIB-4 models.
Keywords: liver stiffness, FIB-4, hepatitis C virus, India, people who inject drugs
Approximately 135 million individuals are chronically infected with hepatitis C virus (HCV), with the highest burden occurring in low- and middle-income countries (LMICs) [1–3]. Highly efficacious direct-acting antivirals (DAAs) have achieved cure rates upwards of 95% [4]; however, treatment delivery remains challenging due to high cost and low access to healthcare in LMICs [5]. As a result, staging liver disease will remain critical in prioritizing treatment need and identifying individuals with cirrhosis who might require different treatment regimens [4, 6] and ongoing screening for hepatocellular cancer and esophageal varices.
Liver biopsy has been considered the gold standard of staging liver disease in patients chronically infected with HCV [7]. However, due to its invasive nature, sampling error, potential for adverse events, and high intra- and interobserver variability [8], noninvasive diagnostic methods have begun to replace the biopsy in many settings [9]. Transient elastography (eg, FibroScan), which provides liver stiffness measurements (LSMs), is a promising alternative due to its high accuracy in detecting severe fibrosis (FIB) and cirrhosis [10]. Elastography has been used in Europe and the United States for over a decade and is US Food and Drug Administration-approved for liver disease staging; however, the prohibitive cost of the machine itself (approximately $120 000) makes its adoption in LMICs challenging.
Noninvasive, serum biomarker panels, which have also been validated against biopsy, are an attractive alternative for staging patients with chronic HCV infection in LMICs [11]. Numerous biomarker panels have been assessed and generally perform best in predicting and excluding severe FIB and cirrhosis [11, 12]. However, most studies were conducted in high-income settings, and data are lacking on the accuracy of low-cost serum biomarker panels in LMICs, where individuals have varying ethnicities, background coinfections, and comorbidities that may impact performance [13].
India has an estimated HCV population prevalence of 1%–2.4%, 3 million opiate users [14, 15], and 1.1 million people who inject drugs (PWID) [16]. We previously documented a high burden of HCV infection [17] and liver stiffness among PWID in India [13] Moreover, we recently demonstrated a strong association between significant and severe stiffness and mortality. However, there is a paucity of research on the performance of validated inexpensive and noninvasive markers, such as FIB-4 [18] and aminotransferase to platelet ratio index (APRI) [19]. Accordingly, we evaluated performance of these panels and explored additional serum markers that might enhance prediction of severe liver stiffness and exclusion of significant stiffness among PWID chronically infected with HCV in Chennai, India.
METHODS
Study Population and Data Collection Procedures
As described previously [13], the Chennai HIV, HCV and Eeral (liver disease) Study (CHHEERS) was conducted by YR Gaitonde Centre for Substance Abuse Research in Chennai, India, with enrollment beginning in 2012 and currently ongoing [20]. A convenience sample of current and former PWID was recruited by community outreach. Eligibility criteria were (1) being at least 18 years of age, (2) self-reported injection drug use in the past 5 years, and (3) mentally fit to understand study/consent procedures. In total, 1324 participants were screened, 1062 of whom were eligible, and 1042 were enrolled. At baseline, all participants underwent a blood draw (after overnight fast) followed by an interview-administered questionnaire. Self-reported data were collected on sociodemographics, past and current substance use, human immunodeficiency virus (HIV), HCV, and hepatitis B virus (HBV) testing and treatment history. A physician examined participants for signs or symptoms of liver disease and assessed FIB/cirrhosis and steatosis by transient elastography and ultrasonography, respectively.
Human immunodeficiency virus-negative and HIV-positive participants not on treatment were observed semiannually, and HIV-positive participants on treatment were observed quarterly. At semiannual visits, all participants underwent a follow-up questionnaire, a nonfasting blood draw, and elastography. Mortality data and cause were reported from other participants and field workers who actively tracked participants when they missed visits. In general, participants were tracked within 1 month of a missed visit.
This analysis was limited to individuals with chronic HCV infection (detectable HCV ribonucleic acid [RNA]; N = 281) at baseline. Of these 281 individuals, 244 (79%) had at least 1 additional follow-up visit. Participants were observed for a median 2.9 years (interquartile range [IQR], 2.2–3.5). The study was approved by the Institutional Review Boards at Johns Hopkins University and YR Gaitonde Centre for AIDS Research and Education in Chennai, India.
Liver Stiffness Measurement
Transient elastography was conducted using a FibroScan machine (Echosens, Paris, France). Measurements were recorded using a small transducer located on the end of an ultrasound probe that emits a shear wave into the liver. Liver stiffness was measured from the velocity of the wave as it went through the liver and then expressed in terms of kilopascals (kPa) [21]. Three operators, trained and certified by the manufacturer, conducted measurements in the clinic. We considered valid examinations to have (1) at least 10 discrete valid measurements, (2) greater than a 60% success rate (number of valid measurements/number of total measurements), and (3) minimum variability (IQR/median value <0.30). Per previous established cutoffs in India and the United States, an LSM ≥12.3 kPa was considered to be indicative of severe liver stiffness or cirrhosis [22, 23], and an LSM ≥8.5 kPa was considered to be indicative of significant liver stiffness (FIB) [13, 24].
Laboratory and Other Clinical Measures
Steatosis was assessed by ultrasound (Logic 400; GE, Milwaukee, WI), conducted by a radiologist, and graded according to established criteria: none, mild, moderate, severe. Human immunodeficiency virus serostatus was ascertained by double enzyme-linked immunosorbent assay (ELISA) testing (Murex HIV-1.2.O [Abbott Murex, Dartford, Kent, United Kingdom] and Vironostika HIV Uni-form II Ag/Ab [Biomerieux, Marcy L'Etoile, France]). Hepatitis C virus antibody testing was conducted using the Genedia HCV ELISA 3.0 (Green Cross Medical Science, Chungbuk, Korea). Chronic HBV infection was determined by detection of the hepatitis B surface antigen (Hepanostika HBsAg Uniform II; Biomérieux, The Netherlands). In addition, fasting plasma glucose, triglycerides, total cholesterol, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol were measured using enzymatic methods (Olympus AU400; Olymps Diagnostica, Tokyo, Japan). Plasma insulin was detected by immunoassay (ELISA) using a Bioscience kit (Monobind kit; Monobind Inc., Lake Forest, CA). Homeostasis model assessment (homeostatic model assessment insulin resistance [HOMA-IR]) was calculated as [fasting plasma glucose (mg/dL) × fasting insulin (µU/mL)/405]. We used established formulas to calculate noninvasive FIB markers: (1) FIB-4 (age [years] × aspartate aminotransferase [AST] (IU/L))/(platelet count (109 cells/L) × √(alanine aminotransferase (IU/L))) [18]; and (2) APRI (AST (IU/L)/AST (upper limit of normal))/(platelet count (109 cells/L) × 100) [19].
Statistical Analyses
We first directly evaluated the performance of FIB-4 and APRI in predicting severe (≥12.3 kPa) liver stiffness by calculating the area under the receiver operating characteristic curve (AUC) and sensitivity and specificity for validated cutoffs (FIB-4 >3.25 and APRI >1.5, respectively) [18, 19]. We then systematically considered inclusion of other markers, all of which had been considered in other validated panels [25–27]. In addition, we considered the performance of cutoffs of FIB-4 < 1.45 and APRI <0.5 in excluding significant stiffness.
Separate models were constructed for prediction of severe stiffness/cirrhosis using APRI and FIB-4. We used logistic regression with the best subset algorithm to find a specified number of models with the highest likelihood score. Base models included either APRI or FIB-4, and then other variables were sequentially added. The final multivariable models were based on diminishing returns where increasing the number of covariates in the model did not significantly increase the difference in the score statistic (similar to the likelihood ratio test) between model j and model j + 1 [28]. We performed a leave-one-out cross-validation where each observation was withheld and the remaining observations used to fit the model [29]. Performance of these models was evaluated by calculating the AUC for all multivariate logistic regression models, and cutoffs values were selected that optimized sensitivity and specificity [30].
Due to criticism of automated variable selection algorithms in logistic regression, we used random forests to confirm that we captured the most important variables that could enhance prediction of severe liver stiffness beyond FIB-4 and APRI. In brief, a random forest is a supervised, nonparametric, ensemble classification method that randomly creates decision trees by bootstrapping from a training dataset and then applying a random set of predictors at each node [31]. Trees are then aggregated to create the random forest, a process known as bagging. Variable importance for correct classification was measured by the mean decrease in classification accuracy (see Supplementary Data). Fibrosis 4 and APRI could not be computed for 3 individuals (1%); however, the rfimpute function was used to impute missing values [32]. Because random forests can detect nonlinear relationships, we used the random forest variable importance plots to ensure that we were not neglecting any important interactions when creating the models based on the logistic regression analysis.
Because our goal was to develop a parsimonious model that could be used in clinical settings in India, consideration of variables to include was based on (1) overlap between variables selected in the best subset logistic regression and variable importance from the random forest analysis and (2) feasibility and practicality of obtaining serum markers in LMICs. Sensitivity analyses included evaluating the performance of the models based on (1) exclusion of heavy drinkers (alcohol use disorders identification test [AUDIT] ≥15) because alcohol use can impact both serum markers and LSM and (2) stratification based on HIV status. A similar process was used to explore the accuracy of FIB-4 and APRI in excluding significant stiffness.
To further validate the accuracy of FIB-4, APRI, and other combinations of markers, we ascertained associations between predicted significant and severe stiffness and mortality. Person-time at risk was calculated as the time between the enrollment date and either date of death or the last date of contact for the participants. Models were adjusted for factors previously associated with mortality in this population (unpublished data) and sequentially included (1) predicted severe stiffness/cirrhosis according to FIB-4 and APRI, (2) prediction of significant stiffness (or more severe stiffness/cirrhosis) based on the final model from the best subset, (3) and a simplified score that included FIB-4, γ-glutamyl transpeptidase (GGT), and body mass index (BMI). We tested for violation of proportional hazards assumption by examining martingale residuals and conducting the Kolmogorov-type supremum test. The randomForest [33] and caret [34] packages in R (Foundation for Statistical Computing, Vienna, Austria) were used to tune parameters and perform cross-validation. SAS version 9.4 (SAS Institute Inc., Cary, NC) was used to compute descriptive statistics and perform logistic and Cox proportional hazards regression.
RESULTS
Baseline characteristics are shown in Table 1. The median age was 42 years (IQR, 37–46) and all participants were male. Over half (55%) had an AUDIT score ≥15, suggestive of alcohol dependence. Of the 30% (N = 85) who were HIV coinfected, 55% had HIV RNA levels ≥4 log10 copies/mL. Mild or moderate steatosis was detected in 140 (50%) individuals, and 74 (26%) individuals had severe liver stiffness. The median LSM was 7.9 kPa (IQR, 6.1–13.3), median FIB-4 was 1.97 (IQR, 1.16–3.37), and median APRI was 0.82 (IQR, 0.46–1.67). Concordance among FIB-4, APRI, and LSM is shown in Figure 1.
Table 1.
Baseline Characteristics of Individuals With Chronic Hepatitis C Virus Infection
| Characteristic | N% or Median (IQR) |
|---|---|
| Male | 281 (100) |
| Age | 41.6 (37.4–45.8) |
| Alcohol use (AUDIT) | |
| No/mild alcohol use | 90 (32) |
| Harmful/hazardous alcohol use | 37 (13) |
| Alcohol dependence | 154 (55) |
| Years of injection drug use (years) | 16.9 (13.0–21.4) |
| Body mass index | 19.8 (17.7–23.1) |
| HIV | |
| Negative | 196 (70) |
| Positive | 85 (30) |
| CD4 count (HIV positives only) | |
| ≥500 cells/mm3 | 16 (19) |
| 350–499 cells/mm3 | 26 (31) |
| 200–349 cells/mm3 | 29 (34) |
| <200 cells/mm3 | 14 (16) |
| HIV RNA (HIV positives only) | |
| <2.6 log10 copies/mL | 19 (23) |
| 2.6–3.9 log10 copies/mL | 18 (22) |
| ≥4.0 log10 copies/mL | 46 (55) |
| Hepatitis B Surface Antigen | |
| Negative | 268 (95) |
| Positive | 13 (5) |
| Aspartate aminotransferase (U/L) | 61 (37–101) |
| Alanine aminotransferase (U/L) | 49 (31–87) |
| γ-glutamyl transpeptidase (U/L) | 64 (32–161) |
| Alkaline phosphatase (U/L) | 87 (71–112) |
| Total platelet count (×109/L) | 186 (135–232) |
| Insulin resistance (HOMA-IR) | 1.5 (0.6–2.6) |
| High-density lipoproteins (mg/dL) | 37 (29–45) |
| Very low-density lipoproteins (mg/dL) | 16 (13–21) |
| Median log10 plasma HCV RNA (log IU/mL) | 6.4 (5.9–6.7) |
| Steatosis | |
| None | 141 (50) |
| Mild | 118 (42) |
| Moderate | 22 (8) |
| FIB-4 | |
| <1.45 | 103 (37) |
| 1.45–3.25 | 105 (37) |
| >3.25 | 73 (26) |
| APRI | |
| <0.5 | 84 (30) |
| 0.5–1.5 | 117 (42) |
| >1.5 | 80 (28) |
| LSM (kPa) | |
| <8.5 | 127 (45) |
| 8.5–12.3 | 80 (28) |
| ≥12.3 | 74 (26) |
Abbreviations: APRI, aspartate aminotransferase to platelet ratio index; AUDIT, alcohol use disorders identification test; FIB-4, fibrosis 4; HIV, human immunodeficiency virus; HCV, hepatitis C virus; HOMA-IR, homeostatic model assessment insulin resistance; IQR, interquartile range; LSM, liver stiffness measure; RNA, ribonucleic acid.
Figure 1.
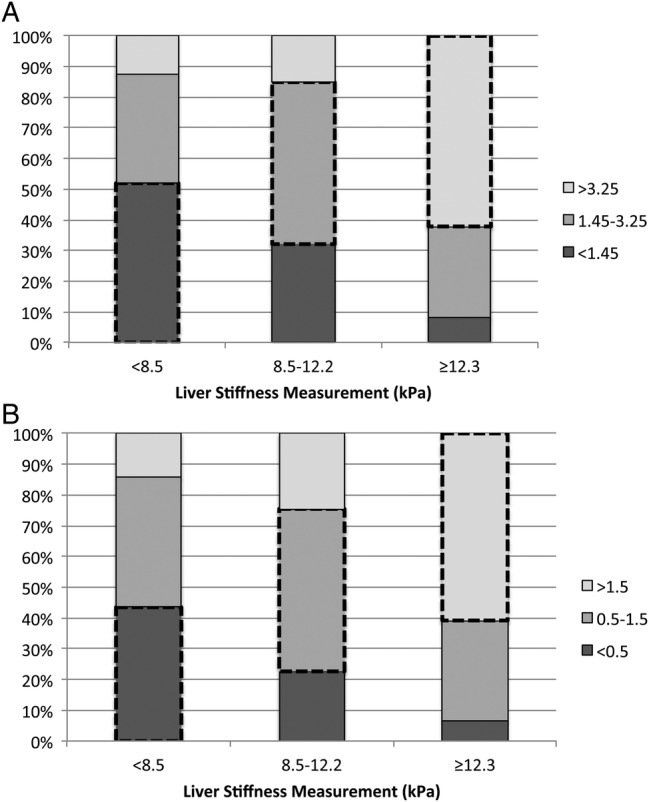
(A) Concordance between FIB-4 cutoffs and liver stiffness measurement. (B) Concordance between aspartate aminotransferase to platelet ratio index (APRI) cutoffs and liver stiffness measurement. Dashed boxes denote concordance between liver stiffness measurement and FIB-4 or APRI cutoff.
Prediction of Severe Stiffness/Cirrhosis
For prediction of severe liver stiffness, AUCs for FIB-4 and APRI were 0.80 (95% confidence interval [CI], .74–.85) and 0.77 (95% CI, .71–.82), respectively (Table 2). At their respective high cutoffs, both panels performed well in excluding those who did not have severe liver stiffness (FIB-4 specificity 87% and negative predictive value [NPV] 87%; APRI specificity 83% and NPV 86%).
Table 2.
Predictive Accuracy of FIB-4 and APRI Alone and in Combination With Other Markers for Identification of Liver Stiffness
| Serum Marker | Severe Stiffness (≥12.3 kPa) vs Mild/Moderate Stiffness (<12.3 kPa) | P Valuea | At Least Significant Stiffness (≥8.5 kPa) vs No Significant Stiffness (<8.5 kPa) | P Valuea |
|---|---|---|---|---|
| FIB-4 alone | ||||
| AUC (95% CI) | 0.80 (.74–.85) | 0.72 (.66–.78) | ||
| Cutoff | 3.25 | 1.45 | ||
| Sensitivity/Specificity (%) | 62/87 | 82/52 | ||
| PPV/NPV (%) | 63/87 | 58/78 | ||
| FIB-4, best subset model | .0012 | <.0001 | ||
| AUC (95% CI) | 0.87 (.82–.91)b | 0.83 (.78–.88)c | ||
| Cutoff probability | 0.39 | 0.51 | ||
| Sensitivity/Specificity (%) | 70/87 | 69/84 | ||
| PPV/NPV (%) | 67/89 | 78/76 | ||
| FIB-4 simplified model | .0037 | <.0001 | ||
| AUC (95% CI) | 0.83 (.78–.89)d | 0.81 (.76–.87)d | ||
| Cutoff probability | 0.25 | 0.48 | ||
| Sensitivity/Specificity (%) | 74/78 | 72/79 | ||
| PPV/NPV (%) | 55/89 | 74/77 | ||
| Modified FIB-4 score | <.0001 | <.0001 | ||
| AUC (95% CI) | 0.86 (.81–.91)e | 0.81 (.75–.86)e | ||
| Cutoff | 88 | 60 | ||
| Sensitivity/Specificity (%) | 77/80 | 80/70 | ||
| PPV/NPV (%) | 58/91 | 69/81 | ||
| APRI alone | ||||
| AUC (95% CI) | 0.77 (.71–.82) | 0.72 (.66–.77) | ||
| Cutoff | 1.5 | 0.5 | ||
| Sensitivity/Specificity (%) | 61/83 | 87/44 | ||
| PPV/NPV (%) | 56/86 | 56/80 | ||
| APRI best subset model | <.0001 | .0022 | ||
| AUC (95% CI) | 0.87 (.82–.91)f | 0.83 (.78–.88)g | ||
| Cutoff probability | 0.27 | 0.45 | ||
| Sensitivity/Specificity (%) | 74/82 | 76/79 | ||
| PPV/NPV (%) | 60/90 | 75/80 | ||
| APRI simplified model | .0002 | .0005 | ||
| AUC (95% CI) | 0.83 (.77–.88)h | 0.81 (.76–.86)h | ||
| Cutoff probability | 0.22 | 0.44 | ||
| Sensitivity/Specificity (%) | 82/70 | 77/75 | ||
| PPV/NPV (%) | 49/92 | 72/80 | ||
Abbreviations: ALP, alkaline phosphatase; APRI, aminotransferase to platelet ratio index; AUC, area under receiver operating characteristic curve; BMI, body mass index; CI, confidence interval; FIB-4, fibrosis 4; GGT, γ-glutamyl transpeptidase; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; HOMA-IR, homeostatic model assessment insulin resistance; NPV, negative predictive value; PPV, positive predictive value; VLDL, very low-density lipoprotein.
a Compared to FIB-4 or APRI cutoffs only.
b Best subset model: FIB-4, HOMA-IR, HDL, GGT.
c Best subset model FIB-4, HDL, GGT, BMI.
d Simplified model: FIB-4, GGT, BMI.
e Calculated as FIB-4 × BMI × log10(GGT), where FIB-4 is 1 = low range (<1.45), 2 = medium range (1.45–3.25), 3 = high range (>3.25).
f Best subset model: APRI, age, GGT, HDL, HOMA-IR, ALP.
g Best subset model: APRI, age, GGT, BMI, VLDL, HDL, HIV viral load.
h Simplified model: APRI, age, GGT, BMI.
In the FIB-4 base model, the best subset for prediction of severe stiffness included HOMA-IR, HDL, and GGT. These variables were also among the most important identified in the random forest analysis (Figure 2). These models had a significantly higher AUC than FIB-4 and APRI alone (P = .0012). At the optimum cutoff probability, sensitivity increased from 62% in FIB-4 alone to 70% when these covariates were added. The APRI best subset model included age, GGT, HDL, HOMA-IR, and alkaline phosphatase, which were also identified to be among the most important variables in the random forest analysis. Similar to the FIB-4 best subset model, the AUC was significantly higher than APRI alone (P < .0001) with improved sensitivity and positive predictive value, 74% and 60%, respectively.
Figure 2.
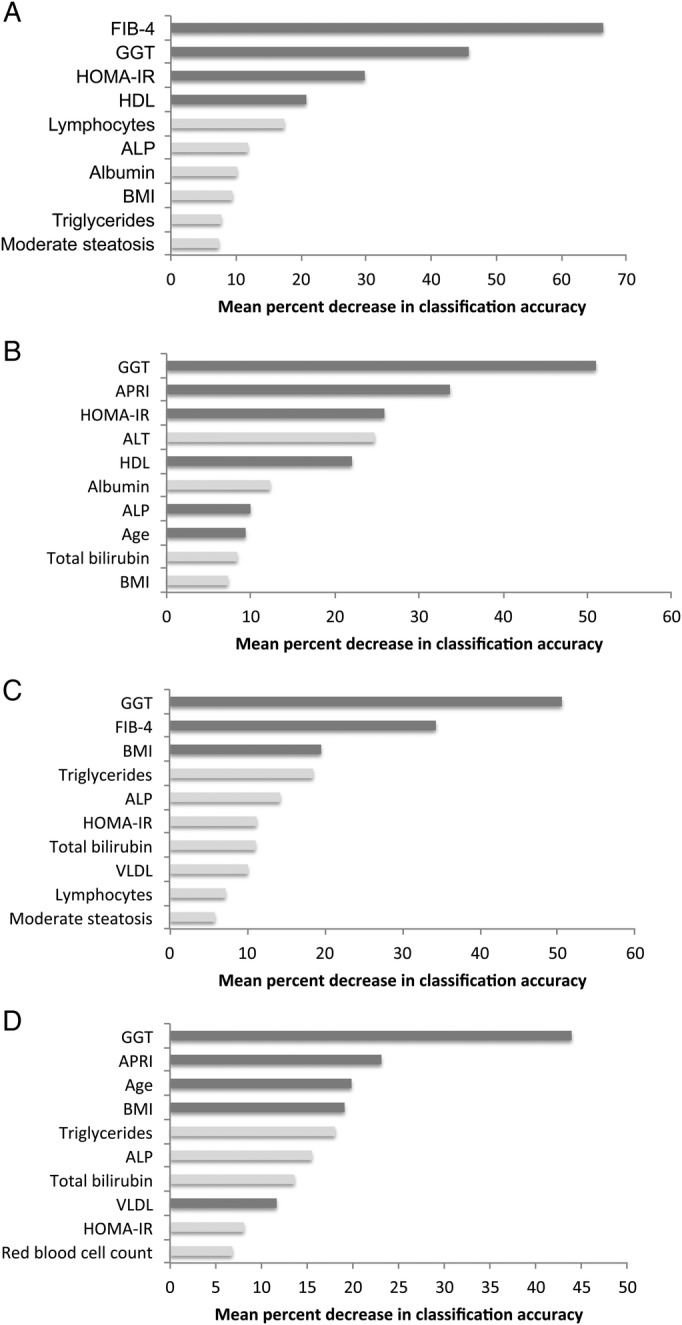
(A) Variable importance plot of FIB-4 random forest model predicting severe liver stiffness (≥12.3 kPa). (B) Variable importance plot of aminotransferase to platelet ratio index (APRI) random forest model predicting severe liver stiffness (≥12.3 kPa). (C) Variable importance plot of FIB-4 random forest model predicting at least significant liver stiffness (≥8.5 kPa). (D) Variable importance plot of APRI random forest model predicting at least significant liver stiffness (≥8.5 kPa). Only the 10 most important variables are shown. Dark gray bars indicate overlap with best subset logistic regression model.
Exclusion of Significant Stiffness
Predictive accuracy for excluding significant stiffness was lower than for severe stiffness/cirrhosis (FIB-4 AUC = 0.72, 95% CI = .66–.78; APRI AUC = 0.72, 95% CI = .66–.77). Seventy-eight percent (NPV) of those with no significant liver stiffness had a FIB-4 < 1.45. Exclusion of significant liver stiffness (or more severe) was improved with the addition of GGT, BMI, and HDL for the FIB-4 base model and GGT, BMI, age, very LDL, HDL, and HIV RNA for the APRI base model. The AUCs for the FIB-4 (0.83; 95% CI, .78–.88) and APRI (0.83; 95% CI, .78–.88) models were significantly higher than the FIB-4 (P < .0001) and APRI base models alone (P = .0005).
Simplified Model and Modified Fibrosis-4 Score Development
Simplified models included the addition of GGT, BMI, and age (for APRI only). The sensitivity for the simplified FIB-4 and APRI models was >70% for identification of severe stiffness/cirrhosis and exclusion of significant stiffness. Although the simplified APRI score performed better than APRI alone, all FIB-4 models outperformed APRI. Thus, we developed a new score incorporating FIB-4, GGT, and BMI. The modified FIB-4 score was calculated using the following equation: FIB-4 × BMI × log10(GGT), where FIB-4 was coded as 1 = low range (<1.45), 2 = medium range (1.45–3.25), and 3 = high range (>3.25). For example, someone with a FIB-4 >3.25, BMI of 23, and GGT of 75 (U/L) would have a score of 3 × 23 × log10(75) = 129.4. This score performed significantly better than FIB-4 alone in predicting severe stiffness (AUC = 0.86; 95% CI, .81–.91; P < .0001) and excluding significant stiffness (AUC = 0.81; 95% CI, .75–.86; P < .0001). In an exploratory analysis, a cutoff of 88 had a sensitivity of 77% and a specificity of 80%. At a cutoff of 60, the specificity was 70% for predicting the exclusion of significant stiffness. In addition, using this lower cutoff, 81% of those without significant stiffness would have been correctly excluded. In sensitivity analyses, performance of the models was comparable when heavy alcohol users were excluded and when participants were stratified by HIV status (Supplementary Data).
Mortality
A total of 39 deaths was observed over 603.4 years of follow-up (6.5 deaths per 100 person-years). After adjusting for BMI, alcohol use, and CD4 count, severe liver stiffness was associated with a 5.11 increased risk of death (95% CI, 2.33–11.18). Compared with those with FIB-4 < 1.45 and APRI < 0.5, those with a FIB-4 >3.25 or APRI >1.5 had significantly increased mortality (adjusted hazard ratio[aHR] = 3.45, 95% CI = 1.43–8.32 and aHR = 2.67, 95% CI = 1.15–6.21, respectively) (Table 3). In addition, those predicted to have severe stiffness at baseline based on the best subset FIB-4 (aHR, 3.20; 95% CI, 1.67–6.14) and APRI (aHR, 4.09; 95% CI, 2.10–7.97) models had significantly increased mortality compared with those not predicted to have severe stiffness. Using the modified FIB-4 score (FIB-4, GGT, and BMI), those with a score ≥88 had a 2.07 (95% CI, 1.00–4.25) increased mortality compared with those with a score <60.
Table 3.
Association Between LSM, FIB-4, APRI, Predictive Models, and Mortality
| Variable | HR (95% CI) | P Value | aHR (95% CI)a | P Value |
|---|---|---|---|---|
| LSM (kPa) | ||||
| <8.5 | Referent | Referent | ||
| 8.5–12.2 | 1.25 (.43–3.59) | .6828 | 1.78 (.60–5.16) | .3064 |
| ≥12.3 | 4.55 (2.21–9.34) | <.0001 | 5.11 (2.33–11.18) | <.0001 |
| FIB-4 | ||||
| <1.45 | Referent | Referent | ||
| 1.45–3.25 | 2.01 (.81–4.99) | .1313 | 2.71 (1.04–7.08) | .0424 |
| >3.25 | 3.90 (1.63–9.34) | .0023 | 3.45 (1.43–8.32) | .0059 |
| APRI | ||||
| <0.5 | Referent | Referent | ||
| 0.5–1.5 | 1.28 (.53–3.09) | .5812 | 1.66 (.66–4.19) | .2823 |
| >1.5 | 2.55 (1.11–5.88) | .0279 | 2.67 (1.15–6.21) | .0230 |
| FIB-4 best subset modelb | ||||
| Predicted no/mild stiffness | Referent | Referent | ||
| Predicted at least significant stiffness | 3.01 (1.55–5.87) | .0012 | 2.68 (1.36–5.29) | .0044 |
| FIB-4 best subset modelc | ||||
| Predicted no/mild/significant stiffness | Referent | Referent | ||
| Predicted severe stiffness/cirrhosis | 3.88 (2.06–7.32) | <.0001 | 3.20 (1.67–6.14) | .0005 |
| Modified FIB-4 score modeld | ||||
| <60 | Referent | Referent | ||
| 60–87 | 1.59 (.63–4.04) | .3296 | 2.03 (.78–5.43) | .1584 |
| ≥88 | 2.28 (1.11–4.67) | .0242 | 2.07 (1.00–4.25) | .0487 |
Abbreviations: aHR, adjusted hazard ratio; APRI, APRI, aminotransferase to platelet ratio index; BMI, body mass index; CI, confidence interval; FIB-4, fibrosis 4; GGT, γ-glutamyl transpeptidase; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment insulin resistance; HR, hazard ratio; LSM, liver stiffness measure.
a The aHR in each row represents a distinct model that adjusted for alcohol use, BMI, and CD4 count.
b Predicted to have at least significant liver stiffness at baseline (≥8.5 kPa) based on optimal cutoff using model with FIB-4, GGT, BMI, and HDL.
c Predicted to have at least severe liver stiffness at baseline (≥12.3 kPa) based on optimal cutoff using model with FIB-4, GGT, HOMA-IR, and HDL.
d Score calculated as FIB-4 × BMI × log10(GGT), where FIB-4 is 1 = low range (<1.45), 2 = medium range (1.45–3.25), 3 = high range (>3.25).
DISCUSSION
New developments in HCV treatment will dramatically change management of chronic HCV; however, access remains a challenge, particularly in LMICs. Although treatment costs are lower in many LMICs including India where generic versions of DAAs such as sofosbuvir, ledipasvir, and daclatasvir are already available [35], most governments are not subsidizing HCV treatment. Thus, a cure will remain out of reach for most in need. Although the goal is to treat everyone, resources are limited and some form of triage will be needed to maximize resources. Moreover, diagnosis of cirrhosis will continue to be important because these patients need to be monitored for development of hepatocellular carcinoma even after cured [36] and may require different regimens than those without cirrhosis [4]. Elastography, which is primarily used in the United States and Europe, has also been used in research settings in LMICs; however, access to elastography may not be realistic globally given the cost of the machine. FibroTest/Fibrosure is also used, but it is expensive and includes some markers that are unavailable in routine laboratories [11]. Panels using simple, easy obtainable laboratory markers such as FIB-4 and APRI may hold the most promise in LMICs.
Although these panels have been validated extensively [18, 19, 37, 38], nearly all studies derive from high-income settings. Our data provide some of the first evidence that these panels have high diagnostic accuracy for the diagnosis of liver disease among persons chronically infected with HCV in LMICs where there is significant diversity in underlying conditions that could impact accuracy (eg, diabetes, obesity, steatosis, tuberculosis treatment, other infections). Overall, FIB-4 had higher diagnostic accuracy than APRI, which is consistent with a recent meta-analysis that found a statistically significant difference in AUCs between FIB-4 vs APRI (AUC = 0.04; 95% CI, .02–.05) for predicting cirrhosis in persons infected with HCV [39]. Performance of FIB-4 in predicting severe liver stiffness/cirrhosis (AUC = 0.80) was within the range (95% CI, .76–.90) of other studies [18, 37]. Moreover, consistent with other studies [38, 40], FIB-4 and APRI levels suggestive of severe stiffness/cirrhosis were associated with significantly increased mortality, providing additional assurance that FIB-4 and APRI are useful in detecting severe liver disease in LMICs.
One of the concerns with using these marker panels and cutoffs in LMICs is that behavioral or biological differences unrelated to liver disease could impact performance. Indeed, our modeling analyses suggested that the performance of FIB-4 and APRI could be enhanced with other serum biomarkers. The additional markers that were identified through both methods are consistent with what has been included in other validated marker panels (eg, GGT [41]) as well as what is known about the phenotype of liver disease in India (eg, insulin resistance). For example, Indians have a genetic predisposition to insulin resistance, which has been previously demonstrated to be associated with severe FIB [13, 42]. The median BMI of our sample was 19.8 compared with approximately 24 [23, 43] in comparable populations in the United States and Western Europe. Thus, it is not surprising that the inclusion of BMI significantly improved predictive accuracy.
Although the optimal panels for excluding significant liver stiffness and predicting severe stiffness/cirrhosis in this population had high diagnostic accuracy, they also included several laboratory markers that may be difficult to obtain in routine clinical practice—either because of cost, laboratory capacity, or the requirement of fasting. Thus, it was encouraging that our modified FIB-4 score, which included only FIB-4, BMI, and GGT, also performed with high accuracy (AUC = 0.86) and could serve as a useful tool to enhance identification of persons with severe liver disease at no additional cost. γ-Glutamyl transpeptidase has been used previously in other noninvasive algorithms [41], and BMI has been previously identified as an independent predictor of chronic liver disease [38].
It is noteworthy to mention that the association between the modified FIB-4 score and mortality was not as strong as FIB-4 or the best subset model. This is most likely because those with low BMI (<18.5) were at an increased risk of mortality; however, when calculating the modified FIB-4 score, higher BMI is associated with a higher score. The increased risk of death due to being underweight reinforces the notion that PWID in India chronically infected with HCV may have different competing mortality risks that are distinct from North American or European risks.
Our study has several limitations. First, we did not have data on liver biopsy, which has long been considered the gold standard for diagnosis of FIB and cirrhosis. However, it is important to note that liver stiffness determination is replacing the biopsy in several high-income settings due to its noninvasive nature and high accuracy. In addition, misclassification of disease stage by liver stiffness would only reduce the apparent accuracy of the blood tests, which may well have been even more accurate than reported. Some of the factors that we identified as important for predicting liver disease in this population (eg, high BMI, alcohol use) have also been identified as factors that affect the performance of elastography [44], and their association could reflect an impact on performance rather than identification of disease. However, in our sample, over 96% of patients had a BMI < 30 (the cutoff recommended for the standard probe of FibroScan), and results were unchanged when excluding those with heavy alcohol use. In addition, HIV has shown to be associated with liver disease progression; however, in our sample, no substantial differences in performance of the models between HIV-negative and HIV-positive individuals were observed.
Although we had several levels of internal validation, we did not have an external validation set from another LMIC to test performance of our new models, such as the modified FIB-4. In addition, it is possible that the FIB-4 and APRI cutoffs used in this analysis may not be the optimum cutoffs given genetic and sociodemographic differences between populations in high-income and low- or middle-income countries. We also did not have a reliable cause of death because these data were collected by verbal autopsy from family and friends. Despite this, only 1 death (homicide) was due to a nonchronic disease-related cause. Finally, it was encouraging that there was significant overlap in the variables chosen from 2 different methods. However, additional validation among populations with different sociodemographics and/or comorbidities is needed.
CONCLUSIONS
In sum, both FIB-4 and APRI had high diagnostic accuracy for the diagnosis of significant and severe stiffness/cirrhosis in this community-based sample of PWID chronically infected with HCV living in Chennai, India. Additional biomarkers, such as BMI and GGT, should also be considered because they may enhance prediction of liver stiffness as shown by the performance of the modified FIB-4.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank the study staff and the participants in the study.
Author contributions. J. A. C. and S. H. M. conceived of and designed the analyses. S. S. S., P. N., P. B., M. S. K., D. L. T., and M. S. S. provided feedback and assisted with revising the manuscript. A. K. S. collected the data. J. A. C. and S. H. M. wrote the manuscript.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was funded by the National Institute on Drug Abuse (grant numbers R01DA026727 [to S. H. M.], DP2DA040244 [to S. S. S.], and R37DA013806 [to D. L. T.]); and the National Institute of Allergy and Infectious Diseases (grant number P30AI094189 [to the Johns Hopkins Center for AIDS Research] and institutional training grant T32AI102623).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013; 57:1333–42. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Hepatitis C. Fact sheet. Updated July 2014. Available at: http://www.who.int/mediacentre/factsheets/fs164/en/. Accessed 1 February 2016.
- 3.Messina JP, Humphreys I, Flaxman A et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015; 61:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curry MP, O'Leary JG, Bzowej N et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med 2015; 373:2618–28. [DOI] [PubMed] [Google Scholar]
- 5.Jayasekera CR, Barry M, Roberts LR, Nguyen MH. Treating hepatitis C in lower-income countries. N Engl J Med 2014; 370:1869–71. [DOI] [PubMed] [Google Scholar]
- 6.Ghany MG, Strader DB, Thomas DL et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009; 49:1335–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedossa P. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994; 20:15–20. [PubMed] [Google Scholar]
- 8.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003; 38:1449–57. [DOI] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2011; 55:245–64. [DOI] [PubMed] [Google Scholar]
- 10.Foucher J, Chanteloup E, Vergniol J et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006; 55:403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 2012; 142:1293–302. [DOI] [PubMed] [Google Scholar]
- 12.Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med 2013; 158:807–20. [DOI] [PubMed] [Google Scholar]
- 13.Solomon SS, Srikrishnan AK, McFall AM et al. Burden of liver disease among community-based people who inject drugs (PWID) in Chennai, India. PLoS One 2016; 11:e0147879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United Nations Office on Drugs and Crime. 2008 World Drug Report. Available at: https://www.unodc.org/documents/wdr/WDR_2008/WDR_2008_eng_web.pdf. Accessed 21 March 2016.
- 15.Marx MA, Murugavel KG, Sivaram S et al. The association of health-care use and hepatitis C virus infection in a random sample of urban slum community residents in southern India. Am J Trop Med Hyg 2003; 68:258–62. [PubMed] [Google Scholar]
- 16.Aceijas C, Rhodes T. Global estimates of prevalence of HCV infection among injecting drug users. Int J Drug Policy 2007; 18:352–8. [DOI] [PubMed] [Google Scholar]
- 17.Solomon SS, Mehta SH, Srikrishnan AK et al. Burden of hepatitis C virus disease and access to hepatitis C virus services in people who inject drugs in India: a cross-sectional study. Lancet Infect Dis 2015; 15:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterling RK, Lissen E, Clumeck N et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–25. [DOI] [PubMed] [Google Scholar]
- 19.Wai CT, Greenson JK, Fontana RJ et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–26. [DOI] [PubMed] [Google Scholar]
- 20.Solomon SS, Srikrishnan AK, Mehta SH et al. High prevalence of HIV, HIV/hepatitis C virus coinfection, and risk behaviors among injection drug users in Chennai, India: a cause for concern. J Acquir Immune Defic Syndr 2008; 49:327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afdhal NH. Fibroscan (transient elastography) for the measurement of liver fibrosis. Gastroenterol Hepatol (N Y) 2012; 8:605. [PMC free article] [PubMed] [Google Scholar]
- 22.Kirk GD, Astemborski J, Mehta SH et al. Assessment of liver fibrosis by transient elastography in persons with hepatitis C virus infection or HIV-hepatitis C virus coinfection. Clin Infect Dis 2009; 48:963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta SH, Kirk GD, Astemborski J et al. Stability of liver fibrosis among HCV-infected injection drug users. Antivir Ther 2012; 17:813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das K, Sarkar R, Ahmed SM et al. “Normal” liver stiffness measure (LSM) values are higher in both lean and obese individuals: a population-based study from a developing country. Hepatology 2012; 55:584–93. [DOI] [PubMed] [Google Scholar]
- 25.Koda M, Matunaga Y, Kawakami M et al. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology 2007; 45:297–306. [DOI] [PubMed] [Google Scholar]
- 26.Sud A, Hui JM, Farrell GC et al. Improved prediction of fibrosis in chronic hepatitis C using measures of insulin resistance in a probability index. Hepatology 2004; 39:1239–47. [DOI] [PubMed] [Google Scholar]
- 27.Adams LA, Bulsara M, Rossi E et al. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem 2005; 51:1867–73. [DOI] [PubMed] [Google Scholar]
- 28.Miao Y, Cenzer IS, Kirby KA, Boscardin WJ, eds. Estimating Harrell's optimism on predictive indices using bootstrap samples. In: Proceedings of the SAS Global Forum, 2013, San Francisco. Paper 504-2013. Available at: http://support.sas.com/resources/papers/proceedings13/504-2013.pdf. Accessed 10 February 2016. [Google Scholar]
- 29.Kohavi R. A study of cross-validation and bootstrap for accuracy estimation and model selection. IJCAI 1995; 14:1137–45. [Google Scholar]
- 30.Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3:32–5. [DOI] [PubMed] [Google Scholar]
- 31.Breiman L. Random forests. Springer In: Machine Learning 2001; pp 45:5–32. [Google Scholar]
- 32.Breiman L. Manual on setting up, using, and understanding random forests V3. 1. Berkeley, CA: Statistics Department University of California, 2002. Available at: https://www.stat.berkeley.edu/~breiman/Using_random_forests_v4.0.pdf. Accessed 19 January 2016. [Google Scholar]
- 33.Liaw A, Wiener M. Classification and regression by randomForest. R News 2002; 2:18–22. Available at: http://www.bios.unc.edu/~dzeng/BIOS740/randomforest.pdf. Accessed 22 January 2016. [Google Scholar]
- 34.Kuhn M. Building predictive models in R using the caret package. J Stat Softw 2008; 28:1–26. [Google Scholar]
- 35.Cadila Healthcare. LediHep 2015. Available at: http://ledihep.com/patients.html Accessed 16 March 2016.
- 36.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virologic response in Veterans with HCV-infection. Hepatology 2016; 64:130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zarski JP, Sturm N, Guechot J et al. Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C: the ANRS HCEP-23 study. J Hepatol 2012; 56:55–62. [DOI] [PubMed] [Google Scholar]
- 38.Vergniol J, Foucher J, Terrebonne E et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology 2011; 140:1970–9. [DOI] [PubMed] [Google Scholar]
- 39.Houot M, Ngo Y, Munteanu M et al. Systematic review with meta-analysis: direct comparisons of biomarkers for the diagnosis of fibrosis in chronic hepatitis C and B. Aliment Pharmacol Ther 2016; 43:16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nunes D, Fleming C, Offner G et al. Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am J Gastroenterol 2010; 105:1346–53. [DOI] [PubMed] [Google Scholar]
- 41.Imbert-Bismut F, Ratziu V, Pieroni L et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001; 357:1069–75. [DOI] [PubMed] [Google Scholar]
- 42.Petersen KF, Dufour S, Feng J et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci U S A 2006; 103:18273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castera L, Le Bail B, Roudot-Thoraval F et al. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol 2009; 50:59–68. [DOI] [PubMed] [Google Scholar]
- 44.Foucher J, Castera L, Bernard PH et al. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol 2006; 18:411–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


