Abstract
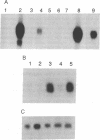
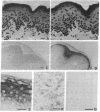
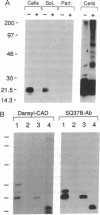
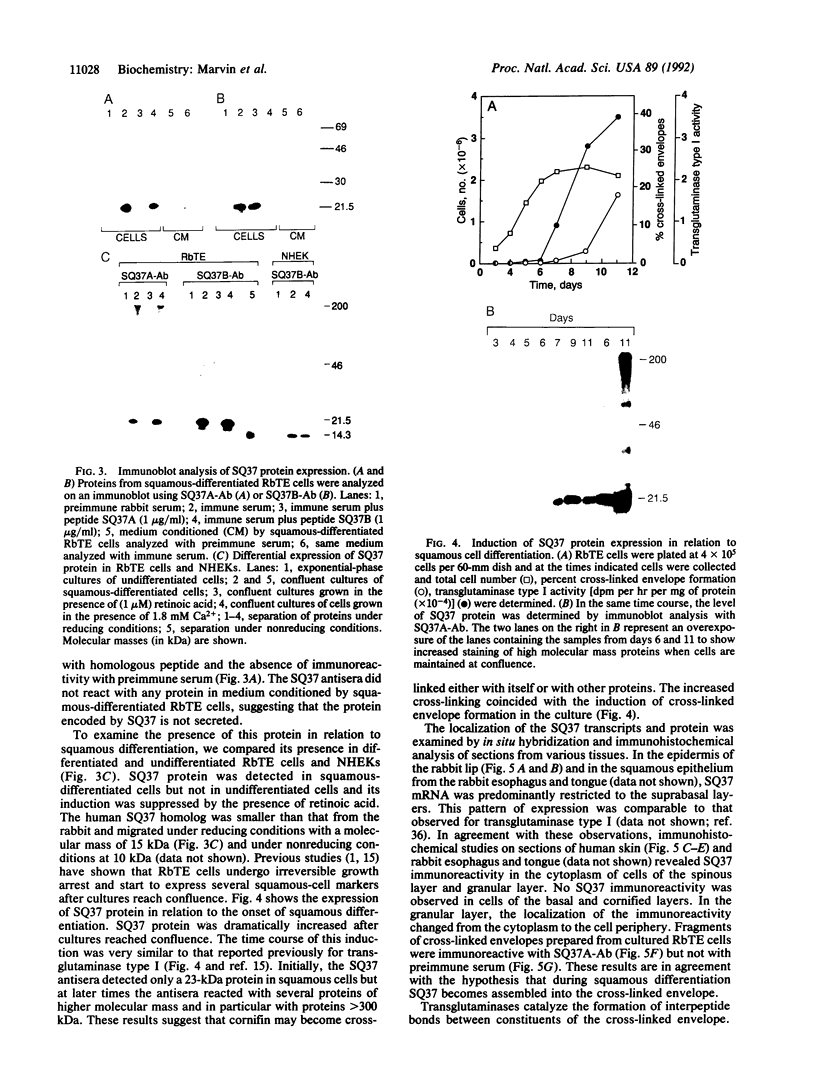
In this study, we have characterized the cDNA clone SQ37 that was isolated previously from a rabbit squamous cell library. The gene encodes a 14-kDa protein that appears to function as a component of the cross-linked envelope in squamous differentiating cells. The protein, which has been named cornifin, has a high content of proline (31%), glutamine (20%), and cysteine (11%) and contains 13 repeats of an octapeptide (consensus sequence, EPCQPKVP) at its C terminus. SQ37 mRNA and protein are induced during squamous differentiation of rabbit tracheal (RbTE) cells and human epidermal keratinocytes. This induction is repressed by retinoids. Immunohistochemical studies reveal SQ37 immunoreactivity in fragmented cross-linked envelopes from squamous-differentiated RbTE cells and in the suprabasal layers of the epidermis. In situ hybridization analysis showed that the presence of SQ37 mRNA is restricted to the suprabasal layers. Treatment of RbTE cells with a Ca2+ ionophore induces cross-linking of the SQ37 protein into higher molecular weight complexes. This cross-linking reaction appears to be mediated by transglutaminase type I. Our observations suggest that the protein encoded by SQ37 participates in the assembly of the cross-linked envelope.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abernethy J. L., Hill R. L., Goldsmith L. A. epsilon-(gamma-Glutamyl)lysine cross-links in human stratum corneum. J Biol Chem. 1977 Mar 25;252(6):1837–1839. [PubMed] [Google Scholar]
- Baden H. P., Kubilus J., Phillips S. B., Kvedar J. C., Tahan S. R. A new class of soluble basic protein precursors of the cornified envelope of mammalian epidermis. Biochim Biophys Acta. 1987 Jul 16;925(1):63–73. doi: 10.1016/0304-4165(87)90148-6. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Haron J. A., Stone E. M., Dennison O. E., Rothblum K. N., Schwartz R. J. Cloning and sequencing of a deoxyribonucleic acid copy of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry. 1983 Mar 29;22(7):1605–1613. doi: 10.1021/bi00276a013. [DOI] [PubMed] [Google Scholar]
- Eckert R. L., Green H. Structure and evolution of the human involucrin gene. Cell. 1986 Aug 15;46(4):583–589. doi: 10.1016/0092-8674(86)90884-6. [DOI] [PubMed] [Google Scholar]
- Eckert R. L., Rorke E. A. Molecular biology of keratinocyte differentiation. Environ Health Perspect. 1989 Mar;80:109–116. doi: 10.1289/ehp.8980109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs S., Lohman F., Teubel W., van de Putte P., Backendorf C. Characterization of the human spr2 promoter: induction after UV irradiation or TPA treatment and regulation during differentiation of cultured primary keratinocytes. Nucleic Acids Res. 1990 Aug 11;18(15):4401–4407. doi: 10.1093/nar/18.15.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl D., Mehrel T., Lichti U., Turner M. L., Roop D. R., Steinert P. M. Characterization of human loricrin. Structure and function of a new class of epidermal cell envelope proteins. J Biol Chem. 1991 Apr 5;266(10):6626–6636. [PubMed] [Google Scholar]
- Jetten A. M., Bernacki S. H., Floyd E. E., Saunders N. A., Pieniazek J., Lotan R. Expression of a preprorelaxin-like gene during squamous differentiation of rabbit tracheobronchial epithelial cells and its suppression by retinoic acid. Cell Growth Differ. 1992 Aug;3(8):549–556. [PubMed] [Google Scholar]
- Jetten A. M. Multistep process of squamous differentiation in tracheobronchial epithelial cells in vitro: analogy with epidermal differentiation. Environ Health Perspect. 1989 Mar;80:149–160. doi: 10.1289/ehp.8980149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Nervi C., Vollberg T. M. Control of squamous differentiation in tracheobronchial and epidermal epithelial cells: role of retinoids. J Natl Cancer Inst Monogr. 1992;(13):93–100. [PubMed] [Google Scholar]
- Jetten A. M., Shirley J. E. Characterization of transglutaminase activity in rabbit tracheal epithelial cells. Regulation by retinoids. J Biol Chem. 1986 Nov 15;261(32):15097–15101. [PubMed] [Google Scholar]
- Kartasova T., van de Putte P. Isolation, characterization, and UV-stimulated expression of two families of genes encoding polypeptides of related structure in human epidermal keratinocytes. Mol Cell Biol. 1988 May;8(5):2195–2203. doi: 10.1128/mcb.8.5.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoltsy A. G. Desmosomes, filaments, and keratohyaline granules: their role in the stabilization and keratinization of the epidermis. J Invest Dermatol. 1975 Jul;65(1):127–142. doi: 10.1111/1523-1747.ep12598093. [DOI] [PubMed] [Google Scholar]
- Mehrel T., Hohl D., Rothnagel J. A., Longley M. A., Bundman D., Cheng C., Lichti U., Bisher M. E., Steven A. C., Steinert P. M. Identification of a major keratinocyte cell envelope protein, loricrin. Cell. 1990 Jun 15;61(6):1103–1112. doi: 10.1016/0092-8674(90)90073-n. [DOI] [PubMed] [Google Scholar]
- Michel S., Bernerd F., Jetten A. M., Floyd E. E., Shroot B., Reichert U. Expression of keratinocyte transglutamine mRNA revealed by in situ hybridization. J Invest Dermatol. 1992 Mar;98(3):364–368. doi: 10.1111/1523-1747.ep12499806. [DOI] [PubMed] [Google Scholar]
- Michel S., Schmidt R., Robinson S. M., Shroot B., Reichert U. Identification and subcellular distribution of cornified envelope precursor proteins in the transformed human keratinocyte line SV-K14. J Invest Dermatol. 1987 Mar;88(3):301–305. doi: 10.1111/1523-1747.ep12466177. [DOI] [PubMed] [Google Scholar]
- Nagae S., Lichti U., De Luca L. M., Yuspa S. H. Effect of retinoic acid on cornified envelope formation: difference between spontaneous envelope formation in vivo or in vitro and expression of envelope competence. J Invest Dermatol. 1987 Jul;89(1):51–58. doi: 10.1111/1523-1747.ep12580383. [DOI] [PubMed] [Google Scholar]
- Phelps W. C., Howley P. M. Transcriptional trans-activation by the human papillomavirus type 16 E2 gene product. J Virol. 1987 May;61(5):1630–1638. doi: 10.1128/jvi.61.5.1630-1638.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. B., Kubilus J., Grassi A. M., Goldaber M. L., Baden H. P. The pancornulins: a group of basic low molecular weight proteins in mammalian epidermis and epithelium that may function as cornified envelope precursors. Comp Biochem Physiol B. 1990;95(4):781–788. doi: 10.1016/0305-0491(90)90317-m. [DOI] [PubMed] [Google Scholar]
- Ponec M., Havekes L., Kempenaar J., Vermeer B. J. Cultured human skin fibroblasts and keratinocytes: differences in the regulation of cholesterol synthesis. J Invest Dermatol. 1983 Aug;81(2):125–130. doi: 10.1111/1523-1747.ep12542979. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979 Nov;18(3):681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell. 1977 Jun;11(2):417–422. doi: 10.1016/0092-8674(77)90059-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M., Green H. Enzymatic cross-linking of involucrin and other proteins by keratinocyte particulates in vitro. Cell. 1985 Mar;40(3):677–683. doi: 10.1016/0092-8674(85)90216-8. [DOI] [PubMed] [Google Scholar]
- Simon M., Green H. Participation of membrane-associated proteins in the formation of the cross-linked envelope of the keratinocyte. Cell. 1984 Apr;36(4):827–834. doi: 10.1016/0092-8674(84)90032-1. [DOI] [PubMed] [Google Scholar]
- Smits H. L., Floyd E. E., Jetten A. M. Molecular cloning of gene sequences regulated during squamous differentiation of tracheal epithelial cells and controlled by retinoic acid. Mol Cell Biol. 1987 Nov;7(11):4017–4023. doi: 10.1128/mcb.7.11.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Stellmach V. M., Fuchs E. Exploring the mechanisms underlying cell type-specific and retinoid-mediated expression of keratins. New Biol. 1989 Dec;1(3):305–317. [PubMed] [Google Scholar]
- Sun T. T., Green H. Differentiation of the epidermal keratinocyte in cell culture: formation of the cornified envelope. Cell. 1976 Dec;9(4 Pt 1):511–521. doi: 10.1016/0092-8674(76)90033-7. [DOI] [PubMed] [Google Scholar]
- Thacher S. M., Rice R. H. Keratinocyte-specific transglutaminase of cultured human epidermal cells: relation to cross-linked envelope formation and terminal differentiation. Cell. 1985 Mar;40(3):685–695. doi: 10.1016/0092-8674(85)90217-x. [DOI] [PubMed] [Google Scholar]
- Watt F. M., Boukamp P., Hornung J., Fusenig N. E. Effect of growth environment on spatial expression of involucrin by human epidermal keratinocytes. Arch Dermatol Res. 1987;279(5):335–340. doi: 10.1007/BF00431227. [DOI] [PubMed] [Google Scholar]
- Wong Y. C., Buck R. C. An electron microscopic study of metaplasia of the rat tracheal epithelium in vitamin A deficiency. Lab Invest. 1971 Jan;24(1):55–66. [PubMed] [Google Scholar]
- Yuspa S. H., Ben T., Steinert P. Retinoic acid induces transglutaminase activity but inhibits cornification of cultured epidermal cells. J Biol Chem. 1982 Sep 10;257(17):9906–9908. [PubMed] [Google Scholar]
- Zettergren J. G., Peterson L. L., Wuepper K. D. Keratolinin: the soluble substrate of epidermal transglutaminase from human and bovine tissue. Proc Natl Acad Sci U S A. 1984 Jan;81(1):238–242. doi: 10.1073/pnas.81.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]