Abstract
Background
Brazil requires the performance of both a test for hepatitis B surface antigen (HBsAg) and a test for antibodies to the core of hepatitis B for blood donor screening. Blood centres in regions of high HBV endemicity struggle to maintain adequate stocks in face of the high discard rates due to anti-HBc reactivity. We evaluated the potential infectivity of donations positive for anti-HBc in search of a rational approach for the handling of these collections.
Study Design and Methods
We tested anti-HBc reactive blood donations from the state of Amazonas for the presence of HBV DNA and for titres of anti-HBs. The study population consists of village-based donors from the interior of Amazonas state.
Results
Among 3600 donations, 799 were anti-HBc reactive (22·2%). We were able to perform real-time PCR for the HBV S gene on specimens from 291 of these donors. Eight of these samples were negative for HBsAg and positive for HBV DNA and were defined as occult B virus infections (2·7%). Six of those eight specimens had anti-HBs titres above 100 mIU/ml, indicating the concomitant presence of the virus with high antibody titres.
Conclusion
A small proportion of anti-HBc reactive donors carry HBV DNA and anti-HBs testing is not useful for predicting viremia on them. This finding indicates the possibility of HBV transmission from asymptomatic donors, especially in areas of high HBV prevalence. Sensitive HBV DNA nucleic acid testing may provide another level of safety, allowing eventual use of anti-HBc reactive units in critical situations.
Keywords: blood donors, Brazilian Amazon, HBV DNA, occult hepatitis B
Introduction
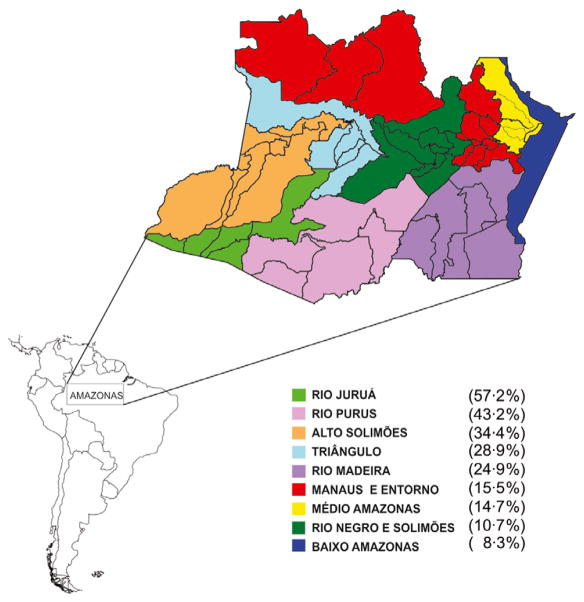
Brazilian blood system is organized by state, each having a central co-ordinating blood centre. The Amazonas state faces particular challenges due to its geographical features. It is the largest Brazilian state (1 577 820 km2) with 3 350 773 inhabitants [1]. Its 62 municipalities are subdivided into nine subregions according to the main rivers [2] (Fig. 1). The Hematology and Hemotherapy State of Amazonas Foundation (FHEMOAM) is the institution responsible for co-ordinating the hemotherapy in the state, collecting approximately 5000 donations/month. Laboratory screening of all donations is performed in a central laboratory at FHEMOAM facilities in Manaus.
Fig. 1.
Prevalence of isolated anti-HBc positive donors in subregions (excluding Manaus) of the Amazon state in the period of June 2011–June 2012. N = 799/3600 (22·2%).
A population-based survey of prevalence of hepatitis viruses in urban populations of Brazil showed a national prevalence of 0·37% for hepatitis B surface antigen (HBsAg), 7·4% for the antibody to the core antigen of hepatitis B (anti-HBc) and 76·7% for the antibody to the surface antigen of hepatitis B (anti-HBs) [3, 4]. The northern region, encompassing completely the Brazilian state of Amazonas, showed the highest prevalence of both anti-HBc (14·7%) and HBsAg (0·6%), a level of endemicity characterized as medium by the World Health Organization (WHO). Noticeably, areas of very high endemicity exist across the country on two distinct clusters: (i) small villages on the northern region [5] and (ii) cities with a strong background of Italian colonization in the states of Santa Catarina and Paraná, in the southern region [6]. In recent studies, the prevalence of HBsAg in the general population of the state of Amazonas ranged from 2 to 9%, while the prevalence of anti-HBc ranged from 5 to 76% [7–9]. The discrepancy between rates observed in the serosurvey cited above (0·6%) and the higher rates here cited may be explained by the different populations investigated. The serosurvey was conducted in state capitals and represents urban populations, while the epidemiological investigations cited were carried out in rural settings. Indeed, in some small villages, HBsAg prevalence was very high and was frequently accompanied by coinfection with the hepatitis delta virus (HDV), justifying the choice of this region to pioneer the HBV immunization programme in Brazil in 1989 [9]. Unfortunately, 46% and 4% of the vaccines are nowadays reactive for anti-HBc and HBsAg, respectively. More relevant to blood collections is the 89% prevalence of anti-HBc in persons above 20 years of age, highlighting the challenge to recruit sero-negative donors.
According to Brazilian federal law, blood centres must discard donations reactive on any of the required infectious disease screening tests, unless the risk of transmission of the infectious agent is lower than the risk of death due to lack of blood. FHEMOAM adopted in the past 15 years a different criterion for acceptance of donors from areas with high anti-HBc prevalence, taking into consideration the mentioned legal requirements and the need to ensure availability of blood. Essentially, FHEMOAM measured the titre of antibodies to the surface antigen of HBV and distributed anti-HBc reactive/HBsAg-negative donations with titres of anti-HBs above 100 mIU/ml, a policy similar to that adopted by Japan for many years [10].
Many countries with medium and high HBV endemicity such as Italy, Greece, Spain and various Asian nations chose not to test donors for anti-HBc [11]. The major risk of HBV transmission by transfusion in the absence of screening for anti-HBc stems from carriers of occult hepatitis B infection (OBI). OBI is characterized by the presence of HBV DNA in the circulation of individuals who are serologically negative for HBsAg and harbour viral loads usually below 200 IU/ml or <103 copies/ml [11]. The number of studies of prevalence of OBI in regions of high endemicity in Brazil is small. Silva et al. [12] found an OBI prevalence of 3·3% among blood donors reactive for anti-HBc in Porto Alegre in southern Brazil, while Arraes et al. found 2·7% of OBI [13] among anti-HBc reactive blood donors in Recife, a north-eastern city.
We examined the prevalence of HBV DNA among anti-HBc and anti-HBs reactive donors in the state of Amazonas, an area of medium to high HBV endemicity in Brazil. To mitigate the risk of HBV transmission associated with the use of anti-HBc reactive/HBV DNA-positive blood in the Amazonas region, we propose the screening of anti-HBc reactive units by ID-NAT for HBV as a safer alternative algorithm.
Materials and methods
Study groups
This cross-sectional study was conducted between June 2011 and June 2012 at Collection and Transfusion Units co-ordinated by FHEMOAM and included volunteer blood donors living in the interior of the state of Amazonas (Amazonas state excluding the state capital Manaus) who were negative for HBsAg, were reactive for anti-HBc and were reactive or non-reactive for anti-HBs. Donors from Manaus, the state capital, were excluded as they may have presented distinct epidemiological characteristics. The ethics committee of FHEMOAM approved the study protocol.
Anti-HBc reactive volunteer blood donors provided follow- up samples that were divided into two groups: group A (HBsAg-negative/anti-HBc reactive/anti-HBs ≥ 100 mIU/ml) and group B (HBsAg-negative/anti-HBc reactive/anti-HBs < 100 mIU/ml). The titre of anti-HBs was determined in the sample from the index donation.
Serological tests
According to regulations, all blood donations in Brazil are screened for syphilis, anti-HIV-1/2, anti-HTLV-I/II, anti-HCV, anti-Trypanosoma cruzi, anti-HBc and HBsAg. Samples found to be reactive on any of these screening tests are retested in duplicate; if both results are negative, the corresponding blood unit is released for transfusion, otherwise the unit is discarded and the donor invited to provide a follow-up sample for confirmatory testing.
Nucleic acid testing (NAT) for HIV, HCV and less frequently for HBV, is performed on a voluntary basis. Public blood banks are currently performing NAT for HIV and HCV RNA using a kit produced by Bio-Manguinhos/FIOCRUZ (Rio de Janeiro, Brazil).
During the study period, FHEMOAM utilized ELISA tests for HBsAg and anti-HBc (Murex Biotech Limited, ABBOTT, Dartford, UK) and chemiluminescence (CMIA, ARCHITECT, ABBOTT, Wiesbaden, Germany), according to the manufacturer’s recommendations. Samples found to be serologically reactive for anti-HBc in the absence of HBsAg were tested for anti-HBs using CMIA (ARCHITECT, ABBOTT).
Detection of HBV DNA
HBV DNA was extracted from 1 ml of serum using the QIAamp UltraSens Virus Kit (Qiagen, Valencia, CA, USA). DNA adequacy was evaluated by real-time PCR targeting the human β-actin gene via the SYBR Green method (GoTaq qPCR Master Mix; Promega Corporation, Madison, WI, USA) using a StepOne Plus Real-Time PCR equipment (Applied Biosystems, Warrington, UK). Cycling conditions were as follows: 10 min at 95°C to activate the Taq polymerase; 40 cycles of denaturation at 59°C for 15 s; and annealing and extension at 60°C for 60 s. Sequences of the primers for β-actin were as follows: sense (5′ TGACAAAACCTAACTTGCGC 3′) and antisense (5′ATAAAGCCATGCCAATCTCA 3′). A Ct of 33–36 is normally verified from serum samples submitted to this protocol.
Real-time PCR
A small fragment of the HBV surface antigen gene was amplified using real-time PCR with the following oligonucleotides: HBV forward primer (5′-GTGTCTGCGGCGTTTTATCAT-3′), co-ordinates 225–245; HBV reverse primer (5′-GGACAAACGGGCAACATACCT-3′), co-ordinates 303–323; and HBVPROBE: (5′FAM-ATCCTGCTGCTATGCCTC – NFQ 3′) and co-ordinates 256–273, resulting in a product of 98 bp (GenBank: af129506.1) [14]. PCRs were performed on a StepOne Plus Real-Time PCR equipment (Applied Biosystems) using an initial step of 95°C for 10 min, followed by three cycles of 95°C for 15 s, 60°C for 1 min and 45 cycles of 94°C for 15 s, and 60°C for 1 min. The 95% detection rate of this assay was established as 24 IU/ml by probit analysis with the HBV DNA WHO standard (data not shown). All samples were tested in duplicate. A standard curve allowed the estimation of viral load on HBV DNA reactive samples.
Results
FHEMOAM collected 3600 blood donations in the interior of the state of Amazonas during the study period, and 835 (23·2%) of these donors had serological markers for hepatitis B. Eight (0·9%) were HBsAg reactive, 799 (95·7%) were only reactive for anti-HBc, and 28 (3·4%) were reactive for both, anti-HBc and HBsAg. Two hundred and ninety-one donors agreed to provide a follow-up sample for serological (anti-HBs) and molecular tests (HBV DNA). They were divided into two groups according to their anti-HBs results: group A with 216 donors with anti-HBs ≥100 mIU/ml) and group B with 75 donors with anti-HBs < 100 mIU/ml. Eight samples, six from group A and two from group B, were positive for HBV DNA. The overall prevalence of occult hepatitis B among blood donors from the interior of the state of Amazonas was 2·7%.
The eight donors identified as OBI were men, with a mean age of 44·1 years; five had been previously vaccinated against HBV. Information regarding risk factors, viral load and S/CO rate of the serological tests is presented in Table 1.
Table 1.
Demographic, clinical, epidemiological and laboratory data from blood donors classified as OBIs
| Variables Identification of donor | Group A (anti-HBc+/anti-HBs+) (n = 216)
|
Group B (anti-HBc+/anti-HBs−) (n = 75)
|
||||||
|---|---|---|---|---|---|---|---|---|
| (D1) | (D2) | (D3) | (D4) | (D5) | (D6) | (D7) | (D8) | |
| Municipality | Manacapuru | Fonte Boa | Tefé | Manicoré | Apǔ́ | Coari | Humaitá | Fonte Boa |
| Sex | Ma | M | M | M | M | M | M | M |
| Age | 41 | 33 | 51 | 50 | 37 | 42 | 63 | 36 |
| First donation | No | No | No | No | No | No | Yes | No |
| Marital status | Married | Married | Married | Married | Stabled | Married | Stabled | Stabled |
| Health care worker | No | No | No | No | No | Yes | No | No |
| Received blood transfusion | No | No | No | No | No | No | No | No |
| Past hepatitis | No | No | No | No | No | No | No | No |
| Family history HBV | No | No | No | No | No | No | No | No |
| Partner had hepatitis | No | No | No | No | No | No | Unknown | No |
| Mother had hepatitis | No | No | Unknown | No | No | No | No | No |
| Use injectable drugs | No | No | No | No | No | No | No | Unknown |
| Past surgery | No | No | No | No | No | No | Yes | No |
| Hep B vaccine | No | No | Yes | Yes | Yes | Yes | Unknown | Yes |
| Has a tattoo or piercing | No | No | No | Yes | No | No | No | No |
| Sharing of manicure/shaving equipment | No | No | No | No | No | No | Yes | No |
| Did acupuncture | No | No | No | No | No | No | No | No |
| Number of sexual partners in the last 12 months | 2 | 1 | 1 | 1 | 0 | 1 | 3 | 0 |
| Use of alcoholic beverages | No | Yes | Yes | No | No | No | Yes | Yes |
| Anti-HBc positive (S/CO) | 0·059b | 8·87c | 5·480c | 6·13c | 8·19c | 0·080b | 4·96c | 10·99c |
| Anti-HBs mIU/ml | >1000 | 608·79 | 281·53 | 284·09 | >1000 | >1000 | 33·94 | 0·05 |
| HBV DNA IU/ml | 4·5 | 3·5 | 29·0 | 6751 | 21·5 | 6·5 | 9·6 | 3·6 |
M, Male.
ELISA (competitive assay Murex, S/CO values <1 are considered reactive).
Chemiluminescence.
Living together in a monogamous relationship.
Discussion
The Brazilian blood system suffers from a chronic shortage of blood components. Several reasons contribute to this, among them are the high rate of first-time donors and the discard rate for infectious markers, being anti-HBc the most prevalent. While in some areas of the country, these factors are well managed; in the HBV endemic regions, an important amount of resources are wasted by collecting blood from first-time anti-HBc reactive donors. The problem is more prominent in the state of Amazonas interior. This area is scarcely populated, but has blood needs that FHEMOAM has to fulfil. Until recently, the risk of transmission of HBV by transfusion was lower than the risk of death associated with the lack of blood, justifying the decision to transfuse anti-HBc reactive blood with anti-HBs titres above 100 mIU/ml. This procedure is controversial because the presence of anti-HBs does not assure the absence of HBV DNA. The transfusion risk is particularly important for immunocompromised recipients, who may acquire HBV even by donations with anti-HBs that contain low levels of HBV DNA [15]. Currently, FHEMOAM discards all collected units that are anti-HBc reactive.
The relationship between infectivity and HBV viral load may be related to several factors, such as the immune status of the donor and recipient; stage of infection; volume of blood products transfused; and the presence of neutralizing antibodies against HBV [15]. In our study, no relationship was found between anti-HBs titres and HBV viral load.
Although the Amazon region is endemic for hepatitis B and the epidemiological scenario is favourable towards the emergence of OBI, no other study to date has evaluated the risk of HBV transmission by OBI in blood banks in the Amazon. The current study showed eight cases of HBV DNA presence from 291 anti-HBc reactive blood donors. Among these, six donors had anti-HBs titres ≥ 100 mIU/l. OBI was more present in the group displaying anti-HBs reactivity, a prevalence of 2·1% (6/291), revealing that the policy adopted for the state of Amazonas interior did not guarantee the safety of blood transfusion. However, the lack of documented cases of HBV transmission associated with blood components sourced from donors with anti-HBs levels exceeding 100 IU/l indicates a negligible risk associated with this policy [10, 15]. To date, the highest anti-HBs level in a donor implicated in an HBV transmission is 29·6 IU/l [16]. A look back study could potentially confirm cases of HBV acquired from blood originated from OBIs in the region, but may be blurred by the high anti-HBc prevalence in the general population, including recipients.
The prevalence of OBI among anti-HBc reactive blood donors in this study (2·7%) proved to be below that observed in other regions where hepatitis B is endemic. In Ganjam, India, the prevalence of anti-HBc is approximately 30%, while OBI represents 30% of those anti-HBc positives or 9% of the overall blood donor population [17]. However, this population is considered of high risk for HIV, not representing the situation for most parts of India. For example, a rate of OBI of 1·1% was described in southern India [18]. The rate for Amazonas resembles those found in other regions with similarly moderate prevalence, such as the Egypt with an OBI rate of 1·26% [19] and the two Brazilian state capitals, Porto Alegre (3·3%) and Recife (2·7%) above cited, meaning that, in despite of a much higher anti-HBc prevalence, the Amazonas OBI rate is similar, suggesting a higher viral clearance in these Amazon blood donors.
It was observed in this study that among donors identified as having OBI, five were previously immunized against hepatitis B virus, and there was no report of exposure to the classical risk factors for infection. The HBV vaccine is known to be effective and is part of Brazil’s National Immunization Programme. However, in the first area in Brazil that started the immunization programme more than 20 years ago, the county of Lábrea in the Amazon, the current prevalence of anti-HBc is 19·8% in children aged 5–9 years and 38·9% among adolescents aged between 10 and 14 years [9]. These data clearly indicate either that the immunization coverage is lower than required and/or that the previously vaccinated now HBsAg carriers represent vaccine breakthroughs. In the US, among nine HBV NAT yield cases detected, six were immunized blood donors, and five of them harboured non-A2 HBV genotypes [20], suggesting vaccine escape may be caused by partial immune protection in persons challenged by HBV genotypes differing from the A2 prototype used in the recombinant HBV vaccine.
The prevailing HBV genotypes in Brazil are A, D and F [21]. Genotype F is thought to be originally from Amerindian populations [22] and, as expected, is the most prevalent in the Amerindian population in the Amazon [23]. It is tempting to speculate that the high rate of anti-HBc and HBsAg observed among vaccines in the Amazon studies cited and among OBIs here described that were also vaccinated, may be attributed to sequence variability in the immunodominant region of the envelope antigen between A and F genotypes, eliciting an immune response unable to fully protect against HBV genotype F strains, what has been observed by Tacke et al. [24]. Unfortunately, we were unable to genotype the OBIs here described, due to very low viremia, but would be important to know whether this group of OBI donors is enriched for F isolates.
Our data demonstrate that using anti-HBs levels as a criterion for releasing anti-HBc reactive units for transfusion has limitations given that we detected HBV DNA in several donors with anti-HBs levels exceeding 100 IU/l – our assumed safe threshold. As noted, there is currently an absence of evidence from look-back studies that anti-HBc reactive units with anti-HBs > 100 IU/l and detectable DNA lead to HBV transmission. However, given the potential infectivity of HBV DNA-positive units, we believe that it would improve safety if these units were identified and removed. Our proposal would be to perform highly sensitive ID-NAT for HBV DNA on repeat donors displaying isolated anti-HBc reactivity. Individuals with this pattern (isolated anti-HBc) comprise an important fraction of the regular blood donors living in the Amazonas interior. Although FHEMOAM policy has changed recently to reject all anti-HBc reactive units, the suggested algorithm may prove safe and cost-effective in some settings of very high HBV endemicity, as the Amazonas state rural area but also in other regions of Brazil with similar prevalence. Moreover, it may guarantee the availability of the blood supply in those areas with logistic complexity and chronic lack of blood. In Brazil, NAT for HBV is currently not widely adopted, its use being limited to certain private blood banks [25]. Brazilian government is currently implementing NAT screening for hepatitis C and HIV in public blood banks by use of a locally produced kit. Priority was given to HCV and HIV, while there is substantial data suggesting that the residual risk for HBV transfusional transmission is considerably higher than for HCV and HIV [25]. Although South Africa does not include anti-HBc testing in their blood screening, after 4 years of ID-NAT introduction, only one HBV transfusion-transmitted case was verified, corresponding to approximately 3 million donations, which highlights the negligible risk of HBV DNA ID-NAT-negative donations [26]. Including HBV DNA as a target will surely provide a high yield of window period cases while allowing the evaluation of the above-proposed algorithm in some regions of Brazil.
Acknowledgments
We acknowledge the Fundação de Hematologia e Hemoterapia do Amazonas (FHEMOAM) for providing the logistics, as well as the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM, D.005-2009) for financial support. We thank Dr Celso Bianco (ISBT) for kindly reviewing this manuscript.
Source of funding
Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM D.005-2009).
Footnotes
Authors contribution
Mônica Nascimento dos Santos Moresco designed, gathered, analysed the data and also wrote the manuscript. Helaine de Araújo Virgolino, Márcia Poinho Encarnação de Morais, Isabella da Motta-Passos, Michele S. Gomes-Gouvêa, Lya Manoele Soares de Assis, Kelly Raphaelle de Luna Aguiar and Suzete Cleusa Ferreira Lombardi gathered and analysed the data. Adriana Malheiro, Norma de Paula Cavalheiro, José Eduardo Levi and Kátia Luz Torres designed the study, reviewed the data and had a major role in writing the paper.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.IBGE - Instituto Brasileiro de Geografia e Estatística. [Last accessed 27 August 2012];Dados do Censo 2010 publicados no Diário Oficial da União. Available at: http://www.censo2010.ibge.gov.br.
- 2.SUSAM - Secretaria de Estado de Saúde do Amazonas. [Last accessed 15 March 2010];Mapa das regionais de saúde do Amazonas. 2010 Available at: http://www.saude.am.gov.br/
- 3.Pereira LMMB, Ximenes RAA, Moreira RC, et al. Estudo de Prevalência de Base Populacional das Infecções pelos vírus das Hepatites A. B e C nas Capitais do Brasil: Ministério da Saúde; 2010. [Last accessed 14 February 2013]. Available at: http://www.aids.gov.br/publicacao/2010/estudo_de_prevalencia_de_base_populacional_das_infeccoes_pelos_virus_das_hepatites_b. [Google Scholar]
- 4.de Ximenes RAA, Pereira LMB, Martelli CMT, et al. Methodology of a nationwide cross-sectional survey of prevalence and epidemiological patterns of hepatitis A, B and C infection in Brazil. Cad Saúde Pública. 2010;26:1693–1704. doi: 10.1590/s0102-311x2010000900003. [DOI] [PubMed] [Google Scholar]
- 5.da Castilho MC, Oliveira CM, Gimaque JB, et al. Epidemiology and molecular characterization of hepatitis B virus infection in isolated villages in the Western Brazilian Amazon. Am J Trop Med Hyg. 2012;87:768–774. doi: 10.4269/ajtmh.2012.12-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertolini DA, Gomes-Gouvêa MS, Guedes de Carvalho-Mello IM, et al. Hepatitis B virus genotypes from European origin explains the high endemicity found in some areas from southern Brazil. Infect Genet Evol. 2012;2:1295–1304. doi: 10.1016/j.meegid.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Braga WS, Silva EB, Souza RA, et al. Seroprevalence of hepatitis B virus and malaria infection in Lábrea, Brazilian western Amazon: estimates of coinfection rates. Rev Soc Bras Med Trop. 2005;38:218–223. doi: 10.1590/s0037-86822005000300002. [DOI] [PubMed] [Google Scholar]
- 8.Ei Khouri M, Cordeiro Q, Luz DA, et al. Endemic hepatitis B and C virus infection in a Brazilian eastern Amazon region. Arq Gastroenterol. 2010;47:35–41. doi: 10.1590/s0004-28032010000100007. [DOI] [PubMed] [Google Scholar]
- 9.Braga WS, da Castilho MC, Borges FG, et al. Prevalence of hepatitis B virus infection and carriage after nineteen years of vaccination program in the Western Brazilian Amazon. Rev Soc Bras Med Trop. 2012;45:13–17. doi: 10.1590/s0037-86822012000100004. [DOI] [PubMed] [Google Scholar]
- 10.Taira R, Satake M, Momose S, et al. Residual risk of transfusion-transmitted hepatitis B virus (HBV) infection caused by blood components derived from donors with occult HBV infection in Japan. Transfusion. 2013;53:1393–1404. doi: 10.1111/j.1537-2995.2012.03909.x. [DOI] [PubMed] [Google Scholar]
- 11.Candotti D, Allain JP. Transfusion-transmitted hepatitis B virus infection. J Hepatol. 2009;51:798–809. doi: 10.1016/j.jhep.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Silva CM, Costi C, Costa C, et al. Low rate of occult hepatitis B virus infection among anti-HBc positive blood donors living in a low prevalence region in Brazil. J Infection. 2005;51:24–29. doi: 10.1016/j.jinf.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Arraes LC, Ximenes R, Andrieu JM, et al. The biological meaning of anti-HBc positive result in blood donors: relation to HBV-DNA and to other serological markers. Rev Inst Med Trop São Paulo. 2003;46:137–140. doi: 10.1590/s0036-46652003000300004. [DOI] [PubMed] [Google Scholar]
- 14.Garson JA, Grant PR, Ayliffe U, et al. Real-time PCR quantitation of hepatitis B virus DNA using automated sample preparation and murine cytomegalo-virus internal control. J Virol Methods. 2005;126:207–213. doi: 10.1016/j.jviromet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Allain JP, Mihaljevic I, Gonzalez-Fraile MI, et al. Infectivity of blood products from donors with occult hepatitis B virus infection. Transfusion. 2013;53:1405–1415. doi: 10.1111/trf.12096. [DOI] [PubMed] [Google Scholar]
- 16.Hanada D, Kino S, Yamauchi S, et al. Transfusion-transmission of hepatitis B virus (HBV) from a NAT-negative occult hepatitis B virus carrier. Vox Sang. 2011;101(Suppl s2):129–134. P-188. [Google Scholar]
- 17.Panigrahi R, Biswas A, Datta S, et al. Anti-hepatitis B core antigen testing with detection and characterization of occult hepatitis B virus by an in-house nucleic acid testing among blood donors in Behrampur, Ganjam, Orissa in southeastern India: implications for transfusion. Virol J. 2010;7:204. doi: 10.1186/1743-422X-7-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ismail AM, Devakumar S, Anantharam R, et al. Low frequency of occult hepatitis B infection in anti-HBc seropositive blood donors: experience from a tertiary care centre in South India. Blood Transfus. 2012;10:230–232. doi: 10.2450/2011.0046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Zayadi AR, Ibrahim EH, Badran HM, et al. Anti-HBc screening in Egyptian blood donors reduces the risk of hepatitis B virus transmission. Trans Med. 2008;18:55–61. doi: 10.1111/j.1365-3148.2007.00806.x. [DOI] [PubMed] [Google Scholar]
- 20.Stramer SL, Wend U, Candotti D, et al. Nucleic acid testing to detect HBV infection in blood donors. N Engl J Med. 2011;36:236–247. doi: 10.1056/NEJMoa1007644. [DOI] [PubMed] [Google Scholar]
- 21.Mello FC, Souto FJ, Nabuco LC, et al. Hepatitis B virus genotypes circulating in Brazil: molecular characterization of genotype F isolates. BMC Microbiol. 2007;7:103. doi: 10.1186/1471-2180-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres C, Piñeiro y Leone FG, Pezzano SC, et al. New perspectives on the evolutionary history of hepatitis B virus genotype F. Mol Phylogenet Evol. 2011;59:114–122. doi: 10.1016/j.ympev.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Devesa M, Loureiro CL, Rivas Y, et al. Subgenotype diversity of hepatitis B virus American genotype F in Amerindians from Venezuela and the general population of Colombia. J Med Virol. 2008;80:20–26. doi: 10.1002/jmv.21024. [DOI] [PubMed] [Google Scholar]
- 24.Tacke F, Amini-Bavil-Olyaee S, Heim A, et al. Acute hepatitis B virus infection by genotype F despite successful vaccination in an immune-competent German patient. J Clin Virol. 2007;38:353–357. doi: 10.1016/j.jcv.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Levi JE, D’Almeida RAP, Polite MBC, et al. One window-period donation in two years of ID-NAT screening for HBV, HCV and HIV. Rev Bras Hematol Hemoter. 2013;35:167–170. doi: 10.5581/1516-8484.20130039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermeulen M, Lelie N, Sykes W, et al. Impact of individual-donation nucleic acid testing on risk of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission by blood transfusion in South Africa. Transfusion. 2009;49:1115–1125. doi: 10.1111/j.1537-2995.2009.02110.x. [DOI] [PubMed] [Google Scholar]