Abstract
The bacterial communities played important roles in the high productivity mangrove ecosystem. In this study, we investigated the vertical distributions of rhizosphere bacteria from three mangrove species (Bruguiera gymnorrhiza, Kandelia candel and Aegiceras corniculatum) in Beilun Estuary, China using high throughput DNA pyrosequencing of the 16S rRNA gene. Phylogenetic analysis showed that bacterial communities from mangrove rhizosphere sediments were dominated by Proteobacteria (mostly Deltaproteobacteria and Gammaproteobacteria), followed by Chloroflexi, Bacteroidetes, Planctomycetes and Acidobacteria. However, the ANOVA analysis on Shannon and Chao1 indices indicated that bacterial communities among sediments of the three mangrove species varied more strongly than the sampling depths. In addition, the PCA result demonstrated that the bacterial communities could be separated into three groups according to the mangrove species. Moreover, the dominated orders Rhodospirillales, GCA004 and envOPS12 were significantly different among sediments of the three mangrove species. The results of this study provided valuable information about the distribution feature of rhizosphere bacteria from Chinese mangrove plants and shed insights into biogeochemical transformations driven by bacteria in rhizosphere sediments.
Introduction
Mangroves are unique intertidal ecosystems in tropical and subtropical regions, where they play an essential roles in providing nursery habitats for aquatic animals, degrading contaminants and protecting the coast [1, 2]. Adapted to intertidal zones, they are subjected to highly variable physicochemical conditions of salinity, flooding, light, temperature and nutrient, which give rise to the high bacterial diversity that characterizes mangrove ecosystems [3, 4]. The diverse microbial communities can continuously transform nutrients from dead mangrove vegetation into sources of nitrogen, phosphorus and other nutrients which can be used by mangrove trees [5]. As a result, bacteria are important to the productivity, conservation and rehabilitation of mangrove ecosystems [5].
Mangrove trees can oxidize the sediment by supplying oxygen to the anaerobic subsediment through their aerial roots [6]. In addition, mangrove root exudates can serve as a nutrient source for bacteria [5]. These changes induced by the trees could influence the proliferation of certain groups of bacteria in the rhizosphere [5, 7]. In terrestrial environments, previous studies confirmed that bacterial communities in rhizosphere were influenced by plant species, and even that the rhizosphere with respect to plant species richness and community size [8–10]. However, in mangrove, bacterial compositions in rhizospheres of different tree species are not well known.
Most of the previous studies focused on the bacterial distribution from mangrove surface sediment in the horizontal direction [11–14]. In addition, seasonal changes of bacterial community in mangrove were also discussed in previous studies [14–16]. The vertical distribution of bacterial composition was fully investigated in terrestrial ecosystem. For example, Yu and Steinberger [17] found vertical changes of soil microbial community under the canopies of Zygophyllumdumosum and Hammadascoparia. Lee et al. [18] also showed that bacterial compositions from rhizosphere soils of a flooded rice paddy differed along a depth gradient and suggested that the oxygen concentration might be a determining factor. But, vertical profiles of bacterial structure in mangrove are still poorly understood.
The aim of the present study was to compare the vertical profiles of bacteria in the rhizospheres of three mangrove tree species (Bruguiera gymnorrhiza, Kandelia candel and Aegiceras corniculatum). In order to reduce possible anthropogenic effects, a pristine site in Beilun Estuary National Nature Reserve (a member of Man and Biosphere Programme, UNESO) was used as the study site, which constructed to protect mangrove located in Guangxi province, China. To thoroughly investigate the bacterial community, a barcoded pyrosequencing analysis of 16S rRNA gene was employed to understand bacterial communities in the mangrove rhizosphere from Beilun Estuary, China.
Materials and Methods
Ethics statement
Beilun Estuary National Nature Reserve approved this study development. The field studies did not involve endangered or protected species.
Study area and sediment sampling
Sediment samples were collected on September 28, 2015 from Beilun Estuary National Nature Reserve, which was located in South China (21°31'00" N-21°37'30" N, 108°00'30" E-108°16'30" E) with an area of 3000 hm2. This reserve contained 15 species of mangrove trees belong to 11 families. In this study, three species of mangrove trees were investigated, including Bruguiera gymnorrhiza (Bru), Kandelia candel (Kan) and Aegiceras corniculatum (Aeg) which were the common and dominant species in this reserve. Three plants of each species (about 1.5 m tall) were selected within distance of 1 to 10 m from each other. For each individual plant, the rhizosphere sediments were sampled vertically along the base of the plant. Then, the rhizosphere sediments corresponding to 0 (surface), 10 and 20 cm depths were collected. Finally, samples of each species in triplicate from each depth were mixed to homogeneity to generate a representative composite sample for further analysis. Samples were kept in sterile plastic bags, maintained in an ice box for transporting to the laboratory, and stored at -20°C for DNA extraction.
Total community DNA extraction and Illumina HiSeq sequencing
Total genomic DNA was extracted directly from 1.0 g of the sample using FastDNA®spin kit (MP bio, Santa Ana, USA) following the manufacturer’s protocol. The bacterial community was analyzed using Illumina HiSeq sequencing of 16S rRNA gene amplicons. PCR amplifications were conducted in triplicate with the primer set 515F (5’-GTGCCAGCAGCCGCGGTAA-3’) and 907R (5’-CCGTCAATTCCTTTGAGTTT-3’) that amplified the V4-V5 region of the 16S rRNA gene. The reverse primer contained a 6-bp error-correcting barcode unique to each sample. DNA was amplified following the protocol described previously [19]. Pyrosequencing was performed on the Illumina HiSeq platforms at Novogene Bioinformatics Technology Co., Ltd, Beijing, China.
Pairs of reads from the original DNA fragments were merged by using FLASH [20]. Sequencing reads were assigned to each sample according to the individual unique barcode. QIIME [21] and UPARSE [22] were used to analyze the sequencing reads and pick operational taxonomic units (OTUs). Sequences were assigned to OTUs at 97% similarity. For each OTU, a representative sequence was selected and used to assign taxonomic composition by using the RDP classifier [23]. In case the read numbers influence the statistical diversity indices, especially for the Chao1 and Shannon indices, the numbers of the bacterial reads in each sample were normalized to 43,632 reads. Finally, Shannon index, Chao1 index and Good’s coverage for the nine samples with the normalized sequencing reads were determined as described previously [24].
Statistical analysis
The significant differences of bacterial composition were analyzed by one-way ANOVA using SPSS 22.0 software package (p<0.05). Principle component analysis (PCA) was applied to compare the bacterial communities among all the samples in R software (version 3.2.3). Moreover, the representative sequences of the most dominant OTUs (>20 sequences) were determined using ternary diagrams to find out the differences of bacterial community from sediments of the three mangrove species. All sequences obtained from this study were deposited in NCBI sequence read archive (SRA) under accession number SRP081285.
Results
Sequencing statistics and diversity estimates
A total of 556,274 qualified pyrosequencing reads were obtained from the nine rhizosphere samples. The numbers of qualified reads per sample ranged from 52,197 (Bru-0) to 68,687 (Kan-0) with a mean of 61,808 (Table 1). The rarefaction curves obtained with the normalized OTUs number nearly reached saturation level for all the samples, which demonstrated bacterial communities from the rhizospheres were well covered by pyrosequencing (S1 Fig). Using 3% sequence cutoff value, the OTUs number ranged from 4,589 to 5,011 across all samples, with A. corniculatum harboring the least number of OTUs among sediments of the three mangrove species (Table 1). The average of Shannon index of bacterial diversity from rhizosphere sediments of B. gymnorrhiza was higher than K. candel or A. corniculatum (Table 1). However, the Chao1 index varied from 5,179 to 5,811, with an average of 5,468 (Table 1). The coverage of each sample was almost similar (from 96.9% to 97.6%), which was consistent with the demonstration of the rarefaction curve (Table 1 and S1 Fig).
Table 1. Sequencing information and diversity estimates for rhizosphere samples collected from three mangrove tree species (B. gymnorrhiza, K. candel and A. corniculatum) obtained by pyrosequencing.
| Samples | No. of qualified reads | OTUs | Shannon | Chao1 | Coverage (%) |
|---|---|---|---|---|---|
| Bru-0 | 52197 | 4621 | 10.38 | 5413 | 97.6 |
| Bru-10 | 66732 | 4953 | 10.34 | 5698 | 97.2 |
| Bru-20 | 68097 | 4928 | 10.26 | 5587 | 97.1 |
| Kan-0 | 68687 | 5011 | 10.33 | 5811 | 96.9 |
| Kan-10 | 67031 | 4866 | 10.17 | 5534 | 97.1 |
| Kan-20 | 63275 | 4609 | 10.17 | 5269 | 97.4 |
| Aeg-0 | 54541 | 4589 | 10.21 | 5179 | 97.6 |
| Aeg-10 | 55325 | 4632 | 10.23 | 5275 | 97.5 |
| Aeg-20 | 60389 | 4733 | 10.24 | 5446 | 97.3 |
Bacterial community structure from different rhizosphere sediments
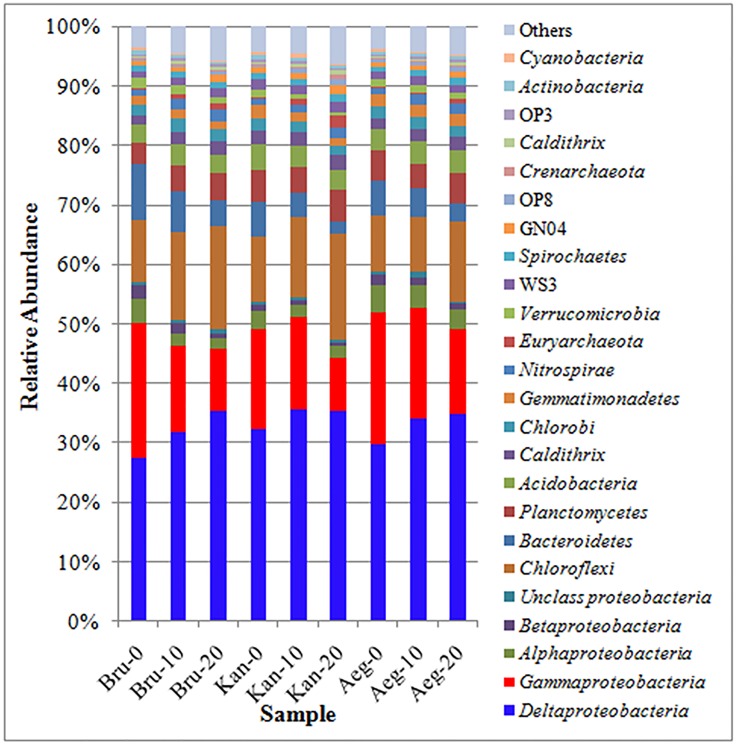
Relative abundance analysis showed that the top 20 phyla of bacteria from each sample accounted for over 90% of the total amplicons (Fig 1). Proteobacteria was the most dominant phylum covering 47.2–58.9% of the total amplicons which detected in all the nine samples. Chloroflexi was the second major phylum observed in this study, followed by Bacteroidetes, Planctomycetes and Acidobacteria. Interestingly, the relative abundance of Chloroflexi (9.1–17.2%) from each mangrove species increased with sampling depth, while Bacteroidetes (3.0–9.4%) decreased with sampling depth. However, the relative abundance of Planctomycetes (3.7–5.5%) and Acidobacteria (3.0–4.2%) showed a little difference between each sample (Fig 1). Besides, phyla Euryarchaeota and Crenarchaeota which belong to kingdom Archaea were found in all samples. In Proteobacteria, the Deltaproteobacteria was the largest class (27.4–35.6% of all amplicons), followed by Gammaproteobacteria (8.9–22.6%) and Alphaproteobacteria (1.8–4.4%). Further, the relative abundance of Gammaproteobacteria apparently decreased with sampling depth. Betaproteobacteria (0.4–2.1%) and unclassified Proteobacteria (0.4–1.2%) were also found in different samples but they accounted for only small portions (Fig 1).
Fig 1. Relative abundance of the dominant bacterial phyla and proteobacterial classes identified through pyrosequencing targeting the 16S rRNA gene.
The effect of mangrove species or sampling depth on bacterial community from rhizosphere sediment
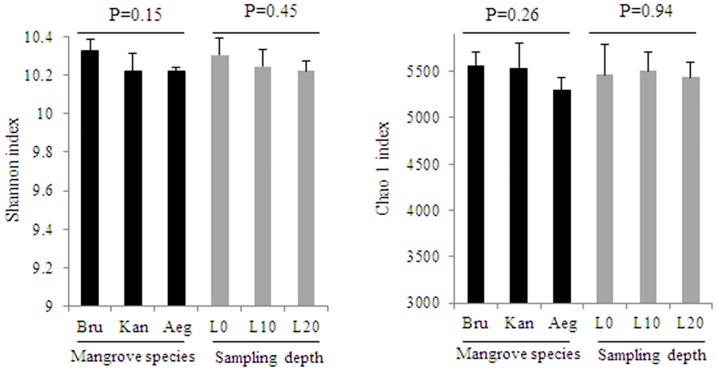
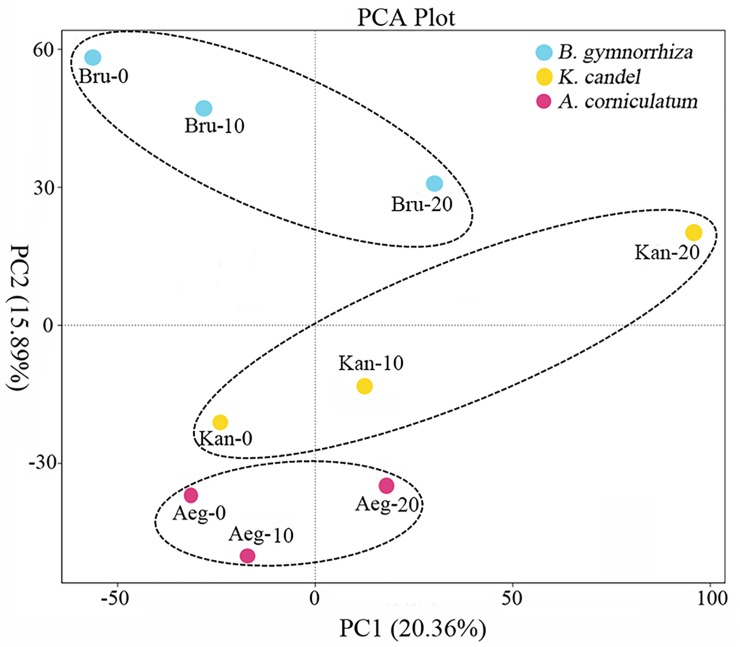
One-way analysis of variance (ANOVA) analysis on Shannon and Chao1 indices showed bacterial communities among sediments of the three mangrove species varied more strongly than the sampling depths (Fig 2). Furthermore, to reduce the number of variables of the data and maintain as much variance as possible, PCA was used to compare bacterial communities between mangrove species and sampling depths. The PCA result also confirmed that the bacterial communities could be divided into three groups corresponding with the mangrove species. In contrast, the bacterial communities were not clearly differentiated along a depth gradient (Fig 3).
Fig 2. The analysis of statistical significant differences about Shannon and Chao1 indices for mangrove species and sampling depths (p<0.05).
Fig 3. Principal coordinates analysis of bacterial communities from rhizosphere sediments.
Comparison of bacterial composition among sediments of the three mangrove species
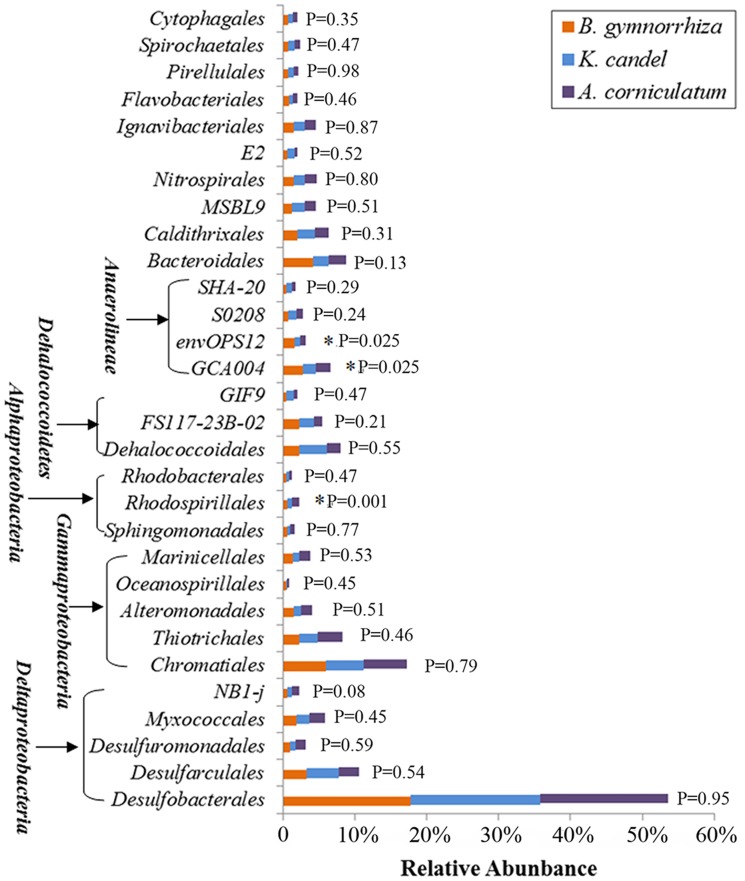
The effect of mangrove tree species on bacterial distribution was further investigated in this study on the order level (Fig 4). Of these dominant orders, Rhodospirillales, and the candidate divisions (GCA004 and envOPS12) were significantly different among sediments of the three mangrove species (p<0.05). Rhodospirillales was highly abundant in rhizosphere sediment from A. corniculatum, while GCA004 and envOPS12 dominated in B. gymnorrhiza. In addition, Bacteroidales and NB1-j showed an obvious difference with the mangrove species (Fig 4). But, Desulfobacterales and Chromatiales, the first two dominant orders of all the investigated mangrove species, were almost invariable which accounted for 17.7–18.0% and 5.2–6.1% of total bacterial sequences, respectively (Fig 4).
Fig 4. Relative abundance of the 30 most dominant bacterial groups on the order level.
Symbols (*) represent significant differences among sediments of the three mangrove species (p<0.05).
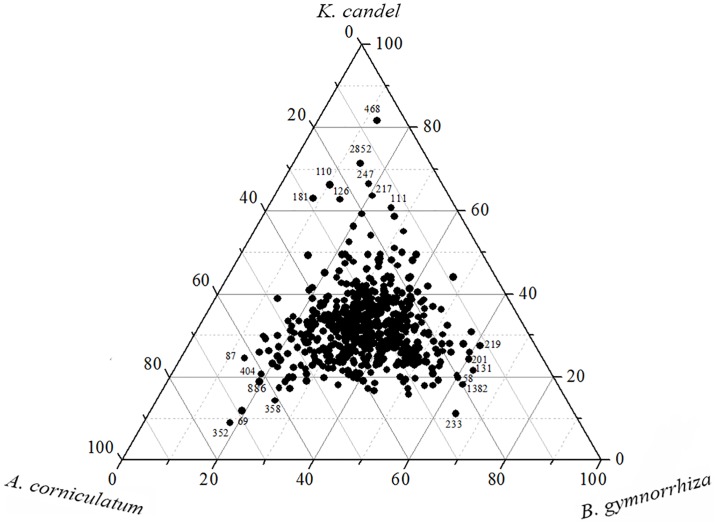
In term of OTUs (3% sequence cutoff value), Venn diagram were plotted to compare bacterial compositions from sediments of the three mangrove species. In rhizosphere sediments, 5,001 OTUs were shared by all the three mangrove species. On the other hand, 776 OTUs were only detected from the rhizosphere of B. gymnorrhiza. But, only 608 and 410 OTUs were only detected from the rhizospheres of K. candel and A. corniculatum, respectively (S2 Fig). The ternary plots of dominant OTUs revealed that different mangrove species contained special OTUs (Fig 5). Six OTUs (58, 131, 201, 219, 233 and 1382) were more prevalent in rhizosphere of B. gymnorrhiza. However, OTUs (110, 111, 126,181, 217, 247, 468 and 2852) were abundant in the rhizosphere of K. candel, while OTUs (69, 87, 352, 358, 404 and 886) dominated in A. corniculatum (Fig 5). From these OTUs, most were similar to 16S rRNA gene sequences reported from uncultured bacteria present in the sediment of marine, estuary or mangrove environment (Table 2). The percentage similarity of the analyzed OTUs with their closest blast hits ranged from 95% to 100%, respectively. What’s more, some OTUs from sediments of the three mangrove species were closely related to bacteria having the capacity to degrade organic pollutants (OTUs 219, 233, 352 and 886) or to recycle nutrients (OTUs 69, 111, 247, 358 and 1382) (Table 2).
Fig 5. Ternary plots showing the ratios of the OTUs (≥20 reads) about sediments of the three mangrove species (B. gymnorrhiza, K. candel and A. corniculatum).
Table 2. Taxonomic assignment of partial OTUs obtained from sediments of the three mangrove species and their closely related sequence identified using BLAST research.
| OTU | Habitat | Database match with accession number in parentheses | Similarity | Origin or known traits |
|---|---|---|---|---|
| 58 | Bru | uncultured Bacteroidetes bacterium (GQ979660) | 97% | an estuary surface sediment |
| 131 | Bru | Uncultured epsilon Proteobacterium (DQ394936) | 99% | the sediment from Victoria Harbourin Hong Kong |
| 201 | Bru | Uncultured Bacteroidetes bacterium (AY822241) | 95% | organically-enriched fish farm sediments |
| 219 | Bru | Desulfatiferula berrensis (NR133737) | 98% | estuarine sediment/a n-alkene-degrading bacterium |
| 233 | Bru | Uncultured Hydrogenophilaceae bacterium (EU266782) | 100% | a tar oil contaminant plume/anaerobic toluene degraders |
| 1382 | Bru | Uncultured Cytophagales bacterium (KC009968) | 97% | the French Guiana coast/carbon dioxide fixing bacteria |
| 110 | Kan | Uncultured Deltaproteobacterium (DQ811827) | 99% | the mangrove sediment |
| 111 | Kan | Uncultured Deltaproteobacterium (LC071293) | 99% | the subsea floor off Hatsushima Island/anaerobic methane oxidization |
| 126 | Kan | Uncultured bacterium (FJ936722) | 98% | volcano mud taken at Avachinsky (Kamtchatka) |
| 181 | Kan | Uncultured Chloroflexi bacterium (FJ902002) | 98% | biomat in the sediment of cenote La Palita |
| 217 | Kan | uncultured archaeon (JX870284) | 99% | the surface sediment of South China Sea |
| 247 | Kan | Uncultured Chloroflexi bacterium (LC070795) | 95% | the subsea floor off Hatsushima Island/anaerobic methane oxidization |
| 468 | Kan | Uncultured Gammaproteobacterium (AB826802) | 95% | the hadopelagic sediments in the Ogasawara Trench |
| 2852 | Kan | Uncultured Deltaproteobacterium (AB433126) | 98% | deep subseafloor sediments at the Brazos-Trinity Basin, Mexico |
| 69 | Aeg | Desulfatitalea tepidiphila (AB719404) | 98% | the tidal flat sediment/ a sulfate-reducing bacterium |
| 87 | Aeg | Uncultured bacterium (GQ246446) | 99% | the North Yellow Sea sediments, China |
| 352 | Aeg | Marinilabiliaceae bacterium Q15 (KR809872) | 100% | degrading different types of hydrocarbons |
| 358 | Aeg | Malonomonas rubra GraMal1 (NR026479) | 99% | sulfur-reducing bacteria |
| 404 | Aeg | Uncultured Deltaproteobacterium (DQ112385) | 99% | the intertidal mudflat sediment from Ganghwa Island, Korea |
| 886 | Aeg | Desulfuromonas michiganensis BB1 (NR114607) | 98% | the freshwater sediment/tetrachloroethene-reducing anaerobic bacteria |
Discussion
In the present study, bacterial communities of different rhizosphere sediments from three mangrove tree species (B. gymnorrhiza, K. candel and A. corniculatum) were examined using high throughput DNA pyrosequencing of the 16S rRNA gene. However, bacterial composition from rhizosphere sediments mainly focused on the mangrove tree species, such as Rhizophora mangle, Avicennia schaueriana, Laguncularia racemosa and Avicennia marina [12, 15, 16, 25, 26]. Unfortunately, the discussion of bacteria from B. gymnorrhiza, K. candel or A. Corniculatum is lacking, while the three mangrove species are common in Guangxi Province of China.
Bacterial community structures from rhizosphere sediments of B. gymnorrhiza, K. candel and A. corniculatum
In general, proteobacteria (mostly Deltaproteobacteria and Gammaproteobacteria) was found to be the most abundant phylum in the rhizosphere sediment from B. gymnorrhiza, K. candel or A. corniculatum (Fig 1). Previous studies also showed that Deltaproteobacteria and Gammaproteobacteria were dominated in rhizosphere of A. schaueriana, L. racemosa and A. marina [16, 25, 26]. Andreote et al. [27] also revealed the dominance of Deltaproteobacteria and Gammaproteobacteria from four distinct mangrove areas in Brazil. Deltaproteobacteria and Gammaproteobacteria were higher due to anaerobic condition of the mangrove sediment which drove selection of specific microbial groups such as sulfur-oxidizing bacteria [4, 28]. In this study, the high relative abundance of orders Desulfobacterales and Chromatiales from rhizosphere sediments belonging to sulfur-oxidizing bacteria dominated in classes Deltaproteobacteria and Gammaproteobacteria, respectively, which indicated mangrove plants played a key role in sulphur cycling (Fig 4). In the same, the two orders were reported to be prevalent from oil-contaminated soil of Brazilian pristine mangrove sediment [29].
Besides, Chloroflexi was the second dominant phylum in the three investigated mangrove species, which was not consistent with previous studies. Alzubaidy et al. [26] suggested that Bacteroidetes was the second largest phylum in A. marina from the Red Sea. However, second most abundant phylum from A. marina in Bay of Bengal was Cyanobacteria/Chloroplast in monsoon, whereas Acidobacteria in summer [16]. The phylum Chloroflexi was particularly widely distributed in many deep-sea sediments, representing up to 80% of the total bacterial 16S rRNA gene sequences at some sites [30]. Members of the phylum Chloroflexi were critical in the decomposition of organic matter [31]. This phylum can be divided into at least six major classes: Chloroflexi, Dehalococcoidetes, Thermomicrobia, Caldilineae, Anaerolineae and a clone cluster called SAR202 cluster [32]. The two classes (Dehalococcoidetes and Anaerolineae) were prevalent in this study (Fig 4). Dehalococcoidetes is strictly anaerobic and slow-growing which uses organohalide respiration via reductive dehalogenases (Rdh) as their sole mode of energy conservation [30]. Anaerolineae appears to take key role in electron transfer to anodes; however, it is presently unclear whether they are directly involved or whether they produce metabolic intermediates from root exudates or soil organic matter, utilized subsequently by other directly anode-coupling microorganisms [33].
Furthermore, some dominated phyla involving in nitrogen cycle were found in mangrove rhizosphere sediments, such as Planctomycetes, nitrospirae and Cyanobacteria (Fig 1). Anammox bacteria belonging to phylum Planctomycetes had the unique metabolic ability to combine ammonium and nitrite or nitrate to form nitrogen gas under anoxic conditions [34, 35]. However, Phylum nitrospirae was one of the key players in the nitrogen cycle referring to nitrite oxidizing bacteria [36, 37]. In addition, phylum Cyanobacteria has been proved to contribute to nitrogen fixation in mangrove by many previous studies [38, 39].
The influence of mangrove species on bacterial composition from rhizosphere sediments
The ANOVA analysis on Shannon and Chao1 indices and the PCA result indicated that bacterial communities from rhizosphere sediments were influenced much more by mangrove species than sampling depths (Figs 2 and 3). To compare with bulk sediment, the aboveground mangrove vegetation showed an important role in shaping rhizosphere bacterial community [13, 26, 40]. Besides, Gomes et al. [25] assessed bacterial compositions between the rhizospheres of two mangrove tree species and suggested that A. schaueriana and L. racemosa roots appeared to be able to impose a selective force on the bacterial communities from mangrove sediments and this phenomenon appeared to be plant species specific. Therefore, the significant influence of mangrove species on the rhizosphere bacterial community was further confirm in this study.
The differences of bacterial composition from the three investigated mangrove species showed that the dominant orders Rhodospirillales, GCA004 and envOPS12 were significantly different between each mangrove species (Fig 4). Members of Rhodospirillales belonged to a kind of photosynthetic anoxygenic bacteria which contained bacteriochlorophyll a as their major pigment and can use light to grow [4]. Basak et al. [14] also found Rhodospirillales was abundant in mangrove sediments of Sundarbans among the class Alphaproteobacteria. The predominant photosynthetic bacteria in anaerobic environments may contribute to the productivity of the mangrove ecosystems [41]. Orders GCA004 and envOPS12 belonged to class Anaerolineae which was found in a wide range of environments, including arctic permafrost, marine and freshwater sediments, sponges, the mammalian gastrointestinal tract and anaerobic sludge bioreactors [42]. The three orders Rhodospirillales, GCA004 and envOPS12 were all anaerobic bacteria, which may be related to the feature of mangrove sediment that was composed of thick organic matter and was anaerobic except for the surface sediment.
The ternary plots of dominant OTUs also confirmed that special OTUs associated with different mangrove species (Fig 5). Furthermore, most of the special OTUs from sediments of the three mangrove species were similar to uncultured bacteria, and some OTUs were closely related to bacteria with the ability of degrading organic pollutants or recycling nutrients (Table 2). These results demonstrated that rhizosphere bacteria in mangrove were highly diverse and made an essential contribution to the productivity of the mangrove ecosystem.
In conclusion, we provided the first insights into the vertical distribution of rhizosphere bacteria from three mangrove species in Beilun Estuary, China. Results indicated that the influence of mangrove tree species on the distribution of rhizosphere bacterial community was more strongly than sampling depths. Further studies are necessary to investigate whether any environmental factors can influence the bacterial community in mangrove. However, the bacterial community from each mangrove species in this study showed potential important ecological functions in mangrove ecosystems.
Supporting Information
(TIF)
(TIF)
Data Availability
All sequences obtained from this study were deposited in NCBI sequence read archive (SRA) under accession number SRP081285.
Funding Statement
This work was supported by Guangdong Natural Science Foundation (2014A030310495, 2014A030313783), and National Natural Science Foundation of China (41406179), as well as the Key Laboratory for Ecological Environment in Coastal Areas, State Oceanic Administration (201508, 201608), and sponsored by the Director Fund of Marine Science and Technology, South China Sea Branch, State Oceanic Administration (1516, 1511). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Duke NC, Meynecke JO, Dittmann S, Ellison AM, Anger K, Berger U, et al. A world without mangroves? Science. 2007; 317: 41–42. 10.1126/science.317.5834.41b [DOI] [PubMed] [Google Scholar]
- 2.Aburto-Oropeza O, Ezcurra E, Danemann G, Valdez V, Murray J, Sala E. Mangroves in the Gulf of California increase fishery yields. Proc Natl Acad Sci USA. 2008; 105: 10456–10459. 10.1073/pnas.0804601105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feller IC, Lovelock CE, Berger U, McKee KL, Joye SB, Ball MC. Biocomplexity in mangrove ecosystems. Ann Rev Mar Sci. 2010; 2: 395–417. 10.1146/annurev.marine.010908.163809 [DOI] [PubMed] [Google Scholar]
- 4.Thatoi H, Behera BC, Mishra RR, Dutta SK. Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: a review. Ann Microbiol. 2013; 63: 1–19. 10.1007/s13213-012-0442-7 [DOI] [Google Scholar]
- 5.Holguin G, Vazquez P, Bashan Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: an overview. Biol Fertil Soils. 2001; 33: 265–278. 10.1007/s003740000319 [DOI] [Google Scholar]
- 6.Sherman RE, Fahey TJ, Howarth RW. Soil-plant interactions in a neotropical mangrove forest: iron, phosphorus and sulfur dynamics. Oecologia. 1998; 115: 553–563. 10.1007/s004420050553 [DOI] [PubMed] [Google Scholar]
- 7.Bashan Y, Holguin G. Plant growth-promoting bacteria: a potential tool for arid mangrove reforestation. Trees. 2002; 16: 159–166. 10.1007/s00468-001-0152-4 [DOI] [Google Scholar]
- 8.Grayston SJ, Wang S, Campbell CD, Edwards AC. Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol Biochem. 1998; 30: 369–378. 10.1016/S0038-0717(97)00124-7 [DOI] [Google Scholar]
- 9.Berg G, Smalla K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol. 2009; 68: 1–13. 10.1111/j.1574-6941.2009.00654.x [DOI] [PubMed] [Google Scholar]
- 10.Mendes R, Garbeva P, Raaijmakers JM. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev. 2013; 37: 634–663. 10.1111/1574-6976.12028 [DOI] [PubMed] [Google Scholar]
- 11.Zhang YY, Dong JD, Yang B, Ling J, Wang YS, Zhang S. Bacterial community structure of mangrove sediments in relation to environmental variables accessed by 16S rRNA gene-denaturing gradient gel electrophoresis fingerprinting. Sci March 2009; 73: 487–498. 10.3989/scimar.2009.73n3487 [DOI] [Google Scholar]
- 12.Gomes NCM, Cleary DFR, Pinto FN, Egas C, Almeida A, Cunha A, et al. Taking root: enduring effect of rhizosphere bacterial colonization in mangroves. PLoS ONE. 2010; 5(11): e14065 10.1371/journal.pone.0014065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang XT, Peng X, Deng GH, Sheng HF, Wang Y, Zhou HW, et al. Illumina sequencing of 16S rRNA tag revealed spatial variations of bacterial communities in a mangrove wetland. Microb Ecol. 2013; 66: 96–104. 10.1007/s00248-013-0238-8 [DOI] [PubMed] [Google Scholar]
- 14.Basak P, Majumder NS, Nag S, Bhattacharyya A, Roy D, Chakrabarty A, et al. Spatiotemporal analysis of bacterial diversity in sediments of Sundarbans using parallel 16s rRNA gene tag sequencing. Microb Ecol. 2015; 69: 500–511. 10.1007/s00248-014-0498-y [DOI] [PubMed] [Google Scholar]
- 15.Colares GB, Melo VMM. Relating microbial community structure and environmental variables in mangrove sediments inside Rhizophora mangle L. habitats. Appl Soil Ecol. 2013; 64: 171–177. 10.1016/j.apsoil.2012.12.004 [DOI] [Google Scholar]
- 16.Loganathachetti DS, Sadaiappan B, Poosakkannu A, Muthuraman S. Pyrosequencing-based seasonal observation of prokaryotic diversity in pneumatophore-associated soil of Avicennia marina. Curr Microbiol. 2016; 72:68–74. 10.1007/s00284-015-0920-9 [DOI] [PubMed] [Google Scholar]
- 17.Yu J, Steinberger Y. Vertical Distribution of Microbial Community Functionality under the Canopies of Zygophyllum dumosum and Hammada scoparia in the Negev Desert, Israel. Microb Ecol. 2011; 62: 218–227. 10.1007/s00248-011-9846-3 [DOI] [PubMed] [Google Scholar]
- 18.Lee HJ, Jeong SE, Kim PJ, Madsen EL, Jeon CO. High resolution depth distribution of Bacteria, Archaea, methanotrophs, and methanogens in the bulk and rhizosphere soils of a flooded rice paddy. Front Microbiol. 2015; 6: e639 10.3389/fmicb.2015.00639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peiffer JA, Spor A, Koren O, Jin Z, Tringe SG, Dangl JL, et al. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc Natl Acad Sci USA. 2013; 110: 6548–6553. 10.1073/pnas.1302837110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011; 27: 2957–2963. 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010; 7(5): 335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgar R C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013; 10: 996–998. 10.1038/nmeth.2604 [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007; 73(16): 5261–5267. 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing Mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009; 75(23): 7537–7541. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomes NCM, Cleary DFR, Pires ACC, Almeida A, Cunha A, Mendonça-Hagler LCS, et al. Assessing variation in bacterial composition between the rhizospheres of two mangrove tree species. Estuar Coast Shelf Sci. 2014; 139: 40–45. 10.1016/j.ecss.2013.12.022 [DOI] [Google Scholar]
- 26.Alzubaidy H, Essack M, Malas TB, Bokhari A, Motwalli O, Kamanu FK, et al. Rhizosphere microbiome metagenomics of gray mangroves (Avicennia marina) in the Red Sea. Gene. 2016; 576: 626–636. 10.1016/j.gene.2015.10.032 [DOI] [PubMed] [Google Scholar]
- 27.Andreote FD, Jimenez DJ, Chaves D, Dias AC, Luvizotto DM, Dini-Andreote F, et al. The microbiome of Brazilian mangrove sediments as revealed by metagenomics. PLoS ONE. 2012; 7(6): e38600 10.1371/journal.pone.0038600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taketani RG, Franco NO, Rosado AS, van Elsas JD. Microbial community response to a simulated hydrocarbon spill in mangrove sediments. J Microbiol. 2010; 48: 7–15. 10.1007/s12275-009-0147-1 [DOI] [PubMed] [Google Scholar]
- 29.Santos HF, Cury JC, Carmo FL, Santos AL, Tiedje J, Elsas JD, et al. Mangrove bacterial diversity and the impact of oil contamination revealed by pyrosequencing: bacterial proxies for oil pollution. PLoS One. 2011; 6(3): e16943 10.1371/journal.pone.0016943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaster AK, Mayer-Blackwell K, Pasarelli B, Spormann AM. Single cell genomic study of Dehalococcoidetes species from deep-sea sediments of the Peruvian Margin. ISME J. 2014; 8: 1831–1842. 10.1038/ismej.2014.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada T, Sekiguchi Y, Imachi H, Kamagata Y, Ohashi A, Harada H. Diversity, localization, and physiological properties of filamentous microbes belonging to Chloroflexi subphylum I in mesophilic and thermophilic methanogenic sludge granules. Appl Environ Microbiol. 2005; 71(11): 7493–7503. 10.1128/AEM.71.11.7493-7503.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blazejak A, Schippers A. High abundance of JS-1- and Chloroflexi-related Bacteria in deeply buried marine sediments revealed by quantitative, real-time PCR. FEMS Microbiol Ecol. 2015; 72: 198–207. 10.1111/j.1574-6941.2010.00838.x [DOI] [PubMed] [Google Scholar]
- 33.Cabezas A, Pommerenke B, Boon N, Friedrich MW. Geobacter, Anaeromyxobacter and Anaerolineae populations are enriched on anodes of root exudate-driven microbial fuel cells in rice field soil. Environ Microbiol Rep. 2015; 7(3): 489–497. 10.1111/1758-2229.12277 [DOI] [PubMed] [Google Scholar]
- 34.Strous M, Fuerst JA, Kramer EH, Logemann S, Muyzer G, van de Pas-Schoonen KT, et al. Missing lithotroph identified as new planctomycete. Nature. 1999; 400: 446–449. 10.1038/22749 [DOI] [PubMed] [Google Scholar]
- 35.Kuenen JG. Anammox bacteria: from discovery to application. Nat Rev Microbiol. 2008; 6: 320–326. 10.1038/nrmicro1857 [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Sheng HF, He Y, Wu JY, Jiang YX, Tam NFY, et al. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of Illumina tags. Appl Environ Microbiol. 2012; 78(23): 8264–8271. 10.1128/AEM.01821-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isobe K, Ohte N. Ecological Perspectives on Microbes Involved in N-Cycling. Microbes Environ. 2014; 29(1): 4–16. 10.1264/jsme2.ME13159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rigonato J, Kent AD, Alvarenga DO, Andreote FD, Beirigo RM, Vidal-Torrado R, et al. Drivers of cyanobacterial diversity in mangrove soils in south-east Brazil. Environ Microbiol. 2013; 15: 1103–1114. [DOI] [PubMed] [Google Scholar]
- 39.Alvarenga DO, Rigonato J, Branco LHZ, Fiore MF. Cyanobacteria in mangrove ecosystems. Biodivers Conserv. 2015; 24: 799–817. 10.1007/s10531-015-0871-2 [DOI] [Google Scholar]
- 40.Pires ACC, Cleary DFR, Almeida A, Cunha A, Dealtry S, Mendonça-Hagler LCS, et al. Denaturing gradient gel electrophoresis and barcoded pyrosequencing reveal unprecedented archaeal diversity in mangrove sediment and rhizosphere samples. Appl Environ Microbiol. 2012; 78(16): 5520–5528. 10.1128/AEM.00386-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saho K, Dhal NK. Potential microbial diversity in mangrove ecosystem: a review. Indian J Mar Sci. 2009; 38(2): 249–256. [Google Scholar]
- 42.Cleary DFR, Polonia ARM, Sousa AI, Lillebø SAI, Queiroa H, Gomes NCM. Temporal dynamics of sediment bacterial communities in monospecific stands of Juncus maritimus and Spartina maritime. Plant Biol. 2016; 10.1111/plb.12459 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
Data Availability Statement
All sequences obtained from this study were deposited in NCBI sequence read archive (SRA) under accession number SRP081285.