Abstract
Nigrosphaerin A, a new isochromene derivative (1), was isolated from the endophytic fungus Nigrospora sphaerica and chemically identified as 3-(3,4-dihydroxyphenyl)-4,6,8-trihydroxy-1H-isochromen-1-one-6-O-β-d-glucopyranoside. In addition nineteen known compounds (2–20) were isolated from the same fungus and chemically identified. Compounds (1–3, 5, and 7–16) were isolated for the first time from this fungus. In vitro antileukemic, antileishmanial, antifungal, antibacterial and antimalarial activities of (1–20) were examined. Compounds 5, 7, 9 and 10 showed good antileukemic activity against HL60 cells with IC50 values of 0.03, 0.39, 0.2 and 0.4 μg/mL, respectively and against K562 cells with IC50 values of 0.35, 0.35, 0.49 and 0.01 μg/mL, respectively. Compounds 3, 4 and 6 showed moderate antileishmanial activity with IC50 values of 30.2, 26.4 and 36.4 μg/ml, respectively. Compound 7 showed moderate antifungal activity against Cryptococcus neoformans with IC50 value of 14.8 μg/mL.
Keywords: Nigrospora sphaerica, Nigrosphaerin A, Isochromene, Antileukemic, Antileishmanial
1. Introduction
Endophytic fungi are prospective producers of an abundant source of bioactive chemically novel compounds with potential for exploitation in a wide variety of medical areas (Tenguria et al., 2011). Fungi belonging to the genus Nigrospora have been a rich source of bioactive secondary metabolites, such as nigrosporolides which found to have plant growth-inhibiting activity (Kim et al., 2001), phomalactones with good anti plant pathogenic fungi effect (Kim et al., 2001), phytotoxic antibacterial nigrosporins (Tanaka et al., 1997), phytotoxic lactones (Fukushima et al., 1998), epoxydons and pyrones (Trisuwan et al., 2008).
The fungus Nigrospora sphaerica has been reported as an endophyte in several plants and marine organisms (Zhang et al., 2009). N. sphaerica has been found to be a source of biologically active secondary metabolites, including diterpenes (Turner and Aldridge, 1983), diketopiperazines (Cutler et al., 1991), lactones (Kim et al., 2001) and nigrosporolides (Zhang et al., 2009).
Chemical and biological investigation for the endophytic fungus N. sphaerica (Fig. 1), led to the isolation of Nigrosphaerin A, a new isochromene derivative (1), along with nineteen known compounds (2–20). The antileukemic, antileishmanial, antifungal and antibacterial activities of the isolated compounds were studied.
Fig. 1.
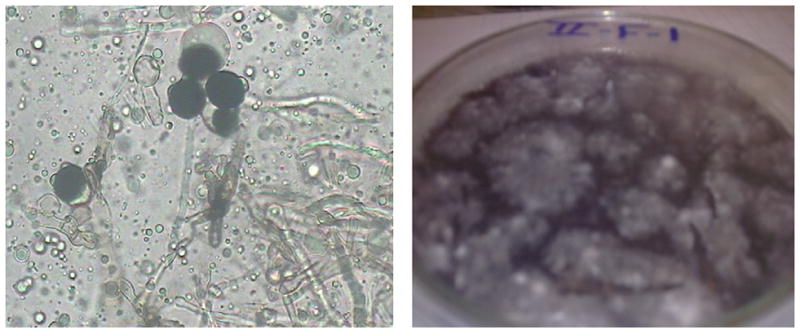
Nigrospora sphaerica.
2. Results and discussion
Compound 1 (Fig. 2A) was isolated as a greenish yellow amorphous powder. The molecular formula C21H20O12 was determined by HR-ESI-MS (+ve mode) showing molecular ion peak [M+H]+ at m/z 465.0990 (calcd. for C21H21O12, 465.1033) indicating twelve degrees of unsaturation. The IR spectrum showed that 1 contained hydroxyl (νmax 3405 cm−1), and carbonyl (νmax 1648 cm−1) functional groups. The UV spectrum showed absorption bands at λmax 255, 290 (sh) and 365.0 nm.
Fig. 2.
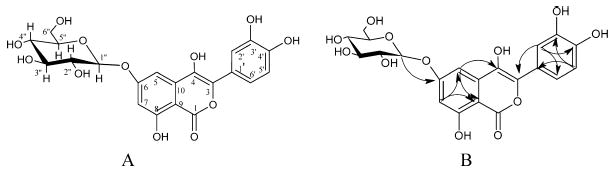
Compound 1 (A) and key HMBC (H → C) of 1 (B).
The 1H NMR spectroscopic data of 1 displayed signals at δH 7.59 (1H, d, J = 2.0 Hz, H-2′), δH 7.45 (1H, dd, J = 8.4, 2.0 Hz, H-6′) and δ 6.86H (1H, d, J = 8.4, H-5′) indicating a 1,3,4-trisubstituted benzene ring. These protons were found to be correlated to δC 114.8, δC 119.7 and δC 115.8 in the HMQC spectrum, respectively. Two meta coupled aromatic protons resonating at δH 6.64(1H, d, J = 1.6 Hz, H-5) and δH 6.75(1H, d, J = 1.6 Hz, H-7) were correlated to δC 97.5 and to δC 103.4 in the HMQC spectrum, respectively. The hydroxyl proton (C-8) found to be resonating at δH 13.48 due to intermolecular hydrogen bonding with the carbonyl group (C-1). The 13C NMR, DEPT and HMQC spectroscopic data of 1 displayed 21 signals, including one methylene, ten methine and ten quaternary carbons. The carbon resonating at δC 171.8 ppm is characteristic for carbonyl group (C-1). The 13C, HMQC and HMBC NMR data further established four phenolic hydroxyl groups at C-4 (δC 157.4), C-8 (δC 163.3), C-3′ (δC 145.3) and C-4′ (δC 147.5). Six carbons for sugar were found to be resonating at δC 103.9 (C-1″), δC 73.8 (C-2″), δC 77.6 (C-3″), δC 69.8 (C-4″), δC 75.8 (C-5″) and δC 60.8 (C-6″). Anomeric proton at δH 4.77 found to be correlated to C-1″ at δC 103.9 in the HMQC spectrum and to δC 158.5 in the HMBC spectrum, indicating that the sugar is attached to C-6. The large coupling constant of the anomeric proton J = 7.2 Hz indicated the β-configuration of the sugar (Avilov et al., 2003; Gao et al., 2008). The following correlations have been found in the HMBC spectrum (Fig. 2B): H-7 (δH 6.75) to C-5 (δC 97.5) and C-9 (δC 106.1), from H-5 (δH 6.64) to C-4 (δC 157.4) and C-9 (δC 106.1), from H-2′ (δH 7.59) to C-3 (δC 143.6), C-4′ (δC 147.5) and C-6′ (δC 119.7), from H-5′ (δH 6.86) to C-1′ (δC 122.2) and C-3′ (δC 145.3) and from H-6′ (δH 7.45) to C-2′ (δC 114.8) and C-4′ (δC 147.5). The hydrolysis of 1 with 0.5 N HCl yielded 3-(3,4-dihydroxyphenyl)-4,6,8-trihydroxy-1H-isochromen-1-one and glucose which was identified by co-chromatography with standard sugars using TLC. Based on the above evidences, the structure of 1 was established as 3-(3,4-dihydroxyphenyl)-4,6,8-trihydroxy-1H-isochromen-1-one-6-O-β-d-glucopyranoside.
Fifteen compounds (2–16, Fig. 3) have been isolated and were chemically identified using 1D NMR (1H, 13C, DEPT135) and 2D NMR (COSY, HMQC, HMBC) as well as HR-ESI-MS and found to be ergosta-6,22-diene-3β,5α,8α-triol (2) (Cateni et al., 2007), ergosta-7,22-diene-3β-ol (3) (Gong et al., 2010), ergosta-4,6,8(14),22-tetraene-3β-ol (4) (Pang and Sterner, 1993), ergosta-4,6,8(14),22-tetraene-3one (5) (Lee et al., 2005), ergosta-5(6),7,22-triene-3β-ol (6) (Li et al., 2007), ergosta-7,9(14),22-triene-3β-ol (7) (Li et al., 2008a), ergosta-7,22-epidioxy-3β-ol (8) (Cateni et al., 2007), 4-(hydroxymethyl)-3,5-dimethyl dihydrofuran-2(3H)-one (9) (Rukachaisirikul et al., 2009), 3-(1-hydroxyethyl)-4-methyl dihydrofuran-2(3H)-one (10) (Choi et al., 2008), uridine (11) (Mantsch and Smith, 1973), adenosine (12) (Liu et al., 2011), 3-benzylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione (13) (Fdhila et al., 2003), 3-methylhexahydro pyrrolo[1,2-a]pyrazine-1,4-dione (14) (Hendea et al., 2006) and methyl 4-hydroxybenzoate (15) (Li et al., 2008b), 4-(2-hydroxyethyl)phenol (16) (Li et al., 2012). Four fatty acids (17–20) have been also isolated and chemically identified as stearic acid (17), oleic acid (18), palmitic acid (19), and myristic acid (20) using 1H NMR and GC/MS after methylation.
Fig. 3.
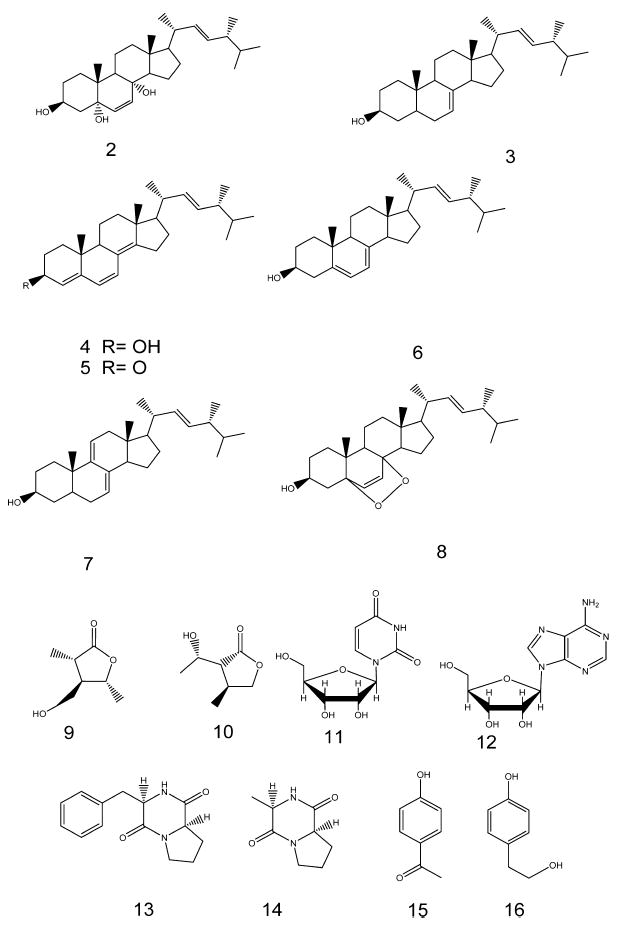
Compounds 2–16.
Compounds 5, 7, 9 and 10 showed good antileukemic activity (Table 1 and Fig. 4) against acute HL60 cells with IC50 values of 0.03, 0.39, 0.2 and 0.4 μM/mL, respectively and against chronic K562 cells with IC50 values of 0.35, 0.35, 0.49 and 0.01 μM/mL, respectively. Standard taxol showed IC50 values of 0.0005 μM/mL and 0.0023 μM/mL, respectively. Compounds 3, 4 and 6 showed moderate antileishmanial activity with IC50 values of 30.2, 26.4 and 36.4 μg/mL, respectively. (Value of IC50 for standard pentamidine was found to be 1.01 μg/mL). Compound 7 showed moderate antifungal activity against Cryptococcus neoformans with IC50 value of 14.81 μg/mL. It was found to be 0.28 μg/mL for standard amphotericin B.
Table 1.
Inhibitory effects of compounds 5, 7, 9 and 10 on the growth of human leukemia cells in vitro (48 h drug exposure for HL60 and K562 cells). Experiments were carried out in triplicate.
| Compounds | Estimated IC50 (μM)
|
|
|---|---|---|
| HL60 cells | K562 cells | |
| Compound 5 | 0.03±30.05 | 0.35±30.002 |
| Compound 7 | 0.39±30.03 | 0.35±30.002 |
| Compound 9 | 0.2±30.001 | 0.49±30.0012 |
| Compound 10 | 0.4±30.001 | 0.01±30.003 |
| Taxol | 0.0005±30.00008 | 0.0023±30.0005 |
Fig. 4.
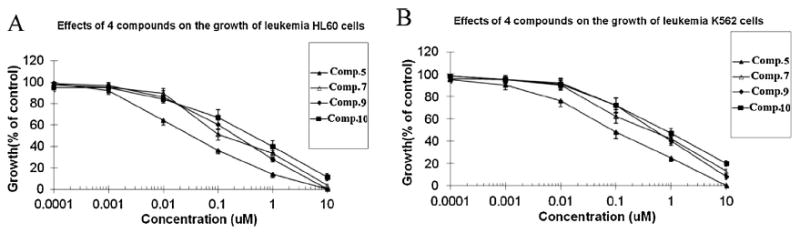
The concentration-dependent effects of compounds 5, 7, 9 and 10 on the growths of acute leukemia HL60 (A) and chronic leukemia K562 (B) cells. Cells were treated with compounds for 48 h and then cell numbers were determined by the trypan blue exclusion test. Results shown are mean ± SD (bars) of triplicate experiments.
3. Experimental
3.1. General
NMR spectra were recorded on a Bruker Avance DRX-500 instrument at 500 (1H) and 125 MHz (13C), and a Varian Mercury 400 MHz spectrometer at 400 (1H) and 100 MHz (13C). The HR-ESI-MS spectra were measured using a Bruker Bioapex-FTMS with electrospray ionization (ESI). The GC–MS was interfaced to a HP 5973 quadrupole mass selective detector. The injector temperature was 250 °C, and 1 μL injections were performed in the split (1:10) mode using helium as carrier gas. Column chromatographic separation was performed on silica gel 60 (0.04–0.063 mm) and sephadex LH-20 (0.25–0.1 mm, Merck). TLC was performed on precoated TLC plates with silica gel 60 F254 (0.2 mm, Merck). Semi preparative HPLC (Waters Delta Prep 4000) was performed using Luna® RP-18 (250,10 mm × 5 μm; flow rate 5 μL/min).
3.2. Fungal material
The fungus N. sphaerica was isolated from surface sterilized fresh leaves of an apparently healthy vinca rosea (Apocynaceae) collected in March 2010 in Cairo, Egypt. The leaves were rinsed with water and followed by surface sterilization in 70% EtOH for 1 min, rinsed with sterilized water, then cut into small pieces (2 cm in length and width) and deposited in on a petri dish containing PDA medium (200 g potato, 20 g glucose, and 15 g agar in 1 L distilled water, supplemented with 100 mg/L chloramphenicol) and cultivated at 28 °C for 3 days. The hypha tips were observed and transferred to new PDA plates and subcultured until pure culture was obtained. The fungus was identified by the regional center for mycology and biotechnology, Cairo, Egypt. Identification was based on The Data Base Identification Program of the Regional Center for Mycology and Biotechnology (RCMB) for fungi, using an Image Analysis System and on current universal keys (Fisher and Cook, 1998; Hoog et al., 2000). After purification the fungus was grown on PDA at 28 °C for 5 days. Ten pieces (0.5 × 0.5 cm2) of mycelial agar plugs were inoculated into ten 1000 mL Erlenmeyer flasks containing sterilized (100 g Asian rice and 100 ml distilled water) at room temperature for 40 days.
3.3. Extraction and Isolation
The fungus was extracted by adding 2 L EtOAc to each flask and homogenized. The homogenized suspensions were collected, filtrated, concentrated under vacuum and partitioned with distilled water. Ethyl acetate portion was evaporated to dryness and fractionated using hexane and 90% MeOH to afford hexane fraction (7.6 g) and MeOH fraction (8.0 g). Water portion was fractionated against n-butanol to afford water fraction (36 g) and butanol fraction (7 g). N. sphaerica MeOH fraction (8 g) was subjected to Si gel VLC eluted with hexane, EtOAc and finally MeOH. Six fractions were collected (500 mL each). Fractions 2–3 (410.9 mg) were chromatographed on sephadex LH-20 using eluent MeOH:CHCl3 (5:5) to yield nine subfractions. Subfraction 2 was chromatographed with Si-SPE column eluted with CHCl3, MeOH in a matter of increasing polarity to afford compound 2 (3 mg), compound 4 (5 mg) and compound 5 (4 mg). Subfraction 3 was chromatographed on Si-SPE column eluted with CHCl3, MeOH to give compounds 6 (12.6 mg) and 8 (1.3 mg). Subfraction 4 was chromatographed on sephadex LH-20 using eluent MeOH:CHCl3 (5:5) to afford compound 7 (2.4 mg) and compound 3 (3.9 mg). Subfractions 5–6 was chromatographed on Si gel eluted with CHCl3, MeOH in a matter of increasing polarity to afford compounds 9 (5.8 mg) and compound 10 (2.7 mg).
The butanol fraction (7.0 mg) was subjected to Si gel VLC gradient eluted with CHCl3/MeOH and finally MeOH. Eight fractions were collected (500 mL each). Fraction 2 (17.8 mg) was chromatographed on sephadex LH-20 eluting with MeOH/H2O, followed by purification with semi-preparative HPLC eluted with 75% MeOH/H2O to give compound 1 (6.3 mg). Fractions 3–4 (702.9 mg) were chromatographed on sephadex LH-20 eluting with MeOH to yield 5 subfractions. Subfraction 2 was chromatographed on RP-Si-SPE (C18) column using eluent H2O:MeOH (6:4) to give compounds 11 (6.7 mg) and 15 (7.3 mg). Subfraction 3 was chromatographed on sephadex LH-20 eluting with MeOH to give compound 12 (15.9 mg). Subfraction 4 was chromatographed with semi-preparative HPLC with linear gradient elution 20–85% aqueous methanol to obtain compounds 13 (7.8 mg), 14 (4.3 mg) and 16 (1.4 mg).
3.4. 3-(3,4-Dihydroxyphenyl)-4,6,8-trihydroxy-1H-isochromen-1-one-6-O-β-d-glucopyranoside (1)
Greenish yellow amorphous powder; UV (MeOH): λmax (log ɛ) nm: 255 (3.93), 290(sh) (3.71) and 365.0 (3.92); IR νmax: 3405, 1648 and 1024 cm−1; HR-ESI-MS m/z 465.0990 [M+H]+ (calcd. for C21H21O12, 465.1033). 1H NMR (DMSO, δ, 400 MHz, ppm): 6.64 (1H, d, J = 1.6 Hz, H-5), 6.75 (1H, d, J = 1.6 Hz, H-7), 7.59 (1H, d, J = 2.0 Hz, H-2′), 7.45 (1H, dd, J = 8.4, 2.0 Hz, H-6′), 6.86 (1H, d, J = 8.4, H-5′), 4.77 (1H, d, J = 7.2 Hz, H-1″), 3.33 (1H, H-2″), 3.30 (1H, H-3″), 3.18 (1H, H-4″), 3.27 (1H, H-5″), 3.65 and 3.70 (2H, H-6″); 13C NMR (DMSO, δ, 125 MHz, ppm): 171.9 (C-1), δ143.6 (C-3), 157.4 (C-4), 97.5 (C-5), 158.5 (C-6), 103.4 (C-7), 163.3 (C-8), 106.1 (C-9), 137.4 (C-10), 122.2 (C-1′), 114.8 (C-2′), 145.3 (C-3′), 147.5 (C-4′), 115.8 (C-5′), 119.7 (C-6′), 103.9 (C-1″), 73.8 (C-2″), 77.6 (C-3″), 69.8 (C-4″), 75.8 (C-5″), 60.8 (C-6″).
3.5. Acid hydrolysis of compound 1
2 mg of compound 1 was refluxed with HCl (0.5 N, 2 mL) for 2 h. The hydrolyzed product was extracted with CH2Cl2/H2O. The sugar was extracted from the aqueous layer using pyridine (1 mL). The sugar was identified as d-glucose by co-chromatography with authentic samples of different sugars using silica gel TLC using solvent system EtOAc/MeOH/HOAc/H2O (11:2:2:2) followed by spraying with anisaldehyde/H2SO4 and heating at 100 °C.
3.6. Antileukemic assay
Human acute leukemia HL60 cells and human chronic leukemia 562 cells were purchased from American Type Culture Collection, Rockville MD, USA. Both cell lines were grown in suspension culture at 37 °C in RPMI-1640 medium supplemented with 10% non-dialysed fetal bovine serum (FBS), 2 mM l-glutamine, 100 units/mL of penicillin and 10 μg/mL of streptomycin. For the cell growth inhibition assay, HL60 and K562 cells were set up at 1 × 105 cells/well in Costar 24-well plates. Cells were allowed to grow undisturbed for 24 h before addition of “compounds”. After 48 h incubation with drugs at 37 °C, viable cell counts were made by using the trypan blue exclusion method to assess cell viability (Roper and Drewinko, 1976).
3.7. Antimicrobial assay
Crude extracts and isolated compounds were tested for antimicrobial activity against Candida albicans ATCC 90028, Candida glabrata ATCC90030, Candida krusei ATCC 6258, Asperigillus fumigates ATCC 90906, Methicillin-resistant Staphylococcus aureus ATCC 33591, Cryptococcus neoformans ATCC 90113, Staphylococcus aureus ATCC 2921, Escherichia coli ATCC 35218, Pseudomonus aeruginosa ATCC 27853, Mycobacterium intracellulare ATCC 23068, Ciprofloxacin and Amphotericin B were used as positive standards (Bharate et al., 2007; Radwan et al., 2009).
3.8. Antimalarial assay
Crude extracts were tested on chloroquine sensitive (D6, Sierraleon) and resistant (W2, Indo-china) strains of Plasmodium falciparum using previously reported method; Artemisinin and Chloroquine were used as positive standards (Bharate et al., 2007).
3.9. Antileishmanial assay
The antileishmanial activity of the isolated metabolites was tested in vitro against a culture of L. donovani promastigotes; Pentamidine and Amphoterecin B were used as positive standards (Abdel-Mageed et al., 2012).
Supplementary Material
Acknowledgments
We are grateful to the Egyptian Government and National Center for Natural Products Research, School of Pharmacy, University of Mississippi, for financial support. We are also thankful for Dr. Baharthi Avula for HRMS, Drs. Melissa Jacob, Babu Tekwani and Shabana Khan for antimicrobial, antileishmanial and antimalarial assays. This investigation was conducted in part in a facility constructed with support from the research facilities improvement program C06 RR-14503-01 from the NIH NCRR. This work is supported in part by United States Department of Agriculture ARS Specific Cooperative Agreement No. 58-6408-2-0009.
Appendix A. Supplementary data
(1H NMR, 13C NMR, DEPT 135, HMQC, HMBC, HR-ESI-MS, UV and IR) spectral data of compound 1.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.phytol.2013.09.001.
References
- Abdel-Mageed WM, Backheet EY, Khalifa AA, Ibraheim ZZ, Ross SA. Antiparasitic antioxidant phenylpropanoids and iridoid glycosides from Tecoma mollis. Fitoterapia. 2012;83:500–507. doi: 10.1016/j.fitote.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Avilov SA, Antonov AS, Silchenko AS, Kalinin VI, Kalinovsky AI, Dmitrenok PS, Stonik VA, Riguera R, Jimenez C. Triterpene glycosides from the Far Eastern sea cucumber Cucumaria conicospermium. J Nat Prod. 2003;66:910–916. doi: 10.1021/np030005k. [DOI] [PubMed] [Google Scholar]
- Bharate SB, Khan SI, Yunus NAM, Chauthe SK, Jacob MR, Tekwani BL, Khan IA, Singh IP. Antiprotozoal and antimicrobial activities of O-alkylated and formylated acylphloroglucinols. Bioorg Med Chem. 2007;15:87–96. doi: 10.1016/j.bmc.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Cateni F, Doljak B, Zacchigna M, Anderluh M, Piltaver A, Scialino G, Banfi E. New biologically active epidioxysterols from Stereum hirsutum. Bioorg Med Chem Lett. 2007;17:6330–6334. doi: 10.1016/j.bmcl.2007.08.072. [DOI] [PubMed] [Google Scholar]
- Choi J-H, Horikawa M, Okumura H, Kodani S, Nagai K, Hashizume D, Koshino H, Kawagishi H. Endoplasmic reticulum (ER) stress protecting compounds from the mushroom Mycoleptodonoides aitchisonii. Tetrahedron. 2008;65:221–224. [Google Scholar]
- Cutler HG, Hoogsteen K, Littrell RH, Arison BH. Epoxyexserohilone, a novel metabolite from Nigrospora sphaerica. Agric Biol Chem. 1991;55:2037–2042. [Google Scholar]
- Fdhila F, Vazquez V, Sanchez JL, Riguera R. dd-Diketopiperazines: antibiotics active against Vibrio anguillarum isolated from marine bacteria associated with cultures of Pecten maximus. J Nat Prod. 2003;66:1299–1301. doi: 10.1021/np030233e. [DOI] [PubMed] [Google Scholar]
- Fisher FW, Cook NB. Fundamentals of Diagnostic Mycology. WB Saunders; Philadelphia, PA: 1998. [Google Scholar]
- Fukushima T, Tanaka M, Gohbara M, Fujimori T. Phytotoxicity of three lactones from Nigrospora sacchari. Phytochemistry. 1998;48:625–630. [Google Scholar]
- Gao Z, Ali Z, Khan IA. Glycerogalactolipids from the fruit of Lycium barbarum. Phytochemistry. 2008;69:2856–2861. doi: 10.1016/j.phytochem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Gong Q, Zhang Y, Tan N, Chen Z. Chemical constituents of three poisonous mushrooms. Tianran Chanwu Yanjiu Yu Kaifa. 2010;22:185–188. [Google Scholar]
- Hendea D, Laschat S, Baro A, Frey W. Diastereoselective alkylation of a proline-derived bicyclic lactim ether. Helv Chim Acta. 2006;89:1894–1909. [Google Scholar]
- Hoog GD, Guarro J, Gené J, Figueras M. Atlas of Clinical Fungi. Centraal-bureau voor Schimmelcultures (CBS); Baarn: 2000. [Google Scholar]
- Kim J-C, Choi GJ, Park J-H, Kim HT, Cho KY. Activity against plant pathogenic fungi of phomalactone isolated from Nigrospora sphaerica. Pest Manag Sci. 2001;57:554–559. doi: 10.1002/ps.318. [DOI] [PubMed] [Google Scholar]
- Lee WY, Park Y, Ahn J-K, Park S-Y, Lee H-J. Cytotoxic activity of ergosta-4,6,8(14),22-tetraen-3-one from the sclerotia of Polyporus umbellatus. Bull Korean Chem Soc. 2005;26:1464–1466. [Google Scholar]
- Li D, Li X, Cui C, Wang B. Chemical constituents of endophytic fungus Hypocreales sp. derived from the red alga Symphyocladia latiuscula. Haiyang Kexue. 2008a;32:51–55. [Google Scholar]
- Li P, Zuo T, Wang X, Zhu L, Wu S, Zhang G. Chemical constituents of Scutellaria barbata D. Don (II) Zhongguo Yaowu Huaxue Zazhi. 2008b;18:374–376. [Google Scholar]
- Li X, Han L, Cao Y-R, Liu J, Jiang Y, Huang X-S. Separation and identification of chemical constituents from a Streptomyces sp. isolated from sika deer feces. Huaxue Yu Shengwu Gongcheng. 2012;29(31/32):49. [Google Scholar]
- Li X, Sun G-Z, Zheng Y-N, Lin W-H, Sattler I. Separation and structures of two steroids from mangrove endophyte Penicillium. Tianran Chanwu Yanjiu Yu Kaifa. 2007;19:420–422. [Google Scholar]
- Liu D, Wang S, Zhang L, Li L. Chemical composition of n-BuOH extract of Potentilla anserina L. and its protective effect of EAhy926 endothelial cells under hypoxia. Lat Am J Pharm. 2011;30:1889–1894. [Google Scholar]
- Mantsch HH, Smith ICP. Solvent effects on the carbon-13 nuclear magnetic resonance spectra of cholesterol pyridine, and uridine. Can J Chem. 1973;51:1384–1391. [Google Scholar]
- Pang Z, Sterner O. The isolation of ergosta-4,6,8(14),22-tetraen-3β-ol from injured fruit bodies of Marasmius oreades. Nat Prod Lett. 1993;3:193–196. [Google Scholar]
- Radwan MM, Rodriguez-Guzman R, Manly SP, Jacob M, Ross SA. Sepicanin A—a new geranyl flavanone from Artocarpus sepicanus with activity against methicillin-resistant Staphylococcus aureus (MRSA) Phytochem Lett. 2009;2:141–143. doi: 10.1016/j.phytol.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper PR, Drewinko B. Comparison of in vitro methods to determine drug-induced cell lethality. Cancer Res. 1976;36:2182–2188. [PubMed] [Google Scholar]
- Rukachaisirikul V, Arunpanichlert J, Sukpondma Y, Phongpaichit S, Sakayaroj J. Metabolites from the endophytic fungi Botryosphaeria rhodina PSU-M35 and PSU-M114. Tetrahedron. 2009;65:10590–10595. [Google Scholar]
- Tanaka M, Fukushima T, Tsujino Y, Fujimori T. Nigrosporins A and B, new phytotoxic and antibacterial metabolites produced by a fungus Nigrospora oryzae. Biosci Biotechnol Biochem. 1997;61:1848–1852. doi: 10.1271/bbb.61.1848. [DOI] [PubMed] [Google Scholar]
- Tenguria RK, Khan FN, Quereshi S. Endophytes—mines of pharmacological therapeutics. World J Sci Technol. 2011;1:127–149. [Google Scholar]
- Trisuwan K, Rukachaisirikul V, Sukpondma Y, Preedanon S, Phongpaichit S, Rungjindamai N, Sakayaroj J. Epoxydons and a pyrone from the marine-derived fungus Nigrospora sp. PSU-F5 J Nat Prod. 2008;71:1323–1326. doi: 10.1021/np8002595. [DOI] [PubMed] [Google Scholar]
- Turner WB, Aldridge DC. Fungal Metabolites. II. Academic Press; New York: 1983. [Google Scholar]
- Zhang Q-H, Tian L, Zhou L-D, Zhang Y, Li Z-F, Hua H-M, Pei Y-H. Two new compounds from the marine Nigrospora sphaerica. J Asian Nat Prod Res. 2009;11:962–966. doi: 10.1080/10286020903339614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


