Abstract
Differential analysis of outer membrane composition of S. Enteritidis strains, resistant to 50% normal human serum (NHS) was performed in order to find factors influencing the resistance to higher concentrations of NHS. Ten S. Enteritidis clinical strains, resistant to 50% NHS, all producing very long lipopolysaccharide, were subjected to the challenge of 75% NHS. Five extreme strains: two resistant and three sensitive to 75% NHS, were chosen for the further analysis of outer membrane proteins composition. Substantial differences were found in the levels of particular outer membrane proteins between resistant and sensitive strains, i.e. outer membrane protease E (PgtE) was present mainly in resistant strains, while sensitive strains possessed a high level of flagellar hook-associated protein 2 (FliD) and significantly higher levels of outer membrane protein A (OmpA).
Introduction
For many years bacteria from the genus of Salmonella have remained the leading cause of gastrointestinal diseases, posing a significant epidemiological threat to the community. Every year 94 million infections, caused by nontyphoidal Salmonella (NTS) organisms, are recorded worldwide [1]. Moreover, these epidemiological data are probably highly underestimated [2], as most salmonellosis cases are not reported due to the short course of the disease and no hospitalization. It is estimated that in the USA only 1–5% of the cases are laboratory confirmed and reported to the Centers for Disease Control and Prevention (CDC) [3]. In addition to the typical course of salmonellosis, an infection with Salmonella can often lead to a parenteral form and eventually to sepsis. Salmonella enterica subsp. enterica Enteritidis (S. Enteritidis) and Salmonella enterica subsp. enterica Typhimurium (S. Typhimurium) are the two most common clinical serovars (serological variants) isolated worldwide as causative agents of salmonellosis [1]. In all regions of the world except North America and Oceania, serovar S. Enteritidis is more widespread than S. Typhimurium (65% and 12% of all isolates worldwide, respectively) [4,5].
In 2012 nearly 77% of the total cases of human salmonellosis was caused by S. Enteritidis—currently the most commonly isolated serovar in Poland [6]. S. Enteritidis—a facultative intracellular pathogen—is the prominent reason responsible for acute gastroenteritis because it is widespread in poultry products and it is highly resistant to harsh and adverse conditions.
Complement resistance is a crucial factor in the development of a systemic infection and bacteremia for many pathogenic bacteria. The complement system is composed from a combination of plasma proteins. The enzymatic cascade activation causes opsonization of the invading microbe and a release of chemotactic compounds that promote leukocyte recruitment and phagocytosis of invading bacteria. In addition, formation of the terminal complement complex leads the lysis of the cell membrane. Three different pathways: classical, alternative and lectin pathway, can activate the complement system. Three homologous glycoproteins: C3, C4 and C5, play the main role in complement activation and are responsible for the interaction with other complement components and precursors for proteins promoting the activation of the complement cascade.
Due to their high virulence and a number of features that predispose Salmonella to survive in the serum, it is necessary to constantly investigate and explore the structural elements of the bacteria, which are responsible for the resistance to the immune response of the host. An important virulence element is the ability to change the structures of the outer envelopes, which enables a survival and multiplication of bacteria in an extremely adverse environment. Salmonella resistance to the bactericidal activity of serum is determined by several factors, like variable lipopolysaccharide (LPS) O-specific chain length, polysaccharide capsules, specific outer membrane proteins (OMPs) or the presence of fimbriae on the cell surface.
The unique chemical structure of LPS is responsible for several specific properties of bacterial cells. LPS consists of three structural domains: lipid A, core oligosaccharide and the O-specific antigen (O-antigen). The O-specific antigen is a polysaccharide, made up from repeating oligosaccharide subunits [7]. It is the distal part of LPS exposed to the external environment and responsible for the serological properties of bacteria, with great chemical diversity among bacterial strains. LPS forms a durable barrier around a bacterial cell, to mask and protect it against the host immune system [8]. The LPS O-specific chain plays an important role in the generation of serum complement resistance of various Gram-negative bacteria: S. Typhimurium [9], Yersinia enterocolitica [10] or Klebsiella pneumoniae [11]. Lipopolysaccharide molecules from some of the Salmonella strains exhibit the so-called modal length distribution. That type of distribution is characterized by the occurrence of three clearly distinct LPS fractions, differing in the number of repeating subunits in the O-specific antigen: low molecular weight LPS (LMW-OAg) with 15 repeating subunits, long (L-OAg) with 16–35 repeating subunits and very long (VL-OAg) comprising more than 100 repeating subunits [12]. The lipopolysaccharide molecules of S. Typhimurium and S. Enteritidis possess modal length distribution, with wzz gene controlling the synthesis of L-OAg and wzzfepE gene responsible for the expression of the VL-OAg [9], while S. Typhi produces LPS without any apparent modal distribution [13]. The long and very long O-specific chains of LPS hamper efficient attachment of complement membrane attack complex (MAC) to the lipid part of the outer membrane, thereby preventing a bacterial cell from lysis. Crawford et al. [14] also show the participation of VL-OAg LPS in the Salmonella survival in bile, which increases the pathogenicity of S. Typhimurium strains. In addition, Bravo et al. report that strains of S. Enteritidis and S. Typhimurium, with L-OAg LPS only, show lower resistance to serum than mutants with VL-OAg LPS only [12].
OMPs act in the cell as active factors against the negative influence of external environment, which can cause various levels of their expression. Due to the peripheral location in a bacterial cell, OMPs are active in the process of cell adaptation to fluctuating habitat conditions [15]. Moreover, they are thought to be a substantial component of the bacterial cell surface that is involved in the inhibition of complement activity and the activation of the complement system. Some OMPs amplify the sensitivity of Gram-negative bacteria to the bacteriolytic action of complement components [16–18]. Binding of OmpK36 from K. pneumoniae to C1q proteins of complement leads to activation of the complement classical pathway and the subsequent deposition of C3b and C5b-9 on the porin [19]. Among OMPs there is a number of proteins involved in Salmonella resistance to serum, including PagC, PgtE, Rck and TraT [20–23]. The mechanisms by which OMPs interact with the complement system have not been fully characterized and are still under investigation. It has been shown that the presence of PagC in the outer membrane of S. Choleraesuis results in serum resistance [20]. Studies of Riva et al. suggest that in S. Typhimurium PgtE mediates in the generation of serum resistance by its ability to degrade the complement components: C3, C3b, C4, C5, and C4b [22]. In turn, Ho et al. show that protein Rck contributes to the inhibition of MAC formation by binding to protein C9 and factor H of the complement system [21]. It has also been shown that protein TraT of S. Typhimurium and S. Enteritidis, can inhibit the formation of MAC by binding to factor 6, which is a part of the complement cascade system [23]. Bugla-Płoskońska et al. confirmed that there are certain OMPs characteristic for the serum-sensitive and serum-resistant phenotypes of Salmonella O48 strains [24]. A number of previous studies have investigated the OMPs profiles of different Salmonella strains [25–28], but only one study on outer membrane proteins of S. Enteritidis has been published [29] to date. As the data concerning virulence factors of S. Enteritidis is generally limited, the objective of the present investigation was a comparative analysis of the outer membrane composition of S. Enteritidis strains, to find a correlation between the expression of specific OMPs and the level of resistance to human serum. Ten strains, which were found to be resistant to normal human serum (NHS, 50%), were further tested for their susceptibility to the increased concentration of NHS (75%). After the analysis of their LPS molecules length distribution, 5 outlying strains, i.e. 2 extremely resistant and 3 extremely sensitive, were chosen for a differential analysis of their OMPs composition. Isolated OMPs were separated in 2D gel electrophoresis and selected spots were identified using mass spectrometry.
Materials and Methods
Bacterial strains
Ten clinical strains of S. Enteritidis were isolated from the faeces of patients with symptoms of diarrhea (Dialab Laboratory). S. Enteritidis were identified, using biochemical tests and serotyping with agglutination tests with specific O and H antisera, and classified according to the Kauffmann-White-Le Minora scheme [30]. The strains are available in the collection of the Department of Microbiology at the University of Wroclaw (Table 1).
Table 1. Salmonella Enteritidis strains from the collection of the Department of Microbiology, the University of Wroclaw.
| No | Date of sampling [year/month] | Number of S. Enteritidis strain | Number of PCM* S. Enteritidis strain |
|---|---|---|---|
| 1 | 2011/04 | 670 | 2813 |
| 2 | 2011/05 | 888 | 2808 |
| 3 | 2012/03 | 27 | 2817 |
| 4 | 2012/04 | 351 | 2814 |
| 5 | 2012/05 | 3686 | 2809 |
| 6 | 2012/08 | 851 | 2812 |
| 7 | 2012/08 | 920 | 2811 |
| 8 | 2012/09 | 1120 | 2815 |
| 9 | 2012/09 | 4574 | 2807 |
| 10 | 2012/11 | 1227 | 2816 |
* S. Enteritidis strains have been submitted to the Polish Collection of Microorganisms (PCM).
Serum
Normal human serum (NHS) from a group of healthy adults, who had not received any antimicrobial drug treatment for 6 months, was obtained from Regional Centre of Transfusion Medicine and Blood Bank in Wroclaw. This was conducted by Elżbieta Klausa according to the principles expressed in the Declaration of Helsinki. Experiments were performed with NHS samples taken from individual donors and mixed together. Blood samples were taken into aseptic dry tubes or tubes with an anticoagulant. After collection, the samples were stored at room temperature for 1 h to allow separation and then centrifuged for 10 min at 1200g to pellet cells and platelets. The serum samples were frozen in 0.5 ml aliquots at -70°C and stored for no longer than 3 months. The required volume of serum was thawed immediately before use and each portion was used only once. For this study, only serum samples lacking hemolysis and with negative test results for HIV, HCV and Treponema pallidum antibodies, a negative HBs antigen test, and a negative viral genome screen (HIV RNA, HBV DNA, HCV RNA) were used.
Serum C3 concentration
The C3 concentration in the mixed serum (NHS) was quantified by a radial immunodiffusion test (Human Complement C3 & C4 ‘Nl’ Bindarid Radial Immunodiffusion Kit; The Binding Site Group Ltd.).
Serum susceptibility assay
Ten S. Enteritidis strains were subjected to the challenge of 50% NHS. All strains were found to be resistant to this concentration of NHS, so the strains were further tested for their susceptibility with an increased concentration of NHS (75%).
The bactericidal activity of NHS was determined as described previously [31] with slight modifications [32]. Bacterial strains were cultured overnight at 37°C in 3 ml of Lysogeny Broth Lennox (LB) (Sigma). After overnight incubation, bacterial cells (500μl) in the early exponential phase were transferred into 5 ml of fresh LB medium and incubated at 37°C for 1 h in a water bath with rotation at 200 rpm to achieve an OD600 of 0.3. Next, bacteria cells were collected by centrifugation (1500g for 20 min at 4°C). The pellet was resuspended in 3 ml of physiological saline (0.9% NaCl) (POCH) and then diluted in the same saline to produce a suspension of approximately 106 cells/ml. Aliquots of the cell suspension were mixed with an appropriate volume of NHS and incubated at 37°C for 0, 60 and 180 min (T0, T1 and T3, respectively) in a water bath with rotation at 200 rpm. Appropriate dilutions were then spread in duplicate onto nutrient agar plates and incubated at 37°C for 24 h. The average number of colony-forming units per milliliter (CFU/ml) was calculated from the replicate plate counts. The survival rate for T1 and T3 was calculated as a percentage of the cell count for T0 (set at 100%). Strains with survival rates of >100% after 180-min incubation with serum were considered resistant, and those with survival rates of <100% were considered susceptible to the bactericidal action of NHS. Strains with survival rates between 50% and 100% were considered to have intermediate (moderate) resistance to NHS. NHS decomplemented by heating at 56°C for 30 min (heat inactivated serum, HIS) was used as a control [33]. Each test was performed three times.
Lipopolysaccharide isolation and analysis by SDS-PAGE
Extraction of LPS was performed using a commercial RNA isolating reagent: Tri-Reagent (Sigma-Aldrich) according to Yi and Hackett [34]. 10 mg of lyophilized bacterial cells were suspended in 200 μl of Tri-Reagent and the cell suspension was then incubated at room temperature for 10 min for complete cell homogenization. After incubation, 200 μl of chloroform was added to create a phase separation. The mixture was then vigorously vortexed and incubated at room temperature for additional 10 min. The resulting mixture was centrifuged at 12 000g (Sigma, Heraeus Fresco 21) for 10 min to separate the aqueous and organic phase. The aqueous phase was transferred to a new 1.5 ml centrifuge tube. Distilled water (100 μl) was added to the organic phase. The mixture was vortexed, incubated at room temperature for 10 min, and centrifuged at 12 000g for 10 min. The upper aqueous phases from both steps were combined. Two additional water extraction steps were repeated. The combined aqueous phase was lyophilized. After lyophilization we used the cold magnesium precipitation procedure according to Darveau and Hancock for the purification of LPS [35]. LPS was dissolved in 500 μl of 0.375 M magnesium chloride (POCH) in 95% ethanol, stored at -20°C, followed by centrifugation at 12 000g for 15 min. The pellet was suspended in 200 μl of distilled water and lyophilized. LPS extracts were analyzed by discontinuous SDS-PAGE using Laemmli buffer system [36]. Samples were applied to the slabs after mixing with Laemmli buffer and heating at 98○C for 7 minutes. Gel electrophoresis was performed using 6% polyacrylamide stacking gel and 15% separating gel. The SDS-PAGE separation of LPS was performed at constant voltage (120V), for 90 minutes using a Mini-Protean Tetra Cell apparatus (Bio-Rad). The separated LPS was visualized using silver staining according to Tsai and Frasch [37] with Fomsgaard [38] and photographed under white light using a GelDoc XR imaging system (Bio-Rad).
OMPs isolation and preparation
The isolation of OMPs was performed according to Murphy and Bartos [39] with own minor modifications [24]. Bacterial strains were cultured overnight at 37°C in 25 ml LB medium. After growth, the cells from the overnight culture were harvested (1500g at 4°C for 20 min) and suspended in 1.25 ml 1 M sodium acetate (POCH), 1mM β-mercaptoethanol (Merck). Subsequently, 11.25 ml water solution containing 5% (w/v) Zwittergent Z 3–14 (Calbiochem) and 0.5 M CaCl2 (POCH) was added. This mixture was stirred at room temperature for 1 h. To precipitate nucleic acids, 3.13 ml of 96% (v/v) cold ethanol (POCH) was added very slowly. The mixture was then centrifuged at 17000g at 4°C for 10 min. The proteins were precipitated from the supernatant by the addition of 46.75 ml of 96% (v/v) cold ethanol and centrifuged at 17000 g at 4°C for 20 min. The pellet was left to dry at ambient temperature and then suspended in 1.5 ml 50 mM Trizma-Base (Sigma-Aldrich) buffer, pH 8.0 containing 0.05% (w/v) Zwittergent Z 3–14 and 10 mM EDTA (Sigma-Aldrich) and stirred at room temperature for 1 h. The solution was kept at 4°C overnight. Insoluble material was removed by centrifugation at 12 000g at 4°C for 10 min, with OMPs present in the supernatant.
Total protein concentration was measured using a commercial BCA Protein Assay Kit (Thermo Scientific).
Two dimensional gel electrophoresis
The OMPs were separated with 4–7 pH immobilized gradient IPG strips (7 cm) (Bio-Rad). 2-DE was carried out with the Mini-PROTEAN Tetra Cell System (Bio-Rad). Isoelectric focusing (IEF) was conducted by stepwise increase of voltage as follows: 250 V, 20 min; 4000 V, 120 min (linear) and 4000 V (rapid), until the total volt-hours reached 14 kVh. IPG strips were then loaded onto the top of 1-mm slabs comprised of a 9% polyacrylamide stacking gel and 12,5% polyacrylamide separating gel, using 0.5% agarose (Bio-Rad) in the running buffer. Electrophoresis was performed at 4°C with constant power current (1 W) until the dye front reached the bottom [40–42]. The protein spots were visualized by the silver staining method [43] and in gels used for mass-spectrometric identification of proteins – by Coomassie Brilliant Blue (Bio-Rad). Band patterns were visualized under white light and photographed using ChemiDoc MP system (Bio-Rad). Image spots of proteins were analyzed by PDQuest software (Bio-Rad).
In-Gel Protein Digestion and MS Protein Identification
All solvents used for digestion, MS preparation and analysis were of LC-MS grade and purchased from Merck Millipore. Ammonium bicarbonate eluent additive for LC-MS, dithiothreitol and iodoacetamide were from Sigma. Sequencing Grade Modified Trypsin was obtained from Promega.
After isolation, 2-DE separation and staining with Coomassie Brilliant Blue method protein spots of interest were excised from the gel and subjected to the in-gel tryptic digestion according to the method described by Shevchenko et al. [44]. Briefly, after destaining (100 mM NH4HCO3/acetonitrile, 1:1, vol:vol), reduction (10 mM dithiothreitol in 100 mM NH4HCO3) and alkylation (55 mM iodoacetamide in 100 mM NH4HCO3), a suitable volume of 13 ng/μl trypsin in 10 mM ammonium bicarbonate containing 10% (vol/vol) acetonitrile was added to the excised gel spot cut into cubes. The obtained peptides were extracted from the gel, concentrated and desalted with the Pierce C18 tip, and subjected to mass spectrometry analysis using the MALDI TOF ultrafleXtreme instrument (Bruker Daltonics). 10 mg/ml of α-cyano-4-hydroxycinnamic acid (Bruker) in acetonitrile/0.1% TFA in H2O (1:1, vol:vol) was used as the eluent of peptides from the Pierce C18 tip directly on a MALDI plate. Spectra were acquired in positive reflector mode, averaging 2000 laser shots per MALDI-TOF spectrum. OMPs identification was achieved using a bioinformatics platform (ProteinScape, Bruker Daltonics) and MASCOT (Matrix Science) as a search engine to search protein sequence databases (NCBI, SwissProt) using the peptide mass fingerprinting method.
Results
Bactericidal Assay of NHS
As C3 is a crucial protein in the activation of the complement cascade, standard analysis of C3 protein levels in NHS used for experiments were performed. C3 concentration was 1330 mg/L, which is in the range of standard values (970–1576 mg/l for males and 1032–1495 for females).
All the ten strains of S. Enteritidis were found to be resistant to NHS in the standard test of the bactericidal activity with 50% NHS (Table 2).
Table 2. Bactericidal activity of 50% NHS and 50% HIS decomplemented by heating at 56°C for 30 min against S. Enteritidis strains.
| Bactericidal activity of 50% NHS1 | Bactericidal activity of 50% HIS2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | CFU/ml3 | Survival rate of cells at T1 [%] | Survival rate of cells at T3 [%] | RP4 | CFU/ml | Survival rate of cells at T3 [%] | |||
| T0 | T1 | T3 | T0 | T3 | |||||
| 27 | 2.36 x 106 ± 2.14 x 105 | 3.71 x 106 ± 8.52 x 105 | 9.07 x 106 ± 2.54 x 106 | 155.8 ± 23.2 | 379.9 ± 77.7 | R | 1.83 x 106 ± 2.32 x 105 | 1.67 x 108 ± 3.00 x 107 | 9092.2 ± 699.3 |
| 851 | 1.80 x 106 ± 1.05 x 105 | 3.28 x 106 ± 4.51 x 105 | 6.96 x 106 ± 6.91 x 105 | 183.2 ± 33.0 | 387.6 ± 48.8 | R | 2.74 x 106 ± 4.31 x 105 | 1.25 x 108 ± 4.26 x 107 | 4462.9 ± 851.2 |
| 670 | 2.40 x 106 ± 1.46 x 105 | 2.96 x 106 ± 3.30 x 105 | 4.84 x 106 ± 2.27 x 106 | 123.3 ± 7.3 | 202.3 ± 97 | R | 2.30 x 106 ± 1.36 x 105 | 3.77 x 107 ± 1.16 x 107 | 1624.9 ± 440.1 |
| 888 | 2.14 x 106 ± 1.75 x 105 | 2.07 x 106 ± 3.75 x 105 | 2.35 x 106 ± 3.27 x 105 | 96.2 ± 10.9 | 109.3 ± 6.6 | R | 3.06 x 106 ± 6.81 x 104 | 5.55 x 107 ± 3.80 x 106 | 1816.1 ± 99.8 |
| 1120 | 2.51 x 106 ± 2.80 x 105 | 2.43 x 106 ± 3.37 x 105 | 2.55 x 106 ± 3.14 x 105 | 96.5 ± 4.6 | 101.4± 2.1 | R | 1.60 x 106 ± 9.85 x 104 | 1.79 x 107 ± 3.67 x 106 | 1112.6 ± 174.2 |
| 351 | 3.15 x 106 ± 3.17 x 105 | 3.71 x 106 ± 2.76 x 105 | 6.77 x 106 ± 1.66 x 105 | 118.3 ± 10.0 | 216.3 ± 23.1 | R | 3.31 x 106 ± 2.88 x 105 | 3.36 x 107 ± 7.61 x 106 | 1016.7 ± 208.0 |
| 920 | 2.06 x 106 ± 7.51 x 104 | 2.53 x 106 ± 2.01 x 105 | 3.60 x 106 ± 5.11 x 105 | 123.0 ± 8.7 | 174.7 ± 22.1 | R | 1.92 x 106 ± 2.80 x 105 | 3.67 x 107 ±1.19 x 107 | 1885.3 ± 421.5 |
| 1227 | 1.54 x 106 ± 8.33 x 104 | 1.89 x 106 ± 1.54 x 105 | 2.57 x 106 ± 2.20 x 105 | 122.4 ± 5.5 | 166.5 ± 14.9 | R | 1.94 x 106 ± 5.16 x 105 | 4.98 x 107 ± 1.68 x 107 | 2568.2 ± 534.1 |
| 3686 | 2.48 x 106 ± 1.81 x 105 | 2.78 x 106 ± 3.50 x 105 | 3.79 x 106 ± 5.69 x 105 | 111. 9 ± 7.2 | 153.3 ± 20.6 | R | 4.02 x 106 ± 1.52 x 105 | 4.71 x 107 ± 3.92 x 106 | 1169.6 ± 82.0 |
| 4574 | 1.73 x 106 ± 1.61 x 105 | 2.02 x 106 ± 5.80 x 105 | 3.62 x 106 ± 1.18 x 106 | 115.5 ± 23.6 | 206.4± 51.2 | R | 2.78 x 106 ± 3.25 x 105 | 6.80 x 107 ± 2.06 x 107 | 2406.7 ± 469.3 |
1 Normal Human Serum
2 Human Inactivated Serum
3 Colony Forming Units/ml
4RP-Resistance phenotype: R – resistant strain, S – sensitive strain, I – intermediate strain.
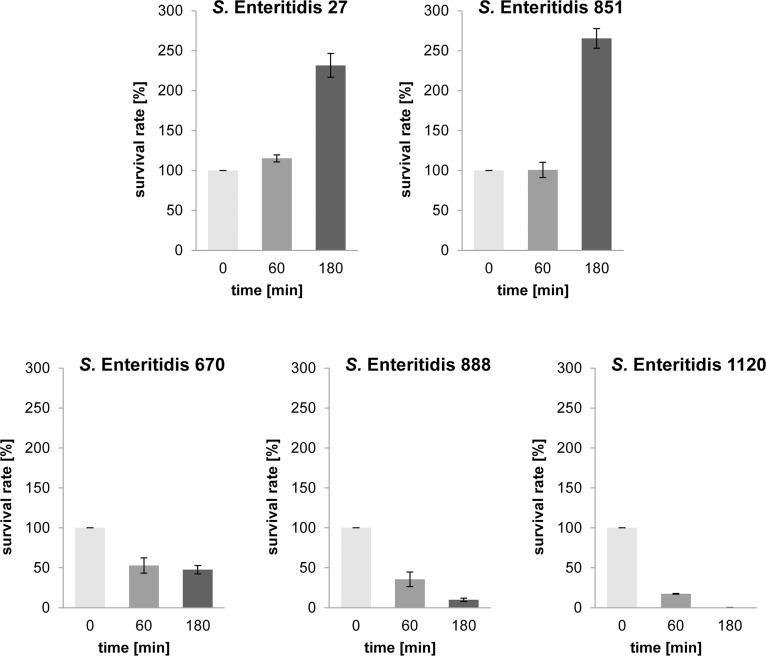
Further analyses showed that resistant strains from the same Salmonella serovar exhibit extremely different reactions to the bactericidal action of NHS after the challenge with a higher concentration of NHS: after a 180 min incubation in 75% NHS the strains revealed significant differences in the level of their resistance (Table 3). Five extreme strains were used for further experiments. Two strains, i.e. S. Enteritidis 27 and S. Enteritidis 851, were resistant to 75% NHS (226.6% and 271.9% survival rate after a 180 min cell incubation in serum, respectively), and three strains, i.e. S. Enteritidis 670, S. Enteritidis 888 and S. Enteritidis 1220, were sensitive (44.5%, 8.8% and 0.2% survival rate, respectively). When the bactericidal activity of NHS was heat-inhibited (HIS, control of experiments), bacterial cells proliferated very intensively and all of the tested strains were resistant to 75% HIS (Table 3, Fig 1).
Table 3. Bactericidal activity of 75% NHS and 75% HIS decomplemented by heating at 56°C for 30 min against S. Enteritidis strains.
| Bactericidal activity of 75% NHS1 | Bactericidal activity of 75% HIS2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain | CFU/ml3 | Survival rateof cells at T1 [%] | Survival rateof cells at T3 [%] | RP4 | CFU/ml | Survival rate of cells at T3 [%] | ||||
| T0 | T1 | T3 | T0 | T3 | ||||||
| 27 | 1.96 x 106 ± 1.59 x 105 | 2.26 x 106 ± 1.85 x 105 | 4.54 x 106 ± 3.36 x 105 | 115.2 ± 4.5 | 231.7 ± 14.8 | R | 2.04 x 106 ± 2.52 x 105 | 1.63 x 108 ± 2.99 x 107 | 8071.5 ± 1720.1 | |
| 851 | 1.47 x 106 ± 1.46 x 105 | 1.49 x 106 ± 2.25 x 105 | 3.90 x 106 ± 2.40 x 105 | 100.7 ± 9.5 | 265.4 ± 12.2 | R | 1.54 x 106 ± 2.14 x 105 | 2.23 x 107 ± 4.89 x 106 | 1446.8 ± 281.5 | |
| 670 | 4.93 x 106 ± 9.56 x 105 | 2.54 x 106 ± 5.20 x 104 | 2.31 x 106 ± 2.23 x 105 | 52.7 ± 9.5 | 47.5 ± 5.2 | S | 2.00 x 106 ± 1.51 x 105 | 3.64 x 107 ± 6.66 x 106 | 1809.9 ± 227.1 | |
| 888 | 5.59 x 106 ± 1.32 x 106 | 1.91 x 106 ± 1.31 x 105 | 5.35 x 105 ± 5.46 x 104 | 35.6 ± 9.1 | 9.9 ± 2.0 | S | 1.90 x 106 ± 4.01 x 105 | 2.14 x 107 ± 5.39 x 106 | 1122.8 ± 149.0 | |
| 1120 | 1.55 x 106 ± 1.10 x 105 | 2.69 x 105 ± 1.49 x 104 | 3.36 x 103 ± 8.50 x 102 | 17.4 ± 0.4 | 0.2 ± 0.1 | S | 2.48 x 106 ± 1.92 x 105 | 1.25 x 107 ± 4.50 x 106 | 497.3 ± 150.5 | |
| 351 | 1.51 x 106 ± 4.66 x 105 | 1.21 x 106 ± 3.53 x 105 | 1.18 x 106 ± 1.18 x 105 | 80.8 ± 2.6 | 81.6 ± 18,0 | I | 4.83 x 106 ± 5.80 x 105 | 3.43 x 107 ± 7.61 x 106 | 705.8 ± 84.7 | |
| 920 | 1.85 x 106 ± 2.59 x 105 | 1.99 x 106 ± 3.54 x 105 | 1.30 x 106 ± 2.78 x 105 | 107.2 ± 7.3 | 69.9 ± 5.8 | I | 1.87 x 106 ± 2.47 x 105 | 7.42 x 107 ±1.35 x 107 | 3981.8 ± 628.9 | |
| 1227 | 3.19 x 106 ± 2.75 x 105 | 1.65 x 106 ± 6.56 x 104 | 2.82 x 106 ± 3.29 x 105 | 52.1 ± 6.2 | 88.2 ± 5.2 | I | 5.99 x 106 ± 4.94 x 105 | 3.34 x 107 ± 2.49 x 107 | 536.6 ± 389.0 | |
| 3686 | 2.00 x 106 ± 5.06 x 105 | 1.87 x 106 ± 4.31 x 105 | 1.57 x 106 ± 3.72 x 105 | 94.2 ± 4.2 | 78.8 ± 1.2 | I | 2.01 x 106 ± 4.92 x 105 | 1.67 x 107 ± 4.61 x 106 | 850.3 ± 248.8 | |
| 4574 | 1.71 x 106 ± 3.93 x 105 | 1.64 x 106 ± 5.66 x 105 | 1.53 x 106 ± 2.84 x 106 | 94.3 ± 10.1 | 90.4 ± 3.8 | I | 2.06 x 106 ± 2.48 x 105 | 4.31 x 107 ± 4.21 x 106 | 2112.9 ± 341.9 | |
1 Normal Human Serum
2 Human Inactivated Serum
3 Colony Forming Units/ml
4RP-Resistance phenotype: R – resistant strain, S – sensitive strain, I – intermediate strain.
Fig 1. Bactericidal activity of 75% NHS against S. Enteritidis strains.
Two strains, i.e. S. Enteritidis 27, S. Enteritidis 851, were resistant to 75% NHS. Three strains, i.e. S. Enteritidis 670, S. Enteritidis 888 and S. Enteritidis 1120, were sensitive to 75% NHS.
Regarding our own results and reports of other research groups, showing that resistance to the bactericidal activity of serum is determined by OMPs and LPS [12, 20, 22, 23], the next stage of research concentrated on the LPS O-antigen chain length distribution and the protein profiles of OMPs.
SDS-PAGE of LPS
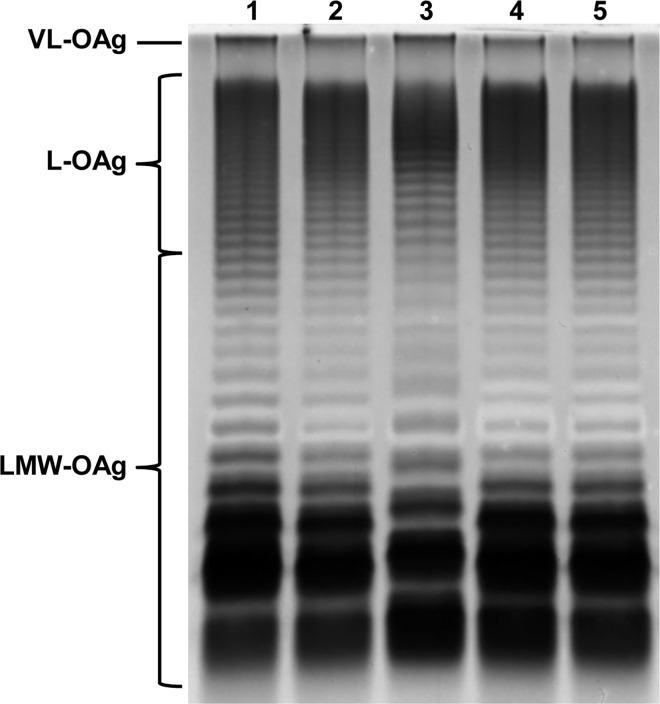
The SDS-PAGE analysis of LPS isolated from bacterial cells showed, that all the five tested strains possessed LPS with a very long O-antigen (VL-OAg LPS, more than 100 O-specific units in the O-chain; Fig 2). That was in accordance with the results of the test with 50% NHS, where all the five strains appeared to be resistant: VL-OAg LPS is described in the literature as an important factor of bacterial resistance to human serum [12]. Although the presence of VL-OAg LPS correlates with the resistance of the strains to 50% NHS, but there is no straight correlation, while 75% NHS has been used for experiments, what indicates the involvement of other factors in the process of bacterial resistance. Additionally the LPS pattern in line 3 (S. Enteritidis 670) in the region of LMW-OAg LPS shows distinct differences comparing to the other lines: the difference in the position of respective bands suggests the increase of the molecular mass of the LPS core or O-antigen structure.
Fig 2. LPS profiles of five extreme strains: Lane 1—S. Enteritidis 27, Lane 2—S. Enteritidis 851, Lane 3—S. Enteritidis 670, Lane 4—S. Enteritidis—888, Lane 5—S. Enteritidis 1220.
Analysis of 2-DE profiles of isolated OMPs
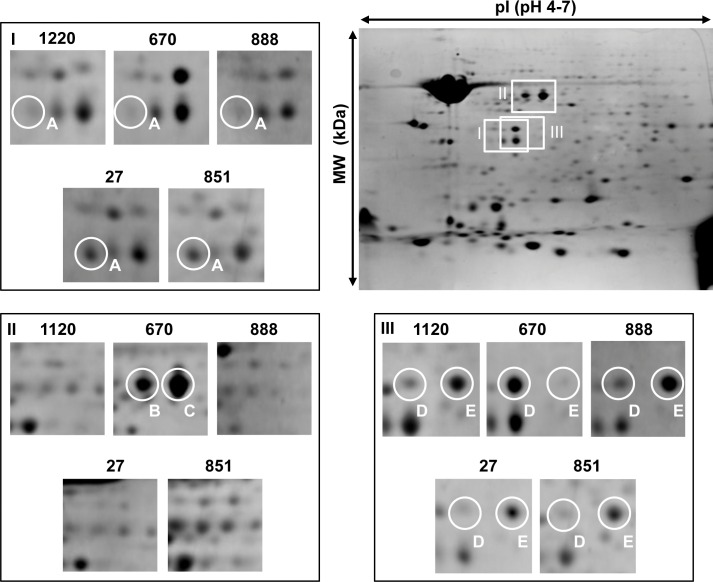
We applied a proteomic approach using the 2-DE and mass spectrometry analysis for the identification of specific proteins that could be involved in the phenomenon of serum resistance of these strains. Protein spots on 2-DE were visualized within the molecular weight (MW) range of 10–159 kDa and isoelectric points (pI) of 4–7. In total, 169–180 spots were counted on the maps of S. Enteritidis strains by the PDQuest software. The comparative protein pattern analysis of S. Enteritidis strains resistant and sensitive to human serum showed differences in the presence of some proteins (Fig 3I–3III). The differential analysis revealed the presence of 5 main protein spots, in which the proteins were subsequently identified (Table 4). MWs of OMPs were distributed in the range of 35.03–49.81 kDa. All the spots were distributed in the range of pI 5.27–5.6. The detailed Mascot search results and MS spectra are provided as supporting information (S1, S2, S3, S4, S5, and S6 Appendices).
Fig 3. Comparative analysis of OMPs from S. Enteritidis strains sensitive and resistant to human serum (75%).
Differential expression of marked protein A in serum-sensitive and serum-resistant strains. Both strains resistant to human serum overexpressed protein A (PgtE) (I). The overexpressed proteins labeled as B and C (both identified as FliD) are only present in strain 670 (II). Differential expression of proteins marked as D and E (both identified as OmpA) in serum-sensitive and serum-resistant strains. Strain 670 overexpressed protein D in contrast to the protein labeled with the letter E which is present in lower amounts (III).
Table 4. Identification of OMPs from S. Enteritidis strains with different sensitivity to 75% serum.
| Spots | Identified proteins | Gene symbols | Molecular weight [kDa] | pI | Average sequence coverage [%] |
|---|---|---|---|---|---|
| A | Outer membrane protease E | pgtE | 35.033 | 5.28 | 59 |
| B | Flagellar hook-associated protein 2 | fliD | 49.805 | 5.27 | 61 |
| C | Flagellar hook-associated protein 2 | fliD | 49.805 | 5.27 | 36 |
| D | Outer membrane protein A | ompA | 37.454 | 5.6 | 46 |
| E | Outer membrane protein A | ompA | 37.454 | 5.6 | 30 |
Outer membrane protease E (PgtE, spot A, Fig 3I) was present in considerably larger quantities in two strains resistant to human serum, i.e. S. Enteritidis 27 and S. Enteritidis 851.
Flagellar hook associated protein 2 (FliD, spots B and C, Fig 3II) was present in a higher amount in S. Enteritidis 670 (strain sensitive to human serum), in contrast to the outer membrane protein A (OmpA, spot E, Fig 3III) which was present in lower amounts in terms of spot intensity in comparison with other strains. In case of OmpA and FliD two post-translational variants were present as two neighboring spots on the gel (spots B and C for FliD, spots D and E for OmpA).
Discussion
Due to its high virulence, and several features that predispose S. Enteritidis to survive and multiply in serum, it is important to explore the role of the surface structures which are involved in the process of avoiding the immune system of the host. All of the tested S. Enteritidis strains were resistant to the bactericidal activity of 50% human serum (Table 2), some of the strains presented resistance even to 75% serum (Table 3). Those findings indicate a relatively high incidence of serum-resistant strains in the population of S. Enteritidis in Poland.
LPS plays an important role in the pathogenesis of a Salmonella infection [8]. Therefore five extreme strains chosen for further experiments after 75% NHS testing were analyzed for their LPS O-specific chains profiles using SDS-PAGE analysis in order to examine the correlation of VL-OAg occurrence with the survival of Salmonella in serum. All of the tested strains showed the presence of VL-OAg LPS, which may indicate that the presence of LPS with a very long O-antigen protects a bacterial cell from the action of 50% NHS [14], but for the resistance against 75% NHS the presence of other factors is required. Serum resistance is a multifactorial phenomenon, wherein LPS plays an important role, but the presence of certain OMPs could be decisive.
There are several papers, concerning 2-DE OMP from Salmonella, mainly from S. Typhimurium strains [25–27]. However, there are no up-to-date data on the 2-DE OMPs analysis from S. Enteritidis strains apart from Cho et al. [29]. Therefore a comparative analysis of OMPs from five strains, resistant and sensitive to 75% NHS, was performed. The experiments showed, that the main difference in the OMP composition was a relatively high level of outer membrane protease E (PgtE, spot A, Fig 3I) and a low level of flagellar hook associated protein 2 (FliD, spots B and C, Fig 3II) in resistant strains: S. Enteritidis 27 and S. Enteritidis 851.
Both proteins take part in the protection of bacterial cells in response to the immunological system activity during the process of pathogenesis. Proteins PgtE from S. Typhimurium and Pla from Yersinia pestis belong to the enterobacterial outer membrane proteases family called omptins. Omptins are structural homologs possessing a very conservative configuration [22,45,46]. Salmonella is a facultative intracellular microorganism preferring the environment of macrophages, where expression of a shortened O-antigen occurs together with PgtE activation [47]. Due to the outer membrane protease E hydrolytic activity of complement components C3, C3b, C4 and C5, this protein is able to mediate in the phenomenon of serum resistance [45, 46]. The finding of our experiments, that two resistant strains have an increased number of PgtE copies in the cell could in consequence have a connection with their special ability to survive in serum.
The S. Enteritidis 670 strain differed significantly from the other four analyzed strains. It had the highest level of two variants of flagellar hook associated protein 2 (FliD, spots B and C, Fig 3II) and also of outer membrane protein A (OmpA, spot D, Fig 3III). Protein FliD is a component of bacterial flagella responsible for adhesion. It is essential for the morphogenesis and the elongation of the flagellar filament by facilitating polymerization of the flagellin monomers at the tip of the growing filament. The second protein present in this strain is OmpA—an important structural protein and a receptor for bacteriophages and colicins. Soulas et al. showed that human and mouse macrophages can be activated after contact with OmpA from K. pneumoniae. They suggested that OmpA recognition by macrophages may initiate an antimicrobial host response (42). Cho et al. analyzing the immunoreactivity of OMPs isolated from S. Enteritidis, showed that FliD and OmpA exhibited strong reactivity with serum of chickens immunized with S. Enteritidis, which can indicate that the presence of both proteins on the cell surface affects the multiplication in the serum and, consequently, the process of pathogenicity of Salmonella [29]. In the analyzed strains, two resistant ones (S. Enteritidis 27 and S. Enteritidis 851) had a high level of OmpA (spot E, Fig 3III), but a different variant than in S. Enteritidis 670 (spot D, Fig 3III). The sensitivity of S. Enteritidis 670 could be due to the presence of that OmpA variant in the cell. However, slight differences in the LPS pattern (Fig 2, lane 3) could also be noted, especially in the low molecular weight region.
In conclusion, during present studies on several strains of S. Enteritidis we have pinpointed some proteins, that could potentially affect the survival of Salmonella strains in serum. In previous results Bugla-Płoskońska et al. [24] presented variability in the survival of Salmonella O48 strains after contact with cord serum, where resistant strains exhibited wide variations in OMPs in comparison to susceptible strains. The present findings confirm, that not only the presence of specific proteins but also the presence of their post-translational variants has an influence on the pathogenicity of S. Enteritidis.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOC)
Acknowledgments
The project was financed from the National Science Center Grant No DEC-2013/11/N/NZ9/00069 and co-financed from the University of Wroclaw Grant number 2109/M/IGM/14 and 1212/M/IGM/15.
Publication supported by Wroclaw Centre of Biotechnology, programme The Leading National Research Centre (KNOW) for years 2014–2018.
The authors thank Malwina Rojek for the contribution in preliminary studies.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The project was financed from the National Science Center Grant No DEC-2013/11/N/NZ9/00069 and co-financed from the University of Wroclaw Grant number 2109/M/IGM/14 and 1212/M/IGM/15.
References
- 1.World Health Organization. Available: http://www.who.int/mediacentre/factsheets/fs139/en/
- 2.Centers for Disease Control and Prevention. Available: http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/salmonellosis-nontyphoidal
- 3.Hoelzer K, Moreno Switt AI, Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet Res. 2011;42: 34 10.1186/1297-9716-42-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fàbrega A, Vila J. Salmonella enterica Serovar Typhimurium Skills To Succeed in the Host: Virulence and Regulation. Clin Microbiol Rev. 2013;26: 308–341. 10.1128/CMR.00066-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’Brien SJ, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2010;50: 882–889. 10.1086/650733 [DOI] [PubMed] [Google Scholar]
- 6.Sadkowska-Todys M, Czarkowski MP. Salmonellosis in Poland in 2012. Przegląd Epidemiol. 2014;68: 243–248, 353–355. [PubMed] [Google Scholar]
- 7.Liu B, Knirel YA, Feng L, Perepelov AV, Senchenkova SN, Reeves PR, et al. Structural diversity in Salmonella O antigens and its genetic basis. FEMS Microbiol Rev. 2014;38: 56–89. 10.1111/1574-6976.12034 [DOI] [PubMed] [Google Scholar]
- 8.Gunn JS. Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2000;2: 907–913. 10.1016/S1286-4579(00)00392-0 [DOI] [PubMed] [Google Scholar]
- 9.Murray GL, Attridge SR, Morona R. Regulation of Salmonella Typhimurium lipopolysaccharide O antigen chain length is required for virulence; identification of FepE as a second Wzz. Mol Microbiol. 2003;47: 1395–1406. 10.1046/j.1365-2958.2003.03383.x [DOI] [PubMed] [Google Scholar]
- 10.Bengoechea JA, Najdenski H, Skurnik M. Lipopolysaccharide O antigen status of Yersinia enterocolitica O:8 is essential for virulence and absence of O antigen affects the expression of other Yersinia virulence factors. Mol Microbiol. 2004;52: 451–469. 10.1111/j.1365-2958.2004.03987.x [DOI] [PubMed] [Google Scholar]
- 11.Tomás JM, Benedí VJ, Ciurana B, Jofre J. Role of capsule and O antigen in resistance of Klebsiella pneumoniae to serum bactericidal activity. Infect Immun. 1986;54: 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bravo D, Silva C, Carter JA, Hoare A, Alvarez SA, Blondel CJ, et al. Growth-phase regulation of lipopolysaccharide O-antigen chain length influences serum resistance in serovars of Salmonella. J Med Microbiol. 2008;57: 938–946. 10.1099/jmm.0.47848-0 [DOI] [PubMed] [Google Scholar]
- 13.Parkhill J, Dougan G, James KD, Thomson NR, Pickard D, Wain J, et al. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature. 2001;413: 848–852. 10.1038/35101607 [DOI] [PubMed] [Google Scholar]
- 14.Crawford RW, Keestra AM, Winter SE, Xavier MN, Tsolis RM, Tolstikov V, et al. Very long O-antigen chains enhance fitness during Salmonella-induced colitis by increasing bile resistance. PLoS Pathog. 2012;8: e1002918 10.1371/journal.ppat.1002918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achouak W, Heulin T, Pagès JM. Multiple facets of bacterial porins. FEMS Microbiol Lett. 2001;199: 1–7. 10.1016/s0378-1097(01)00127-6 [DOI] [PubMed] [Google Scholar]
- 16.Zollinger WD, Boslego J, Frøholm LO, Ray JS, Moran EE, Brandt BL. Human bactericidal antibody response to meningococcal outer membrane protein vaccines. Antonie Van Leeuwenhoek. 1987;53: 403–411. 10.1007/bf00415494 [DOI] [PubMed] [Google Scholar]
- 17.Merino S, Nogueras MM, Aguilar A, Rubires X, Albertí S, Benedí VJ, et al. Activation of the complement classical pathway (C1q binding) by mesophilic Aeromonas hydrophila outer membrane protein. Infect Immun. 1998;66: 3825–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albertí S, Marqués G, Camprubí S, Merino S, Tomás JM, Vivanco F, et al. C1q binding and activation of the complement classical pathway by Klebsiella pneumoniae outer membrane proteins. Infect Immun. 1993;61: 852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albertí S, Marqués G, Hernández-Allés S, Rubires X, Tomás JM, Vivanco F, et al. Interaction between complement subcomponent C1q and the Klebsiella pneumoniae porin OmpK36. Infect Immun. 1996;64: 4719–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishio M, Okada N, Miki T, Haneda T, Danbara H. Identification of the outer-membrane protein PagC required for the serum resistance phenotype in Salmonella enterica serovar Choleraesuis. Microbiol Read Engl. 2005;151: 863–873. 10.1099/mic.0.27654-0 [DOI] [PubMed] [Google Scholar]
- 21.Ho DK, Jarva H, Meri S. Human complement factor H binds to outer membrane protein Rck of Salmonella. J Immunol Baltim Md 1950. 2010;185: 1763–1769. 10.4049/jimmunol.1001244 [DOI] [PubMed] [Google Scholar]
- 22.Riva R, Korhonen TK, Meri S. The outer membrane protease PgtE of Salmonella enterica interferes with the alternative complement pathway by cleaving factors B and H. Front Microbiol. 2015;6 10.3389/fmicb.2015.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pramoonjago P, Kaneko M, Kinoshita T, Ohtsubo E, Takeda J, Hong KS, et al. Role of TraT protein, an anticomplementary protein produced in Escherichia coli by R100 factor, in serum resistance. J Immunol Baltim Md 1950. 1992;148: 827–836. [PubMed] [Google Scholar]
- 24.Bugla-Płoskońska G, Korzeniowska-Kowal A, Guz-Regner K. Reptiles as a source of Salmonella O48-clinically important bacteria for children: the relationship between resistance to normal cord serum and outer membrane protein patterns. Microb Ecol. 2011;61: 41–51. 10.1007/s00248-010-9677-7 [DOI] [PubMed] [Google Scholar]
- 25.Chooneea D, Karlsson R, Encheva V, Arnold C, Appleton H, Shah H. Elucidation of the outer membrane proteome of Salmonella enterica serovar Typhimurium utilising a lipid-based protein immobilization technique. BMC Microbiol. 2010;10: 44 10.1186/1471-2180-10-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Encheva V, Wait R, Gharbia SE, Begum S, Shah HN. Proteome analysis of serovars Typhimurium and Pullorum of Salmonella enterica subspecies I. BMC Microbiol. 2005;5: 42 10.1186/1471-2180-5-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh R, Shasany AK, Aggarwal A, Sinha S, Sisodia BS, Khanuja SPS, et al. Low molecular weight proteins of outer membrane of Salmonella Typhimurium are immunogenic in Salmonella induced reactive arthritis revealed by proteomics. Clin Exp Immunol. 2007;148: 486–493. 10.1111/j.1365-2249.2007.03362.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho Y, Sun J, Han JH, Jang JH, Kang ZW, Hahn T-W. An immunoproteomic approach for characterization of the outer membrane proteins of Salmonella Gallinarum. Electrophoresis. 2014;35: 888–894. 10.1002/elps.201300199 [DOI] [PubMed] [Google Scholar]
- 29.Cho Y, Park S, Barate AK, Truong QL, Han JH, Jung C-H, et al. Proteomic analysis of outer membrane proteins in Salmonella enterica Enteritidis. J Microbiol Biotechnol. 2015;25: 288–295. 10.4014/jmb.1410.10052 [DOI] [PubMed] [Google Scholar]
- 30.Grimont PAD, Weill FX. Antigenic formulae of Salmonella serovars. WHO Collaborating Centre for Reference and Research on Salmonella. 2007 Available: http://www.scacm.org/free/Antigenic%20Formulae%20of%20the%20Salmonella%20Serovars%202007%209th%20edition.pdf
- 31.Bugla-Płoskońska G, Rybka J, Futoma-Kołoch B, Cisowska A, Gamian A, Doroszkiewicz W. Sialic acid-containing lipopolysaccharides of Salmonella O48 strains-potential role in camouflage and susceptibility to the bactericidal effect of normal human serum. Microb Ecol. 2010;59: 601–613. 10.1007/s00248-009-9600-2 [DOI] [PubMed] [Google Scholar]
- 32.Skorek K, Raczkowska A, Dudek B, Miętka K, Guz-Regner K, Pawlak A, et al. Regulatory protein OmpR influences the serum resistance of Yersinia enterocolitica O:9 by modifying the structure of the outer membrane. PloS One. 2013;8: e79525 10.1371/journal.pone.0079525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doroszkiewicz W. Mechanism of antigenic variation in Shigella flexneri bacilli. IV. Role of lipopolysaccharides and their components in the sensitivity of Shigella flexneri 1b and its Lac+ recombinant to killing action of serum. Arch Immunol Ther Exp (Warsz). 1997;45: 235–242. [PubMed] [Google Scholar]
- 34.Yi EC, Hackett M. Rapid isolation method for lipopolysaccharide and lipid A from gram-negative bacteria. The Analyst. 2000;125: 651–656. 10.1039/b000368i [DOI] [PubMed] [Google Scholar]
- 35.Darveau RP, Hancock RE. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella Typhimurium strains. J Bacteriol. 1983;155: 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227: 680–685. 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- 37.Tsai CM, Frasch CE. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119: 115–119. 10.1016/0003-2697(82)90673-x [DOI] [PubMed] [Google Scholar]
- 38.Fomsgaard A, Freudenberg MA, Galanos C. Modification of the silver staining technique to detect lipopolysaccharide in polyacrylamide gels. J Clin Microbiol. 1990;28: 2627–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy TF, Bartos LC. Surface-exposed and antigenically conserved determinants of outer membrane proteins of Branhamella catarrhalis. Infect Immun. 1989;57: 2938–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250: 4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 41.Bednarz-Misa I, Serek P, Dudek B, Pawlak A, Bugla-Płoskońska G, Gamian A. Application of zwitterionic detergent to the solubilization of Klebsiella pneumoniae outer membrane proteins for two-dimensional gel electrophoresis. J Microbiol Methods. 2014;107: 74–79. 10.1016/j.mimet.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 42.Bugla-Płoskońska G, Futoma-Kołoch B, Skwara A, Doroszkiewicz W. Use of zwitterionic type of detergent in isolation of Escherichia coli O56 outer membrane proteins improves their two-dimensional electrophoresis (2-DE). Pol J Microbiol Pol Tow Mikrobiol Pol Soc Microbiol. 2009;58: 205–209. [PubMed] [Google Scholar]
- 43.Gromova I, Celis JE. Protein detection in gels by silver staining: a procedure compatible with mass spectrometry In: Celis JE, Carter N, Hunter T, Simons K, Small JV, Shotton D, editors. Cell Biology: A Laboratory Handbook (3rd ed.), vol. 4: Elsevier Academic Press; 2006. pp. 219–223 [Google Scholar]
- 44.Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1: 2856–2860. 10.1038/nprot.2006.468 [DOI] [PubMed] [Google Scholar]
- 45.Ramu P, Tanskanen R, Holmberg M, Lähteenmäki K, Korhonen TK, Meri S. The surface protease PgtE of Salmonella enterica affects complement activity by proteolytically cleaving C3b, C4b and C5. FEBS Lett. 2007;581: 1716–1720. 10.1016/j.febslet.2007.03.049 [DOI] [PubMed] [Google Scholar]
- 46.Guina T, Yi EC, Wang H, Hackett M, Miller SI. A PhoP-regulated outer membrane protease of Salmonella enterica serovar Typhimurium promotes resistance to alpha-helical antimicrobial peptides. J Bacteriol. 2000;182: 4077–4086. 10.1128/jb.182.14.4077-4086.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lähteenmäki K, Kyllönen P, Partanen L, Korhonen TK. Antiprotease inactivation by Salmonella enterica released from infected macrophages. Cell Microbiol. 2005;7: 529–538. 10.1111/j.1462-5822.2004.00483.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.