Abstract
Background
Fibromyalgia syndrome (FMS) is associated with central alterations, but controversies exist regarding the presence and role of peripheral factors. Microdialysis (MD) can be used in vivo to study muscle alterations in FMS. Furthermore for chronic pain conditions such as FMS, the mechanisms for the positive effects of exercise are unclear. This study investigates the interstitial concentrations of algesics and metabolites in the vastus lateralis muscle of 29 women with FMS and 28 healthy women before and after an exercise intervention.
Methods
All the participants went through a clinical examination and completed a questionnaire. In addition, their pressure pain thresholds (PPTs) in their upper and lower extremities were determined. For both groups, MD was conducted in the vastus lateralis muscle before and after a 15-week exercise intervention of mainly resistance training of the lower limbs. Muscle blood flow and interstitial muscle concentrations of lactate, pyruvate, glutamate, glucose, and glycerol were determined.
Results
FMS was associated with significantly increased interstitial concentrations of glutamate, pyruvate, and lactate. After the exercise intervention, the FMS group exhibited significant decreases in pain intensity and in mean interstitial concentrations of glutamate, pyruvate, and glucose. The decrease in pain intensity in FMS correlated significantly with the decreases in pyruvate and glucose. In addition, the FMS group increased their strength and endurance.
Conclusion
This study supports the suggestion that peripheral metabolic and algesic muscle alterations are present in FMS patients and that these alterations contribute to pain. After an exercise intervention, alterations normalized, pain intensity decreased (but not abolished), and strength and endurance improved, all findings that suggest the effects of exercise are partially peripheral.
Introduction
Fibromyalgia syndrome (FMS) is a common chronic pain condition associated with negative implications [1,2]. FMS is associated with central alterations (e.g., central hyperexcitability with disinhibition [3–7]), but controversies exist regarding the presence and role of peripheral factors. For example, several findings suggest peripheral nociceptive mechanisms such as muscle and nociceptive C-fibre alterations e.g. small fibre neuropathy [8–12] and signs of peripheral nociceptive input [13–17] are at play.
Exercise is beneficial for FMS and chronic widespread pain patients (CWP) [18–21], but the mechanisms for the positive effects of exercise are unclear [22–25].
Microdialysis (MD) is an in vivo technique that can be used to sample biochemical substances in the muscle interstitium (i.e., extra cellular fluid), where nociceptor free nerve endings terminate, providing information on the local biochemical milieu. Several MD studies have found increased concentrations of glutamate, serotonin, lactate, and/or pyruvate in the trapezius muscle in regional chronic neck pain conditions [26,27]. MD has also been used to study muscle alterations in FMS and one study found an increased concentration of serotonin in the myalgic masseter muscle of FMS [28]. Two MD studies investigated alterations of the trapezius muscle in FMS: one study found increased interstitial concentrations of lactate and pyruvate–metabolites and products of glycolysis–in the resting trapezius muscle [29] and one study found significantly increased interstitial concentrations of glutamate and lactate from a cohort of mainly FMS patients (i.e., 15 out of the 17 CWP patients)[30]. The latter study also reported that the concentrations of glutamate and lactate correlated with pressure pain thresholds (PPTs) of trapezius and pain intensity in the FMS/CWP group [30]. For the same FMS/CWP cohort was found that endogenous pain inhibitory substances (N-Acylethanolamines; NAE) were mobilized differently than in controls and in chronic regional neck-shoulder pain patients [31]. A relatively small study found no increase in lactate of the vastus lateralis [32]. To develop effective mechanism-based treatments for FMS will require a better understanding of peripheral muscle alterations and how exercise normalizes alterations and influences aspects of pain.
This study investigates the interstitial concentrations of certain metabolites and algesics in the vastus lateralis of women with FMS and in female healthy controls (CON) before and after a 15-week exercise intervention. In addition, this study investigates relationships between concentrations of the biochemical substances and aspects of pain (group and intensity).
Subjects and Methods
This is a non-randomized sub-study of a multi-centre experimental study comprising women with FMS and healthy women (ClinicalTrials.gov identification number: NCT01226784). The three participating centers were the Pain- and Rehabilitation Medicine, Department of Medical and Health Sciences (IMH), Faculty of Health Sciences, Linköping University, Pain- and Rehabilitation Centre, County Council of Östergötland, Linköping; the Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Göteborg; and the Department of Clinical Sciences, Karolinska Institutet at Danderyd University Hospital, Stockholm, all in Sweden.
Subjects
Recruitment procedures have been described in detail in previous articles [33,34]. The following inclusion criteria for women with FMS were used: between 20 and 65 years old and meeting the American College of Rheumatology (ACR) 1990 classification criteria for FMS. Even though there exist newer criteria these are the criteria generally used in clinical practice and in most research studies both in Sweden and in other countries. Moreover, in clinical practise in Sweden these criteria have to be fulfilled in order to obtain the ICD-10 code for FMS (i.e. M79.7). The following exclusion criteria were used: high blood pressure (>160/90 mmHg), osteoarthritis in hip or knee, other severe somatic or psychiatric disorders, primary causes of pain other than FMS, high consumption of alcohol (i.e. Audit >6 according to the recommendations for women), participation in a rehabilitation program within the past year, regular resistance exercise training or relaxation exercise training more than twice a week, inability to understand or speak Swedish, and not being able to refrain from analgesics, NSAID, or hypnotics for 48 hours before examinations.
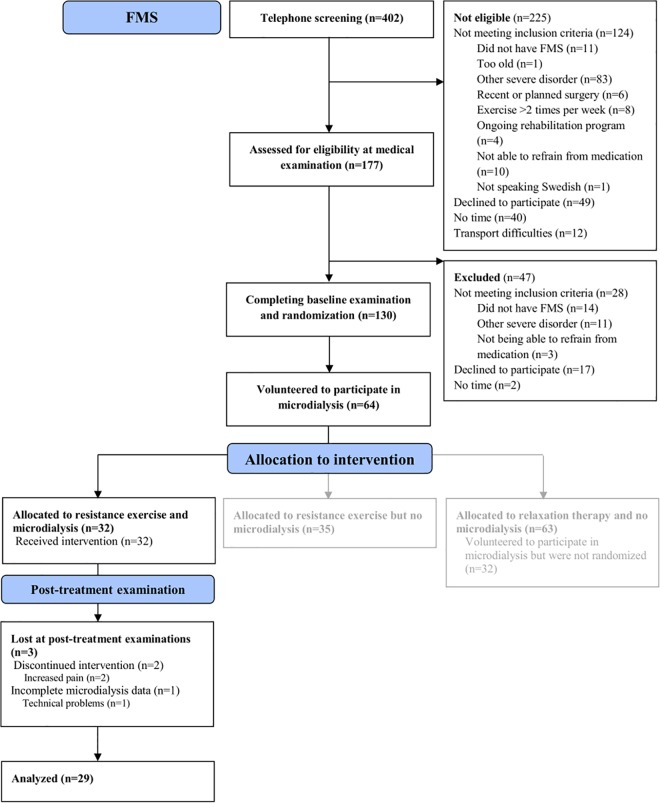
The patients were recruited by newspaper advertisement in the local newspapers of three cities in Sweden (Gothenburg, Stockholm, and Linköping) (Fig 1). In response to these advertisements, 402 women expressed an interest to participate in the study. All 402 were telephone screened for possible eligibility. Out of these women, 225 were not eligible for enrolment. The remaining 177 women were then assessed for eligibility during a medical examination. As a result of this examination, 44 additional women were excluded, leaving 133 women with FMS eligible for a multicentre experimental study, which will be reported elsewhere.
Fig 1. Flow diagram of the recruitment process and participation in the group of patients with fibromyalgia (FMS).
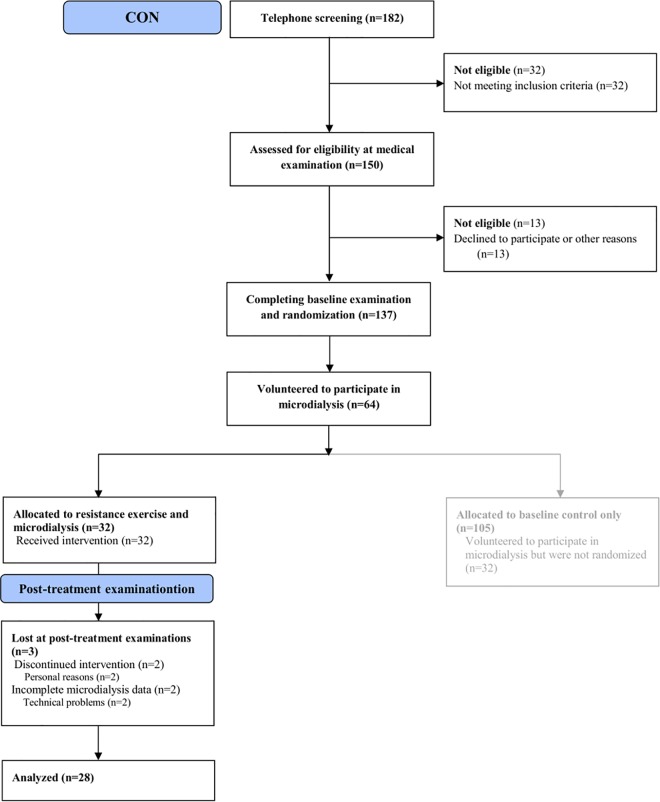
Twenty-eight female controls (CON) participated, approximately coinciding the mean age of the patients with FMS. They were also recruited through advertisements in the local newspapers of the three participating cities (Gothenburg, Stockholm, and Linköping) and formed a subgroup of the 32 controls that participated in the above mentioned trial (Fig 2). The inclusion criteria were age between 20 and 65 years, and female sex. Exclusion criteria were any pain condition, high blood pressure (>160/90 mmHg), osteoarthritis (OA) in hip or knee, other severe somatic or psychiatric disorders, high consumption of alcohol (see above), participation in a rehabilitation program within the past year, regular resistance exercise or relaxation exercise twice a week or more, inability to understand or speak Swedish, and not being able to refrain from analgesics, NSAID or hypnotics for 48 hours prior to examination.
Fig 2. Flow diagram of the recruitment process and participation in the group of healthy controls (CON).
This sub-study reports results from microdialysis of the vastus lateralis of the dominant side before and after a progressive resistance exercise training intervention of the lower limbs (15 weeks) for both the FMS group and for the CON group. This sub-study included data from subjects that took part in both MD sessions i.e. 29 patients with FMS and 28 healthy subjects (CON). Research subjects were recruited and followed up from October 2010 to September 2013.
When estimating the sample size for analyzing differences in the interstitial concentration of glutamate (μmol l-1) between the groups, we assumed based on a recent study [30] that the mean difference should have a standard deviation of 30 μmol l-1. Expectation of a mean difference of 25 μmol l-1, required a sample size of 24 subjects in each group to reject the null hypothesis with a power of 0.80 and a probability of <0.05 (two tailed).
The estimation of sample size for analyzing changes within groups the interstitial concentration of glutamate (μmol l-1) was based on a recent study [35] and we assumed that the mean difference should have a standard deviation of 25 μmol l-1. Expectation of a mean change of 15 μmol l-1, required a sample size of 24 pairs of subjects to reject the null hypothesis with a power of 0.80 and a probability of <0.05 (two tailed).
The Sample size calculations were made using the computer program Power and Sample Size Calculations (v. 3.0.43, http://biostat.mc.vanderbilt.edu/wiki/Main/PowerSampleSize).
Due to the fact that this study was an intervention study with risk for drop-outs approximately 30 subjects in each group was considered as sufficient.
Methods
After receiving verbal and written information about the study, all subjects signed a consent form in accordance with the Declaration of Helsinki. The study, including the economical compensation, was granted ethical clearances by the Regional ethics committee in Stockholm (Dnr: 2010/1121-31/3).
Procedures
CON and FMS participated in a 15-week exercise intervention program that mainly trained the lower limbs. Before the exercise intervention anthropometric data, blood pressures, psychological distress and aspects of quality of life were collected. At this time, the FMS participants were also examined for the number of tender points and pain duration. Before and after the exercise intervention, pressure pain threshold (PPT) and physical capacity (i.e., functional tests for strength of upper and lower limbs and six-minute walking distance) data were collected. In addition, MD was performed of the vastus lateralis muscle. During both these MD sessions, which included a 20 minutes standardised work period, the following data were collected five separate times every 20 minutes: pain intensity, blood flow, and the interstitial muscle concentrations of glucose, lactate, pyruvate, glycerol, and glutamate from vastus lateralis.
Background and Anthropometric data
The age (years), weight (kg), height (cm), Body Mass Index (BMI; kg/m2), and systolic and diastolic blood pressures (mm Hg) were collected for all the subjects. The participants completed a brief questionnaire regarding depressive and anxiety symptoms as well as quality of life (Table 1). At the clinical examination, the number of tender points (ACR criteria) and pain duration was determined for the subjects of the FMS group.
Table 1. Age, anthropometric data, depressive and anxiety symptoms, quality of life and pain duration (only FMS), blood pressure and pressure pain threshold and number of tender points (only FMS) in patients with fibromyalgia syndrome (FMS) and in healthy controls (CON).
| Group | FMS | N = 29 | CON | N = 28 | Statistical comparison |
|---|---|---|---|---|---|
| Variables | Mean | SD | Mean | SD | p-value |
| Age (years) | 53.7 | 8.9 | 54.8 | 8.1 | .737 |
| Height (m) | 1.64 | 0.07 | 1.65 | 0.06 | .994 |
| Weight (kg) | 73.3 | 13.4 | 66.8 | 10.9 | .043* |
| BMI (kg/m2) | 27.2 | 5.2 | 24.7 | 4.6 | .029* |
| BP diastolic (mm Hg) | 83 | 8 | 83 | 7 | .885 |
| BP systolic (mm Hg) | 133 | 19 | 131 | 14 | .797 |
| PPT-all sites (kPa) | 184 | 73 | 336 | 100 | < .001* |
| Nos. tender points | 15.9 | 1.4 | NA | NA | NA |
| Pain duration (years) | 13.3 | 7.9 | NA | NA | NA |
| HAD-Depression | 6.5 | 4.1 | 1.5 | 1.8 | < .001* |
| HAD-Anxiety | 7.0 | 4.4 | 3.3 | 3.1 | .001* |
| SF36-PSC | 31.0 | 7.6 | 54.6 | 4.4 | < .001* |
| SF36-MSC | 42.0 | 11.8 | 51.4 | 6.0 | .003* |
Mean ± one standard deviation (SD) is given. Furthest to the right is the result of the statistical comparison between the two groups (p-value)
* denotes significant group difference.
NA denotes not applicable.
BMI = Body Mass Index; BP = blood pressure; HADS-Anxiety = Hospital Anxiety and Depression Scale—subscale anxiety; HADS-Depression = Hospital Anxiety and Depression Scale—subscale depression; PPT-all sites = pressure pain thresholds–mean for all sites; SF36-PCS = Short Form Health Survey 36-physical summary component; SF36-MSC = Short Form Health Survey 36-mental (psychological) summary component.
The Hospital Anxiety and Depression Scale (HADS), a short self-assessment questionnaire that measures anxiety and depression, comprises seven items in each of the depression (HAD-D) and anxiety (HAD-A) scales [36,37]. Possible subscale scores range from 0 to 21, with the lower score indicating the least depression and anxiety possible. A score of 7 or less indicates a non-case, a score of 8–10 indicates a doubtful case, and a score of 11 or more indicates a definite case.
The Short Form Health Survey (SF36) measures multi-dimensional health concepts and measurements of a full range of health states, including levels of well-being and personal evaluations of health. The instrument has eight dimensions and uses a scale from 0 to 100: physical functioning, role limitations due to physical functioning, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health [38]. Based on these eight scales, the instrument calculates a physical summary component (SF36-PSC) and a mental (psychological) summary component (SF36-MSC). This study uses the two summary components.
Functional tests of physical capacity
Maximal isometric elbow flexion force (kg) in both arms was measured using a dynamometer (Isobex®; Medical Device Solutions AG, Oberburg, Switzerland). The participant was in a seated position without back support and legs stretched out. The upper arm was aligned with the trunk and the elbow in 90° of flexion. The maximum strength obtained during five seconds was recorded [39].
Static knee extension strength (N) was determined using a dynamometer (Steve Strong®; Stig Starke HBI, Gothenburg, Sweden). The participant was in a fixed seated position with back support, knee and hip in 90° of flexion, and legs hanging freely. A non-elastic strap was placed around the ankle and attached to a pressure transducer with an amplifier. The maximum strength obtained during five seconds was recorded. The instrument has been used in previous studies of physical performance [40,41]. The distance that a person can cover in a six minutes (six-minute walk test; WT-6min) was also recorded [42].
Pressure pain thresholds
As a part of the clinical examination, algometry was performed using an electronic pressure algometer (Somedic, Hörby, Sweden) as previously described [43]. The algometry was conducted approximately one week before the microdialysis. The area of the contact area was 1 cm2 and pressure was applied perpendicularly to the skin at a speed corresponding to approximately 50 kPa/s. The subjects were instructed to mark the pressure pain threshold (PPT) by pressing a button as the sensation of “pressure” changed to “pain”. When the button was pressed or when the maximum pressure of 1 500 kPa was reached, the application of pressure ceased. Algometry was performed bilaterally over the supraspinatus muscle (at origins above the scapula spine near the medial border), the lateral epicondyle (2-cm distal to the epicondyles), over the gluteus maximus (in upper outer quadrants of buttocks in anterior fold of muscle), and inside of the knee (at the medial fat pad proximal to the joint line). That is, the algometry was used on eight of the eighteen tender points defined by the ACR criteria [44]. In the present study, a mean value of all eight anatomical sites was used. Before the actual testing of PPT, the subjects were given instructions and were familiarized with the testing procedure.
The 15-week exercise intervention
The exercise intervention has been described in detail elsewhere [34]; here is given a brief description. The main goal of the exercise intervention was to improve muscle strength and health status by progressive resistance exercise, but without risking increased pain while loading the muscles [34]. The exercise intervention lasted 15 weeks and each session was performed twice a week. Each session was performed under the supervision of specially trained physical therapists. It was conducted at physiotherapy premises and at a local gym at the different sites in groups compromising five to seven participants to promote interaction between participants and to facilitate physiotherapeutic guidance. The intervention was proceeded by an individual introductory meeting. Low intensity of exercise adjusted to individual limitations works well in most patients with FMS [45]. For this study, we initiated resistance training at 40% of the one repetition maximum (1 RM) and successively progressed to 70–80% of 1RM. This exercise mode has previously shown to be feasible in FMS [46]. Each session started with a 10-minute warm up (stationary bicycle) followed by a 50-minute resistance training protocol. In order to promote the participant´s sense of control and to avoid possible negative effects related to exercise, the exercise was initiated at low loads, and possibilities for progression of loads were evaluated every 3–4 weeks in dialogue between the physiotherapist and participant. The physiotherapists were not blinded for group belonging (FMS or CON).
Microdialysis and sample preparation
MD mimics the function of a capillary blood vessel by perfusing a thin dialysis tube implanted in the tissue with a physiological saline solution. Substances can pass by simple diffusion across the dialysis membrane along the concentration gradient. The dialysate is analysed chemically. MD allows for continuous sampling of compounds in the muscle interstitial space, where nociceptor-free nerve endings terminate in close proximity to the muscle fibres, providing accurate information on local biochemical changes before such compounds are diluted and cleared by the circulatory system.
The MD session was performed a few days after the clinical examination when the PPTs were determined. The participants were asked not to perform strenuous exercise two days before the study. They were also instructed not to drink any beverages with caffeine and not to smoke on the day of the study. In addition, they were asked not to take paracetamol-medication two days before and NSAID-medication one week before the MD sessions. The participants arrived at the clinical department in the morning after having eaten breakfast. All subjects reported that they had followed the instructions. During the study, they received a standardised meal at the end of the trauma period, but otherwise they were not allowed to eat. They could, however, drink water.
The skin and the subcutaneous tissues above where the catheter entered were anaesthetised with a local injection (0.5 ml) of lidocaine (Xylocain® 20 mg/ml, AstraZeneca, Södertälje, Sweden) without adrenaline. Care was taken not to anaesthetise the underlying muscle. Two commercially available microdialysis catheters–cut-off points of 100 (CMA 71) and 20 kDa (CMA 60), CMA Microdialysis AB, Solna, Sweden; membrane 30-mm length, 0.5-mm diameter–were inserted parallel to the muscle fibres into the vastus lateralis muscle at half the distance between the trochanter and the knee on the dominant side. Typically, a brief involuntary contraction and change of resistance were perceived when the tip of the insertion needle of the catheter entered the fascia and the muscle. To determine the concentrations of small molecules such as lactate, pyruvate, glutamate, and glucose, the catheter with the 20 kDa cut-off was used [47]. The catheters were perfused with a high-precision syringe pump (CMA 107; CMA/Microdialysis AB, Stockholm, Sweden) at a rate of 5 μl/min with a Ringer acetate solution (Fresenius Kabi AB, Uppsala, Sweden) containing 3-mM glucose, 0.5-mM lactate, and 3.0-μM [14C]-lactate (specific activity: 5.81 GBq/mmol, GE Healthcare, Buckinghamshire, UK). This procedure was performed according to the internal reference method [48]. Furthermore, nutritive muscle blood flow was estimated by the microdialysis ethanol technique [49] using 3H2O instead of ethanol [50]. In addition, 0.3-μl/ml 3H2O (specific activity: 37 MBq/g; PerkinElmer Life Sciences, Boston, USA) was added to the perfusate. The ratio of 3H2O in the dialysate and the perfusate (the outflow-to-inflow ratio) varies inversely with the local blood flow in the tissue [49,50].
Immediately after the insertion of catheters, participants rested comfortably in a supine position on a bed for 120 minutes (i.e., the trauma period) to allow the tissue to recover from possible changes induced in the interstitial environment. The samples of dialysate from the trauma period were not considered in the present study, which focussed upon the habitual situation of the muscle tissue. After the trauma period, participants continued to rest for 20 minutes–the baseline period (denoted baseline). The baseline period was followed by a 20-minute period of standardised repetitive dynamic low-intensity exercise of the quadriceps (thigh muscles) (in the following denoted as 20-min work period). The subjects were seated on a bed (back supported) with the knee slightly bent and the lower leg resting on an exercise ball (diameter 55 cm). Participants with current pain intensity in the exercising leg > 40 on a 0–100 visual analogue scale (VAS) started at 15° of knee flexion and those with less pain started at 20° of knee flexion. During the 20-min work period, the participants were asked to slowly extend the knee to a straight position (0° knee angle), slowly lower it on the ball, and then immediately repeat the cycle without resting on the ball. Each repetition took five seconds. The experiment ended with a recovery period of 60 minutes during which participants rested on the bed (denoted recov#1–3). During the MD session, subjects rated the overall pain intensity using a 100 mm VAS (endpoints: 0 = no pain and 100 = worst possible pain). Pain intensity was registered every 20 minutes throughout the experiments except for the exercise period (then every five minutes).
Samples from both catheters were obtained every 20 minutes for the 220 minutes of testing, weighed immediately, and kept on ice throughout the rest of the MD experiment. The samples were then stored as aliquots in -70°C until analysis. All vials were weighed before the experiment started and after each 20-minute interval to confirm that sampling and fluid recovery (FR) was working according to the perfusion rate set. Vials with visible sign of haemolysis were discarded.
Relative recovery (RR) measurements: A 5-μL dialysate or perfusate was pipetted into a counting vial containing 3-ml scintillation fluid (High-flash Point, Universal LSC-Cocktail, ULTIMA GOLD™, PerkinElmer, Inc., MA, U.S.A) and vortexed. β -counting was performed using a liquid scintillation counter (Beckman LS 6000TA, Beckman instruments, Inc., Fullerton, CA, USA). RR was calculated as (dpmp—dpmd)/ dpmp, where dpmp and dpmd are disintegrations per minutes in the perfusate and the dialysate, respectively.
The dialysates from the baseline (140 min), the 20-min work period (160 min), and the recovery period (180, 200 and 220 min) were thawed, centrifugalised, and analysed for the interstitial concentrations of pyruvate, lactate, glutamate, glycerol, and glucose (abbreviated as [pyruvate], [lactate], [glutamate], [glycerol], and [glucose]) with an ISCUSSflex Analyser (CMA Microdialysis, Solna, Sweden; standard range). The detection intervals are as follows: 0.1–12 mmol l-1 for [lactate]; 10–1500 μmol l-1 for [pyruvate]; 1.0–150 μmol l-1 for [glutamate]; 0.1–25 mmol l-1 for [glucose]; and 10–1500 mmol l-1 for [glycerol]. The interstitial concentrations (Ci) were calculated for the metabolites as Ci = (Cd–Cp) / RR + Cp, where Cd was the dialysate concentration and Cp was the perfusate concentration.
Outcomes of the present study
In conclusion, the present study used the following outcomes:
Interstitial muscle concentrations of lactate, pyruvate, glutamate, glucose and glycerol obtained during miscrodialysis.
Pain intensity during microdialysis.
Tests of physical capacity: elbow flexion force right and lefts sides, knee extension strength right and left sides and six-minute walk test (WT-6min).
Statistics
Statistical analyses were made using IBM SPSS (version 20.0; IBM Corporation, Route 100 Somers, New York, USA) and SIMCA-P+ (version 13.0; Umetrics Inc., Umeå) and p≤0.05 was used as level of significance in all analyses. In text and tables, data are presented as mean ± one standard deviation (± 1SD); in figures, data are presented as mean ± one standard error of the mean (± 1SEM). Generally the present study focused upon between group comparisons but also within group analyses were made. For the overall analyses, we used mean values of each substance, blood flow, and pain intensity throughout the MD session (i.e. time points: 140, 160, 180, 200 and 220 min). To compare groups concerning background data and data from the questionnaire, we used non parametric tests for the classical statistical between group (Mann Whitney U-test) and within group analyses (Wilcoxon Matched-Pair Signed-Rank test, Friedman's 2-Way ANOVA by Ranks test), as the biochemical substances, blood flow variables, and pain intensity were not normally distributed. It was not possible to transform these variables so that e.g. mixed models analyses could be applied using the available statistical package (i.e. SPSS).
Classical statistical methods can quantify the level of individual metabolites but disregard interrelationships between different metabolites [51] and thereby ignore the system-wide aspect of metabolism and algesics. Classical methods assume variable independence when interpreting the results [52]. Multivariate data analyses (MVDA) are capable of handling a number of intercorrelated substances and use advanced principal component analyses (PCA) and Partial Least Squares (PLS) regressions as important tools. When investigating the multivariate correlations between the concentrations of metabolites and pain intensities, PPT and group membership PLS were applied using SIMCA-P+ [53]. Before this analysis, PCA was used to check for multivariate outliers (no multivariate outliers were identified). In the PLS analyses, the mean of each MD session for the biochemical substances and blood flow were used.
PLS (i.e., PLS-OPLS/O2PLS) was used for the multivariate regression analysis of PPT, pain intensity (VAS), and group membership (FMS or CON; i.e., PLS-discriminant analysis (PLS-OPLS/O2PLS-DA))[53] using the mean interstitial concentrations of the metabolites as regressors. The VIP variable (variable influence on projection) indicates the relevance of each X-variable pooled over all dimensions and the Y-variables–the group of variables that best explains Y. Hence, variables with the highest VIP were most important as regressors. VIP ≥ 1.0 was considered significant. Coefficients (PLS scaled and centred regression coefficients) were used to note the direction of the relationship (positive or negative; in the text reported after the VIP value).
Multiple linear regression (MLR) is an alternative method when regressing pain intensity and PPT, but it assumes that the regressor (X) variables are independent. If multi-colinearity (i.e., high correlations) occurs among the X-variables in MLR, the regression coefficients become unstable and their interpretability breaks down [53]. MLR also assumes that a high subject-to-variables ratio is present (e.g., >5) and such requirements are not required for PLS; in fact, PLS can handle subject-to-variables ratios < 1 and can handle several Y-variables simultaneously [53].
Results
Background data
The two groups did not differ in age, blood pressure, or height (Table 1). FMS had significantly higher weight and BMI than CON (Table 1). FMS subjects had their pain condition for a considerable time. FMS had significantly lower PPT than CON (Table 1). Even though the two groups differed significantly on the two scales concerning anxiety and depression (Table 1), FMS did not show levels indicating anxiety or depression (i.e. ≥11 on both scales of HADS). Prominent group differences were found for the two quality of life indices: SF36-PCS and SF36-MSC. Both groups had a high participation rate in the 15-week exercise intervention and no significant group difference existed: CON: 93 ± 12% vs. FMS: 92 ± 10%, p = 0.605.
Strength and endurance before and after the 15-week exercise intervention
Tests of strength and endurance are reported in Table 2. No significant group differences in the strength of the lower limbs were found before or after the 15-week exercise intervention (Table 2): before: right side (p = 0.196) and left side (p = 0.598); after: right side (p = 0.127) and left side (p = 0.798).
Table 2. Results of the static strength of the arm flexors and the knee extensor muscles and the six-minute walk test (WT-6min) before and after the 15-week exercise intervention in patients with fibromyalgia syndrome (FMS) and in healthy controls (CON).
| Group | FMS | (N = 29) | CON | (N = 28) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Mean | SD | Mean | SD | p-value | Mean | SD | Mean | SD | p-value |
| Physical capacity | ||||||||||
| Elbow flexion force right (kg) | 12.8 | 4.6 | 15.2 | 5.0 | .001* | 17.0 | 4.5 | 16.9 | 4.5 | .876 |
| Elbow flexion force left (kg) | 12.8 | 4.9 | 15.0 | 5.4 | .003* | 16.4 | 4.1 | 16.8 | 3.1 | .692 |
| Knee extension strength right (N) | 340.8 | 98.1 | 353.5 | 107.6 | .387 | 370.1 | 85.8 | 398.8 | 69.1 | .062 |
| Knee extension strength left (N) | 321.9 | 98.6 | 354.6 | 102.8 | .033* | 334.9 | 84.1 | 354.7 | 78.5 | .063 |
| WT-6min (m) | 567 | 67 | 578 | 59 | .049* | 627 | 57 | 652 | 51 | 0.006* |
Mean ± one standard deviation (SD) is given before and after the 15-week exercise intervention (15-weeks). For each group, the group analyses (before vs. after; p-value) were reported;
* denotes significant difference.
For between group analyses, see text.
Significant group differences existed in strength of the upper extremities before the 15-week exercise intervention (right side: p = 0.002 and left side: p = 0.006)(Table 2). No significant group differences were found after the 15-week exercise intervention (p = 0.136 and p = 0.119) (Table 2).
Within group analysis showed that after the 15-week exercise intervention FMS had improved muscle strength in three out of four strength tests (Table 2).
Significant group differences existed both before (p = 0.001) and after (p<0.001) the 15-week exercise intervention for WT-6min with longer distances walked in CON than in FMS (Table 2). Both CON (p = 0.008) and FMS (p = 0.049) had improved WT-6min after the 15-week exercise intervention (Table 2).
Pain intensity before and after the 15-week exercise intervention
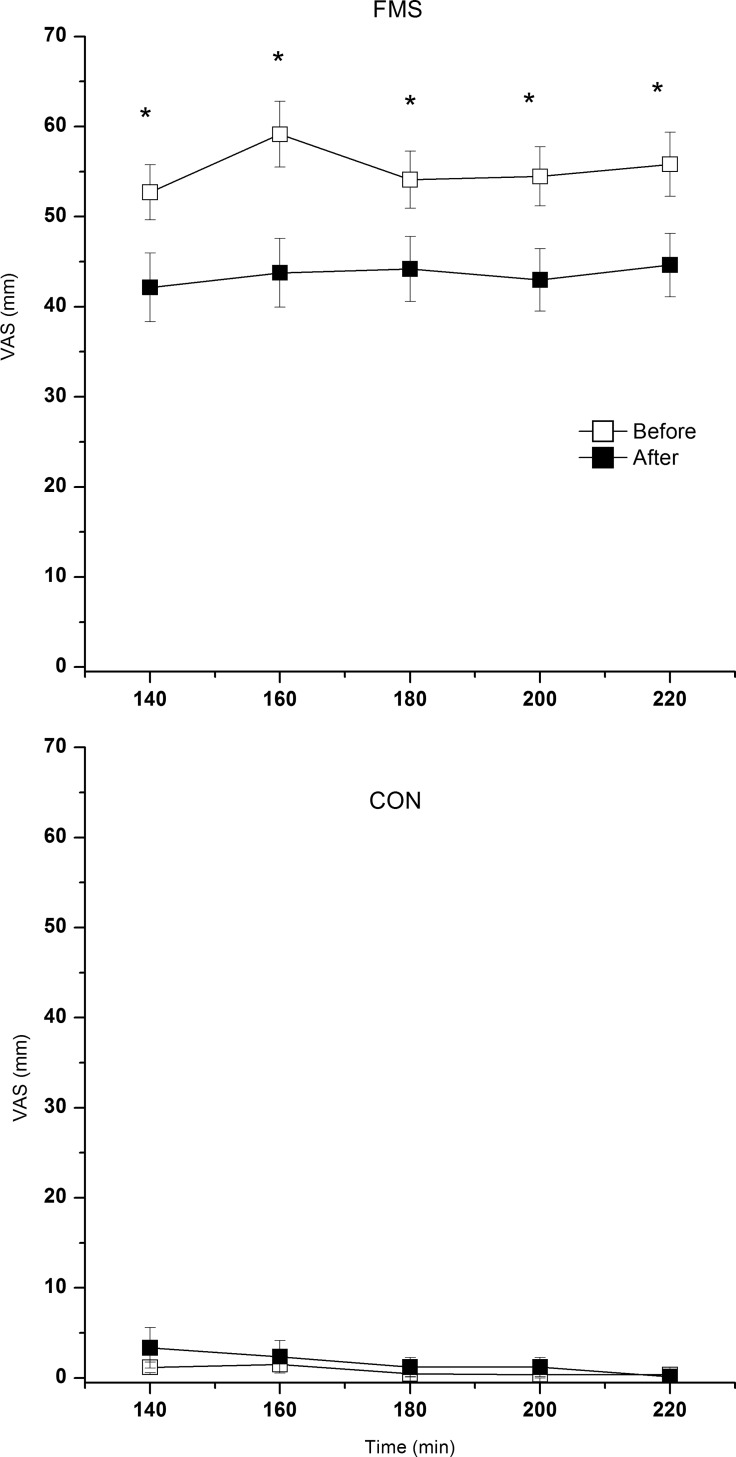
As expected, the mean pain intensity throughout the MD experiment was significantly higher in FMS than in CON both before and after the 15-week exercise intervention (both p>0.001)(Fig 3).
Fig 3. Pain intensity (mean ± SEM) before and after the 15-week exercise intervention in FMS (upper panel) and CON (lower panel) at the time points 140 (baseline), 160 (immediately after the 20-min work period), 180 (recovery), 200 (recovery), and 220 (recovery) min.
Note the different y-axis. * denotes significant difference in pain intensity at that time point between before and after the exercise intervention.
The more detailed analyses revealed that FMS exhibited a significant increase in pain intensity from baseline (i.e., 140 min) to the end of the 20-min work period (i.e., 160 min) at the MD session before (p = 0.006) but not after (p = 0.150) the 15-week exercise intervention (Fig 3). No such significant changes were found in CON (p: 0.581–1.000).
The FMS had significantly lower pain intensities at all time points after the 15-week exercise intervention (p: 0.001–0.007)(Fig 3); no significant changes existed in CON (p: 0.500–0.866).
The interstitial concentrations and blood flow at the two MD sessions
In order to get an overview is reported mean values. In the Supporting information (S1 Appendix and S1–S6 Figs) more detailed analyses of the different time points are presented.
Before the 15-week exercise intervention, there were significant group differences for the mean concentrations of pyruvate and glutamate with significantly higher concentrations in FMS (Table 3). A tendency for higher [lactate] in FMS was also observed (p = 0.088). A regression of group membership was done and confirmed in the multivariate context that the mean values of following substances were of significant importance for group membership before the 15-week exercise intervention (R2 = 0.11; p<0.05): [pyruvate] (VIP = 1.54+); [glutamate] (VIP = 1.40+); and [lactate] (VIP = 1.17+) (Variables with high VIP values are more important regressors than variables with lower VIP. The sign after the VIP value indicates the direction of the correlation between the regressor and the dependent variable). As a result, [lactate] was also important in the multivariate context.
Table 3. The mean concentrations of the five biochemical substances investigated during the two MD sessions–before and after the 15-week exercise intervention–in patients with fibromyalgia syndrome (FMS) and in healthy controls (CON).
| Group | FMS | n = 29 | Statistical comparison within FMS | CON | n = 28 | Statistical comparison within CON | Statistical comparison between groups |
|---|---|---|---|---|---|---|---|
| Substance | Mean | SD | p-value | Mean | SD | p-value | p-value |
| Before exercise intervention | |||||||
| [glucose] (mmol l-1) | 8.5 | 6.4 | 6.7 | 3.2 | .213 | ||
| [lactate] (mmol l-1) | 2.1 | 0.9 | 1.6 | 0.6 | .088 | ||
| [pyruvate] (μmol l-1) | 82.2 | 43.9 | 54.2 | 33.7 | .012* | ||
| [glycerol] (mmol l-1) | 133.7 | 127.7 | 116.0 | 110.6 | .690 | ||
| [glutamate] (μmol l-1) | 72.3 | 47.3 | 47.4 | 23.0 | .014* | ||
| After exercise intervention | |||||||
| [glucose] (mmol l-1) | 6.0 | 1.7 | .039* | 6.8 | 2.3 | .838 | .191 |
| [lactate] (mmol l-1) | 1.9 | 1.1 | .381 | 1.7 | 0.8 | .873 | .371 |
| [pyruvate] (μmol l-1) | 52.0 | 39.4 | .007* | 80.9 | 71.1 | .072 | .155 |
| [glycerol] (mmol l-1) | 120.2 | 91.5 | .940 | 103.6 | 63.7 | .909 | .655 |
| [glutamate] (μmol l-1) | 45.7 | 23.1 | .006* | 51.3 | 28.3 | .946 | .598 |
Mean ± one standard deviation (SD) is given before and after the 15-week exercise intervention. Within group analyses (i.e., before vs. after the exercise intervention) and between group analyses (FMS vs. CON) are reported (p-values)
* denotes significant difference.
The FMS showed significant decreases in [glutamate], [pyruvate], and [glucose] after the 15-week exercise intervention (Table 3), while no significant changes were found in CON. As a consequence, no significant group differences for the mean concentrations of the five biochemical substances were found after the 15-week exercise intervention (Table 3). In order to clarify the importance of these within group differences a multivariate regression of group membership was done using the differences in the biochemical substances as regressors. Hence, this regression confirmed that belonging to the FMS group (R2 = 0.31; p<0.05) was associated with changes (differences between concentration before and after 15-week exercise intervention) in [pyruvate] (VIP = 1.64+) and in [glutamate] (VIP = 1.31+).
No significant differences in mean blood flow between the two groups were found at the two sessions–before: CON: 0.64 ± 0.17 vs. FMS: 0.63 ± 0.17; p = 0.549; after CON: 0.63 ± 0.13 vs. FMS: 0.58 ± 0.17; p = 0.231. No significant within group changes (i.e., before vs. after the 15-week exercise intervention) in mean blood flow were found in either the CON (p = 0.707) or the FMS (p = 0.113).
Regressions of pain intensity, PPT, psychological status and physical tests
Regressions were made in order to explore if the concentrations of the biochemical substances correlated with pain aspects and physical performance; regression of pain intensity was not done in CON as the subjects in this group had no chronic pain.
No significant regressions were obtained when regressing mean pain intensity using the mean values of the five biochemical substances and blood flow in FMS before and after the 15-week exercise intervention. A significant regression was found when regressing the difference in mean pain intensity (before exercise minus after the 15-week exercise intervention) using the changes in biochemical substances and blood flow (before minus after the 15-week exercise intervention) (R2 = 0.13; p<0.05) and the following variables were significant: change in [pyruvate] (VIP = 1.72-) and change in [glucose] (VIP = 1.02-).
No significant regressions were obtained when regressing PPT or psychological status (both subscales of HADS) in each group separately using the mean values of the biochemical substances and blood flow as regressors.
It was not possible to significantly regress differences in the physical tests (i.e., after minus before the 15-week exercise intervention) using the differences in the biochemical concentrations and blood flow (before exercise intervention minus after the exercise intervention) in all subjects taken together or in either of the two groups separately.
Discussion
This study produced three major and novel results:
FMS was associated with higher [glutamate], [pyruvate], and [lactate].
After the 15-week exercise intervention, the FMS subjects had a decrease in pain intensity, decreases in [glutamate], [pyruvate], and [glucose], and an increase in muscular fitness.
The decrease in pain intensity in FMS after the exercise intervention was significantly correlated with the decreases in [pyruvate] and [glucose].
Biochemical substances and blood flow before the 15-week exercise intervention
Four earlier MD studies have investigated muscle algesic and/or metabolic situations in FMS [28–30,32]. The regression of group membership confirmed that [glutamate] and [pyruvate] were important and [lactate] was also important when considering system-wide aspects. Although only three substances had increased concentrations, a relatively massive nociceptive inflow to the CNS may be present if similar alterations are present in several muscles. Muscle nociceptive inputs are believed to be important for maintaining central hyperexcitability [1]. Moreover, although the levels of glutamate, pyruvate and glucose had decreased after the 15-week exercise intervention and the decreases in two of these substances correlated with decreases in pain intensity it must be noted that FMS was not pain free after the exercise intervention. Taken together this may indicate that other peripheral and CNS factors also are important for pain aspects in FMS. A proteomic study of trapezius dialysate in CWP/FMS found that 1/3 of the proteins were significantly up or down regulated [54], which could reflect nociceptive processes, deconditioning, etc. The present and other MD studies pinpoint the need to move from a search of single molecules to a more systematic approach with respect to metabolites, algesics, and non-inflammatory substances [27].
Glutamate
The finding that increased [glutamate] was present before the exercise intervention (Table 3 and S1 Fig) agrees with findings from a study of the trapezius muscle of CWP/FMS [30]. However, in another study [glutamate] during rest did not differ between FMS and controls [29]. The difference in results may be related to methodological differences. Higher [glutamate] in muscles of local/regional pain conditions have also been found [26,27,55], although there is not total consensus [56]. Glutamate can be released from peripheral afferent neurons, resulting in excitation and sensitization of the same or adjacent neurons [55,57,58]. Glutamate acts via N-methyl-D-aspartate (NMDA), AMPA and possibly TRPV1 receptors [59–61]. When NMDA receptors are activated by glutamate, Nitric Oxide (NO)–a reactive oxygen species (ROS)–is generated [62] and can contribute to peripheral hyperalgesia [63,64]. Glutamate can induce experimental pain [55] and hypertonic saline infusion into the masseter and biceps muscles leads to release of glutamate [65,66]. Thus, human studies of e.g. FMS have found that glutamate is a peripheral algesic and contributes to chronic pain.
Lactate, pyruvate, and blood flow
Lactate is produced during anaerobic and aerobic conditions and can be metabolized in the same cell or transported to other cells for metabolic use [67–73]. [Pyruvate] was significantly increased in FMS and [lactate] was multivariately important (Table 3). Increased [lactate] and [pyruvate] were found in the trapezius muscle of FMS [29]; this observation was also confirmed for [lactate] in FMS/CWP [30]. Hence, most studies of FMS show significant alterations in these substances.
Increased [lactate] and [pyruvate] can induce ROS [74–76], which may interact with nociception [77]. On the other hand, it has been suggested that pyruvate is an endogenous antioxidant [78–80]. A human study showed that a combination of metabolites is needed to activate nociceptors [81]. Lactic acid is dissociated at body pH [82]. Lactate together with adenosine triphosphate (ATP) facilitate the response of acid-sensing ion channel 3 (ASIC-3) to low pH [83–86]. Other receptors for low pH are TRPV1 [87], TRPV4 [61], TRPC4, and TRPC5 [88].
Increased [pyruvate] and [lactate] cannot be explained by insufficient oxygen supply since no group difference in blood flow existed either in the present cohort (S6 Fig) or the FMS/CWP cohort [30]; FMS studies using other techniques are inconsistent [89–92]. Altered activation patterns of the fibromyalgic muscle [93–96] may indicate muscular over-activity and thereby higher [lactate] and possibly [pyruvate] [97,98]. Mitochondrial insufficiency is another explanation for increased [lactate] and possibly [pyruvate] [99,100]. Some studies report increased frequencies of muscle fibres with alterations in mitochondrial distribution [101–103]. Decreased levels of ATP and phosphocreatine in vastus lateralis of FMS could indicate mitochondrial insufficiency [104,105]. Mitochondria dysfunction may be linked to the pain per se, but it may also be due to e.g. physical inactivity [99]. In FMS/CWP, muscular fitness is reduced [101,106]; a tendency also noted in this cohort of FMS.
Glycerol and Glucose
Glycerol is a component of cell membranes [107] and insertion of catheters may cause an increase in [glycerol]. No difference in tissue reactions existed between the two groups (Table 3), but on the other hand it could have occurred during the trauma phase (not analysed in the present study). An increase in [glycerol] together with increases in [glutamate] and [serotonin] after hypertonic saline infusion have been reported [65], but their relationship to pain is unclear.
Correlations between elevated [glucose] in plasma and chronic pain have been reported [108,109], while PPT correlated positively with hyperglycemia in healthy men [110]. As in our study, one study found no significant group differences in [glucose] in chronic trapezius myalgia of low severity [111]. The decrease in [glucose] after the exercise intervention may be due to decreased sympathetic activity in FMS [112–114]. Unfortunately indicators of sympathetic drive was not registered using e.g. heart rate analysis in the present study.
The effects of the 15-week exercise intervention
Earlier studies of exercise studies concerning chronic pain including FMS and CWP [18–21,115] also found decreased pain intensity. In addition, our study found improvements in 4/5 fitness tests in FMS. Our study also found that the CON exhibited significant improvement in WT-6min and the strength of the knee extensors nearly reached significant improvements.
The mechanisms for the positive effects of exercise in chronic pain are unclear as mentioned earlier, which hinders the development of exercise interventions aimed at diminishing pain. However, our study found that exercise at least partially is linked to periphearl effects in FMS and that glutamate and pyruvate normalized simultaneous with a decrease in pain intensity. One earlier project has used MD to evaluate the effects of exercise and reported decreased [glutamate] and [substance P] together with increased [beta-endorphin] and [cortisol] in the trapezius of subjects with chronic neck-shoulder pain [35]. Moreover, the effects on two NAEs differed with respect to type of exercise intervention [116]. Hence, two chronic pain conditions showed peripheral changes and decreased [glutamate] after exercise interventions. Karlsson et al. reported that improvements in pain intensity were associated with a decrease in [glutamate] and in an increase in cortisol [35]. In this study, improvements in pain intensity were associated with a decrease in [pyruvate]. The reason for this difference is unclear but may be linked to different pain conditions, muscles, and/or exercise interventions were investigated. Although a significant relationship existed, the explained variation was low (R2 = 0.13), indicating that other substances may be more important with respect to pain intensity. The possibility that the changes in metabolites mainly was linked to improvements in muscular fitness was not confirmed.
Strengths and Limitations
The number of subjects in each group was higher than in other MD studies of FMS. FMS shows considerable heterogeneity, so it is difficult to generalize our results to FMS in the population. Both in this and other FMS cohorts investigated by our group, a low severity of comorbidities was noted and there is a risk that the severe cases of FMS were excluded due to nature of the studies. The design of the 15-week exercise intervention made it impossible to determine when the changes occurred. It is important to elucidate when improvements in pain intensity, physical performance, and biochemical substances occur to optimize exercise interventions. Significant differences in BMI and weight existed between the two groups and it cannot be completely ruled out that the normalization of metabolites in FMS was due to improved fitness after the 15-week exercise intervention. Against this can be argued that we found no significant correlations between changes in physical performance and changes in metabolites. In the present study was made no corrections for multiple statistical testing since the tests were not statistically independent, which is obvious when analysing the different time points e.g. for [glutamate] (S1 Fig) between the two groups. Using Bonferroni corrections in such situations will lead to too strong corrections and increase the risk for false negative results. The use of MVDA in the present study mitigates but does not completely eliminate the problem of multiple testing.
Conclusions
FMS had significantly increased [glutamate], [pyruvate] and [lactate] in the vastus lateralis before the exercise intervention. The increased levels of [glutamate] and [pyruvate] decreased after the 15-week exercise intervention and no group differences were noted. The decrease in pain intensity in FMS after the 15-week exercise intervention was significantly correlated with the decreases in [pyruvate] and [glucose]. As in earlier studies, our study indicates that peripheral muscle alterations may contribute to pain in FMS patients. These alterations can be normalized and pain intensity can be decreased (but not abolished) after an exercise intervention. In addition, the effects of exercise in FMS are partially peripheral.
Supporting Information
(PDF)
In this appendix are MD results reported more in detail i.e. at the five time points during the MD sessions before and after the intervention in the two group of subjects.
(PDF)
Interstitial concentration of glutamate (mean ± SEM; μmol l-1) before and after the 15-week exercise intervention in FMS (upper panel) and CON (lower panel) at the time points 140 (baseline), 160 (immediately after 20-min work period), 180 (recovery), 200 (recovery), and 220 (recovery) min. * denotes significant difference in interstitial concentration of glutamate at that time point between before and after the exercise intervention.
(PDF)
Interstitial concentration of lactate (mean ± SEM; mmol l-1) before and after the 15-week exercise intervention in FMS (upper panel) and CON (lower panel) at the time points 140 (baseline), 160 (immediately after 20-min work period), 180 (recovery), 200 (recovery), and 220 (recovery) min. * denotes significant difference interstitial concentration of lactate at that time point between before and after the exercise intervention.
(PDF)
Interstitial concentration of pyruvate (mean ± SEM; μmol l-1) before and after the 15-week exercise intervention in FMS (upper panel) and CON (lower panel) at the time points 140 (baseline), 160 (immediately after 20-min work period), 180 (recovery), 200 (recovery), and 220 (recovery) min. * denotes significant difference in interstitial concentration of pyruvate at that time point between before and after the exercise intervention.
(PDF)
Interstitial concentration of glycerol (mean ± SEM; mmol l-1) before and after the 15-week exercise intervention in FMS (upper panel) and CON (lower panel) at the time points 140 (baseline), 160 (immediately after 20-min work period), 180 (recovery), 200 (recovery), and 220 (recovery) min. * denotes significant difference in concentration of glycerol at that time point between before and after the exercise intervention.
(PDF)
Interstitial concentration of glucose (mean ± SEM; mmol l-1) before and after the 15-week exercise intervention in FMS (upper panel) and CON (lower panel) at the time points 140 (baseline), 160 (immediately after 20-min work period), 180 (recovery), 200 (recovery), and 220 (recovery) min. * denotes significant difference in concentration of glucose at that time point between before and after the exercise intervention.
(PDF)
Blood flow of the trapezius (mean ± SEM; arbitrary units) before and after the 15-week exercise intervention in FMS (upper panel) and CON (lower panel) at the time points 140 (baseline), 160 (immediately after 20-min work period), 180 (recovery), 200 (recovery), and 220 (recovery) min. * denotes significant difference in blood flow at that time point between before and after the exercise intervention.
(PDF)
(PDF)
Acknowledgments
We would like to thank all colleagues who performed examinations and supervised the groups in Gothenburg, Alingsås, Linköping, and Stockholm.
Data Availability
Data can be found in the Public repository of Linköping University: http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-111796.
Funding Statement
This work was funded by the Swedish Rheumatism Association, the Swedish Research Council, the Health and Medical Care Executive Board of Västra Götaland Region, ALF-LUA at Sahlgrenska University Hospital and Linköping University Hospital, Linköping University, Gothenburg Centre for Person Centred Care (GPCC) and AFA Insurance. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Staud R (2011) Peripheral pain mechanisms in chronic widespread pain. Best Pract Res Clin Rheumatol 25: 155–164. 10.1016/j.berh.2010.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peolsson M, Borsbo B., Gerdle B. (2007) Generalized pain is associated with more negative consequences than local or regional pain: a study of chronic whiplash-associated disorders. J Rehabil Med 39: 260–268. 10.2340/16501977-0052 [DOI] [PubMed] [Google Scholar]
- 3.Petersel DL, Dror V, Cheung R (2011) Central amplification and fibromyalgia: disorder of pain processing. J Neurosci Res 89: 29–34. 10.1002/jnr.22512 [DOI] [PubMed] [Google Scholar]
- 4.Bradley LA (2009) Pathophysiology of fibromyalgia. Am J Med 122: S22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith H, Harris R, Clauw D (2011) Fibromyalgia: an afferent processing disorder leading to a complex pain generalized syndrome. Pain Physician 14: E217–245. [PubMed] [Google Scholar]
- 6.Jensen K, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, et al. (2009) Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain 144: 95–100. 10.1016/j.pain.2009.03.018 [DOI] [PubMed] [Google Scholar]
- 7.Lannersten L, Kosek E (2010) Dysfunction of endogenous pain inhibition during exercise with painful muscles in patients with shoulder myalgia and fibromyalgia. Pain 151: 77–86. 10.1016/j.pain.2010.06.021 [DOI] [PubMed] [Google Scholar]
- 8.Uceyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A, et al. (2013) Small fibre pathology in patients with fibromyalgia syndrome. Brain: a journal of neurology. 10.1093/brain/awt053 [DOI] [PubMed] [Google Scholar]
- 9.Oaklander AL, Herzog ZD, Downs HM, Klein MM (2013) Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 154: 2310–2316. 10.1016/j.pain.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serra J, Collado A, Sola R, Antonelli F, Torres X, Salgueiro M, et al. (2013) Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol. 10.1002/ana.24065 [DOI] [PubMed] [Google Scholar]
- 11.Bennett RM (1999) Emerging concepts in the neurobiology of chronic pain: evidence of abnormal sensory processing in fibromyalgia. Mayo Clin Proc 74: 385–398. 10.4065/74.4.385 [DOI] [PubMed] [Google Scholar]
- 12.Staud R (2008) The role of peripheral input for chronic pain syndromes like fibromyalgia syndrome. J Musculoskelet Pain 16: 67–74. 10.1080/10582450801960339 [DOI] [Google Scholar]
- 13.Schneider GM, Smith AD, Hooper A, Stratford P, Schneider KJ, Westaway MD, et al. (2010) Minimizing the source of nociception and its concurrent effect on sensory hypersensitivity: An exploratory study in chronic whiplash patients. BMC Musculoskelet Disord 11: 29 10.1186/1471-2474-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staud R, Nagel S, Robinson ME, Price DD (2009) Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain 145: 96–104. 10.1016/j.pain.2009.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herren-Gerber R, Weiss S, Arendt-Nielsen L, Petersen-Felix S, Di Stefano G, Radanov BP, et al. (2004) Modulation of central hypersensitivity by nociceptive input in chronic pain after whiplash injury. Pain Med 5: 366–376. 10.1111/j.1526-4637.2004.04055.x [DOI] [PubMed] [Google Scholar]
- 16.Staud R, Robinson ME, Weyl EE, Price DD (2010) Pain variability in fibromyalgia is related to activity and rest: role of peripheral tissue impulse input. J Pain 11: 1376–1383. 10.1016/j.jpain.2010.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosek E, Ekholm J, Hansson P (1996) Modulation of pressure pain thresholds during and following isometric contraction in patients with fibromyalgia and in healthy controls. Pain 64: 415–423. 10.1016/0304-3959(95)00112-3 [DOI] [PubMed] [Google Scholar]
- 18.Busch AJ, Barber KA, Overend TJ, Peloso PM, Schachter CL (2007) Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev: CD003786 10.1002/14651858.cd003786.pub2 [DOI] [PubMed] [Google Scholar]
- 19.Busch AJ, Webber SC, Richards RS, Bidonde J, Schachter CL, Schafer LA, et al. (2013) Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev 12: CD010884 10.1002/14651858.CD010884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassett AL, Williams DA (2011) Non-pharmacological treatment of chronic widespread musculoskeletal pain. Best Pract Res Clin Rheumatol 25: 299–309. 10.1016/j.berh.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 21.Kristensen J, Franklyn-Miller A (2012) Resistance training in musculoskeletal rehabilitation: a systematic review. Br J Sports Med 46: 719–726. 10.1136/bjsm.2010.079376 [DOI] [PubMed] [Google Scholar]
- 22.Ahn AH (2013) Why does increased exercise decrease migraine? Curr Pain Headache Rep 17: 379 10.1007/s11916-013-0379-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen KB, Berna C, Loggia ML, Wasan AD, Edwards RR, Gollub RL (2012) The use of functional neuroimaging to evaluate psychological and other non-pharmacological treatments for clinical pain. Neurosci Lett 520: 156–164. 10.1016/j.neulet.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortega E, Garcia JJ, Bote ME, Martin-Cordero L, Escalante Y, Saavedra JM, et al. (2009) Exercise in fibromyalgia and related inflammatory disorders: known effects and unknown chances. Exerc Immunol Rev 15: 42–65. [PubMed] [Google Scholar]
- 25.Steiger F, Wirth B, de Bruin ED, Mannion AF (2012) Is a positive clinical outcome after exercise therapy for chronic non-specific low back pain contingent upon a corresponding improvement in the targeted aspect(s) of performance? A systematic review. Eur Spine J 21: 575–598. 10.1007/s00586-011-2045-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerdle B, Larsson B (2012) Potential muscle biomarkers of chronic myalgia in humans—a systematic review of microdialysis studies In: Khan T, editor. Biomarker INTECH; open Access publisher. [Google Scholar]
- 27.Gerdle B, Ghafouri B, Ernberg M, Larsson B (2014) Chronic musculoskeletal pain: review of mechanisms and biochemical biomarkers as assessed by the microdialysis technique. J Pain Res 7: 313–326. 10.2147/JPR.S59144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ernberg M, Hedenberg-Magnusson B, Alstergren P, Kopp S (1999) The level of serotonin in the superficial masseter muscle in relation to local pain and allodynia. Life sciences 65: 313–325. 10.1016/s0024-3205(99)00250-7 [DOI] [PubMed] [Google Scholar]
- 29.Gerdle B, Soderberg K, Salvador Puigvert L, Rosendal L, Larsson B (2010) Increased interstitial concentrations of pyruvate and lactate in the trapezius muscle of patients with fibromyalgia: a microdialysis study. J Rehabil Med 42: 679–687. 10.2340/16501977-0581 [DOI] [PubMed] [Google Scholar]
- 30.Gerdle B, Larsson B, Forsberg F, Ghafouri N, Karlsson L, Stensson N, et al. (2014) Chronic widespread pain: increased glutamate and lactate concentrations in the trapezius muscle and plasma. Clin J Pain 30: 409–420. 10.1097/AJP.0b013e31829e9d2a [DOI] [PubMed] [Google Scholar]
- 31.Ghafouri N, Ghafouri B, Larsson B, Stensson N, Fowler CJ, Gerdle B (2013) Palmitoylethanolamide and stearoylethanolamide levels in the interstitium of the trapezius muscle of women with chronic widespread pain and chronic neck-shoulder pain correlate with pain intensity and sensitivity. Pain 154: 1649–1658. 10.1016/j.pain.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 32.McIver KL, Evans C, Kraus RM, Ispas L, Sciotti VM, Hickner RC (2006) NOmediated alterations in skeletal muscle nutritive blood flow and lactate metabolism in fibromyalgia. Pain 120: 161–169. 10.1016/j.pain.2005.10.032 [DOI] [PubMed] [Google Scholar]
- 33.Palstam A, Larsson A, Bjersing J, Lofgren M, Ernberg M, Bileviciute-Ljungar I, et al. (2014) Perceived exertion at work in women with fibromyalgia: Explanatory factors and comparison with healthy women. J Rehabil Med 46: 773–780. 10.2340/16501977-1843 [DOI] [PubMed] [Google Scholar]
- 34.Larsson A, Palstam A, Lofgren M, Ernberg M, Bjersing J, Bileviciute-Ljungar I, et al. (2015) Resistance exercise improves muscle strength, health status and pain intensity in fibromyalgia—a randomized controlled trial. Arthritis Res Ther 17: 161 10.1186/s13075-015-0679-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlsson L, Gerdle B, Ghafouri B, Backryd E, Olausson P, Ghafouri N, et al. (2014) Intramuscular pain modulatory substances before and after exercise in women with chronic neck pain. Eur J Pain. 10.1002/ejp.630 [DOI] [PubMed] [Google Scholar]
- 36.Bjelland I, Dahl AA, Haug TT, Neckelmann D (2002) The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 52: 69–77. 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 37.Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361–370. 10.1037/t03589-000 [DOI] [PubMed] [Google Scholar]
- 38.Sullivan M, Karlsson J., Ware J. (1995) The Swedish 36 Health survey. Evaluation of data quality, scaling assumption, reliability and construct validity across general populations in Sweden. Soc Sci Med 41: 1349–1358. 10.1016/0277-9536(95)00125-q [DOI] [PubMed] [Google Scholar]
- 39.Leggin B, Neuman R, Iannotti J, Williams G, Thompson E (1996) Intrarater and interrater reliability of three isometric dynamometers in assessing shoulder strength. J Shoulder Elbow Surg 5: 18–24. 10.1016/s1058-2746(96)80026-7 [DOI] [PubMed] [Google Scholar]
- 40.Brodin E, Ljungman S, Sunnerhagen K (2008) Rising from a chair: a simple screening test for physical function in predialysis patients. Scand J Urol Nephrol 42: 293–300. 10.1080/00365590701797556 [DOI] [PubMed] [Google Scholar]
- 41.Schaufelberger M, Eriksson B, Grimby G, Held P, Swedberg K (1997) Skeletal muscle alterations in patients with chronic heart failure. Eur Heart J 18: 971–980. 10.1093/oxfordjournals.eurheartj.a015386 [DOI] [PubMed] [Google Scholar]
- 42.Mannerkorpi K, Svantesson U, Carlsson J, Ekdahl C (1999) Tests of functional limitations in fibromyalgia syndrome: a reliability study. Arthritis Care Res 12: 193–199. [DOI] [PubMed] [Google Scholar]
- 43.Sjors A, Larsson B, Persson AL, Gerdle B (2011) An increased response to experimental muscle pain is related to psychological status in women with chronic non-traumatic neck-shoulder pain. BMC Musculoskelet Disord 12: 230 10.1186/1471-2474-12-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. (1990) The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33: 160–172. 10.1002/art.1780330203 [DOI] [PubMed] [Google Scholar]
- 45.Mannerkorpi K, Nordeman L, Ericsson A, Arndorw M, Group GAUS (2009) Pool exercise for patients with fibromyalgia or chronic widespread pain: a randomized controlled trial and subgroup analyses. J Rehabil Med 41: 751–760. 10.2340/16501977-0409 [DOI] [PubMed] [Google Scholar]
- 46.Valkeinen H, Hakkinen A, Hannonen P, Hakkinen K, Alen M (2006) Acute heavy-resistance exercise-induced pain and neuromuscular fatigue in elderly women with fibromyalgia and in healthy controls: effects of strength training. Arthritis Rheum 54: 1334–1339. 10.1002/art.21751 [DOI] [PubMed] [Google Scholar]
- 47.Waelgaard L, Pharo A, Tonnessen TI, Mollnes TE (2006) Microdialysis for monitoring inflammation: efficient recovery of cytokines and anaphylotoxins provided optimal catheter pore size and fluid velocity conditions. Scand J Immunol 64: 345–352. 10.1111/j.1365-3083.2006.01826.x [DOI] [PubMed] [Google Scholar]
- 48.Scheller D, Kolb J (1991) The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. J Neurosci Methods 40: 31–38. 10.1016/0165-0270(91)90114-f [DOI] [PubMed] [Google Scholar]
- 49.Hickner R, Bone D, Ungerstedt U, Jorfeldt L, Henriksson J (1994) Muscle blood flow during intermittent exercise: comparison of the microdialysis ethanol technique and 133Xe clearance. Clin Sci (Lond) 86: 15–25. 10.1042/cs0860015 [DOI] [PubMed] [Google Scholar]
- 50.Stallknecht B, Donsmark M, Enevoldsen LH, Fluckey JD, Galbo H (1999) Estimation of rat muscle blood flow by microdialysis probes perfused with ethanol, [14C]ethanol, and 3H2O. J Appl Physiol 86: 1054–1061. [DOI] [PubMed] [Google Scholar]
- 51.Jansen JJ, Szymanska E, Hoefsloot HC, Jacobs DM, Strassburg K, Smilde AK (2012) Between Metabolite Relationships: an essential aspect of metabolic change. Metabolomics 8: 422–432. 10.1007/s11306-011-0316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pohjanen E, Thysell E, Jonsson P, Eklund C, Silfver A, Carlsson IB, et al. (2007) A multivariate screening strategy for investigating metabolic effects of strenuous physical exercise in human serum. J Proteome Res 6: 2113–2120. 10.1021/pr070007g [DOI] [PubMed] [Google Scholar]
- 53.Eriksson L, Johansson E, Kettaneh-Wold N, Trygg J, Wikström C, Wold S (2006) Multi- and Megavariate Data analysis; part I and II Umeå: Umetrics AB. [Google Scholar]
- 54.Olausson P, Gerdle B, Ghafouri N, Larsson B, Ghafouri B (2012) Identification of proteins from interstitium of trapezius muscle in women with chronic myalgia using microdialysis in combination with proteomics. PLoS One 7: e52560 10.1371/journal.pone.0052560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cairns BE, Dong X (2008) The role of peripheral glutamate and glutamate receptors in muscle pain. J Musculoskelet Pain 16: 85–91. 10.1080/10582450801960388 [DOI] [Google Scholar]
- 56.Ernberg M, Castrillon EE, Ghafouri B, Larsson B, Gerdle B, List T, et al. (2013) Experimental myalgia induced by repeated infusion of acidic saline into the human masseter muscle does not cause the release of algesic substances. Eur J Pain 17: 539–550. 10.1002/j.1532-2149.2012.00216.x [DOI] [PubMed] [Google Scholar]
- 57.Miller KE, Hoffman EM, Sutharshan M, Schechter R (2011) Glutamate pharmacology and metabolism in peripheral primary afferents: physiological and pathophysiological mechanisms. Pharmacol Ther 130: 283–309. 10.1016/j.pharmthera.2011.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lam D, Hu J, Sessle B, Cairns B (2005) Neural mechanisms of temporomandibular joint and masticatory muscle pain: A possible role for peripheral glutamate receptor mechanisms. Pain Research & Management 10: 145–152. 10.1155/2005/860354 [DOI] [PubMed] [Google Scholar]
- 59.Alfredson H, Forsgren S, Thorsen K, Lorentzon R (2001) In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper's knee. J Orthop Res 19: 881–886. 10.1016/S0736-0266(01)00016-X [DOI] [PubMed] [Google Scholar]
- 60.Coggeshall RE, Carlton SM (1998) Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. The Journal of comparative neurology 391: 78–86. [DOI] [PubMed] [Google Scholar]
- 61.Schaible HG, Ebersberger A, Natura G (2011) Update on peripheral mechanisms of pain: beyond prostaglandins and cytokines. Arthritis Res Ther 13: 210 10.1186/ar3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan W, Huang F, Wu Z, Zhu X, Li D, He H (2012) The role of nitric oxide in orofacial pain. Nitric Oxide 26: 32–37. 10.1016/j.niox.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 63.Cury Y, Picolo G, Gutierrez VP, Ferreira SH (2011) Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide 25: 243–254. 10.1016/j.niox.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 64.Benarroch EE (2011) Nitric oxide: A pleiotropic signal in the nervous system. Neurology 77: 1568–1576. 10.1212/WNL.0b013e318233b3e4 [DOI] [PubMed] [Google Scholar]
- 65.Louca S, Christidis N, Ghafouri B, Gerdle B, Svensson P, List T, et al. (2014) Serotonin, glutamate and glycerol are released after the injection of hypertonic saline into human masseter muscles—a microdialysis study. J Headache Pain 15: 89 Epub ahead of print. 10.1186/1129-2377-15-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tegeder L, Zimmermann J, Meller S, Geisslinger G (2002) Release of algesic substances in human experimental muscle pain. Inflamm Res 51: 393–402. 10.1007/pl00000320 [DOI] [PubMed] [Google Scholar]
- 67.Brooks GA (2009) Cell-cell and intracellular lactate shuttles. J Physiol 587: 5591–5600. 10.1113/jphysiol.2009.178350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gladden LB (2004) Lactate metabolism: a new paradigm for the third millenium. J Physiol (Lond) 558: 5–30. 10.1113/jphysiol.2003.058701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Philip A, Macdonald AL, Watt PW (2005) Lactate—a signal coordnating cell and systemic function. Exp Biol 208: 4561–4575. [DOI] [PubMed] [Google Scholar]
- 70.Draoui N, Feron O (2011) Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech 4: 727–732. 10.1242/dmm.007724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robergs RA, Ghiasvand F, Parker D (2004) Biochemistry of exercise-induced metabolic acidosis. American Journal of Physiology Regulatory, Integrative and Comparative Physiology 287: R502–R516. 10.1152/ajpregu.00114.2004 [DOI] [PubMed] [Google Scholar]
- 72.Gjedsted J, Buhl M, Nielsen S, Schmitz O, Vestergaard ET, Tonnesen E, et al. (2011) Effects of adrenaline on lactate, glucose, lipid and protein metabolism in the placebo controlled bilaterally perfused human leg. Acta Physiol (Oxf) 202: 641–648. 10.1111/j.1748-1716.2011.02316.x [DOI] [PubMed] [Google Scholar]
- 73.Allen M (2011) Lactate and acid base as a hemodynamic monitor and markers of cellular perfusion. Pediatr Crit Care Med 12: S43–49. 10.1097/PCC.0b013e3182211aed [DOI] [PubMed] [Google Scholar]
- 74.Cruz RS, de Aguiar RA, Turnes T, Penteado Dos Santos R, de Oliveira MF, Caputo F (2012) Intracellular shuttle: the lactate aerobic metabolism. ScientificWorldJournal 2012: 420984 10.1100/2012/420984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lanza IR, Nair KS (2010) Mitochondrial function as a determinant of life span. Pflugers Arch 459: 277–289. 10.1007/s00424-009-0724-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barbieri E, Sestili P (2012) Reactive oxygen species in skeletal muscle signaling. J Signal Transduct 2012: 982794 10.1155/2012/982794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kallenborn-Gerhardt W, Schroder K, Geisslinger G, Schmidtko A (2012) NOXious signaling in pain processing. Pharmacol Ther. [DOI] [PubMed] [Google Scholar]
- 78.Das UN (2006) Is pyruvate an endogenous anti-inflammatory molecule? Nutrition 22: 965–972. 10.1016/j.nut.2006.05.009 [DOI] [PubMed] [Google Scholar]
- 79.Kao KK, Fink MP (2010) The biochemical basis for the anti-inflammatory and cytoprotective actions of ethyl pyruvate and related compounds. Biochem Pharmacol 80: 151–159. 10.1016/j.bcp.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 80.Fink MP (2007) Ethyl pyruvate: a novel anti-inflammatory agent. J Intern Med 261: 349–362. 10.1097/01.CCM.0000042476.32014.43 [DOI] [PubMed] [Google Scholar]
- 81.Pollak KA, Swenson JD, Vanhaitsma TA, Hughen RW, Jo D, White AT, et al. (2014) Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol 99: 368–380. 10.1113/expphysiol.2013.075812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Birklein F, Weber M, Neundorfer B (2000) Increased skin lactate in complex regional pain syndrome: evidence for tissue hypoxia? Neurology 55: 1213–1215. 10.1212/wnl.55.8.1213 [DOI] [PubMed] [Google Scholar]
- 83.Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, et al. (2010) Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron 68: 739–749. 10.1016/j.neuron.2010.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Immke DC, McCleskey EW (2001) ASIC3: a lactic acid sensor for cardiac pain. ScientificWorldJournal 1: 510–512. 10.1100/tsw.2001.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim TJ, Freml L, Park SS, Brennan TJ (2007) Lactate concentrations in incisions indicate ischemic-like conditions may contribute to postoperative pain. J Pain 8: 59–66. 10.1016/j.jpain.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 86.Deval E, Gasull X, Noel J, Salinas M, Baron A, Diochot S, et al. (2010) Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther 128: 549–558. 10.1016/j.pharmthera.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 87.Lee Y, Lee CH, Oh U (2005) Painful channels in sensory neurons. Mol Cells 20: 315–324. [PubMed] [Google Scholar]
- 88.Eisenhut M, Wallace H (2011) Ion channels in inflammation. Pflugers Arch 461: 401–421. 10.1007/s00424-010-0917-y [DOI] [PubMed] [Google Scholar]
- 89.Elvin A, Siosteen AK, Nilsson A, Kosek E (2006) Decreased muscle blood flow in fibromyalgia patients during standardised muscle exercise: a contrast media enhanced colour Doppler study. Eur J Pain 10: 137–144. 10.1016/j.ejpain.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 90.Lund N, Bengtsson A, Thorborg P (1986) Muscle tissue oxygen pressure in primary fibromyalgia. Scand J Rheumatol 15: 165–173. 10.3109/03009748609102084 [DOI] [PubMed] [Google Scholar]
- 91.Sandberg M, Lindberg LG, Gerdle B (2004) Peripheral effects of needle stimulation (acupuncture) on skin and muscle blood flow in fibromyalgia. Eur J Pain 8: 163–171. 10.1016/S1090-3801(03)00090-9 [DOI] [PubMed] [Google Scholar]
- 92.Shang Y, Gurley K, Symons B, Long D, Srikuea R, Crofford LJ, et al. (2012) Noninvasive optical characterization of muscle blood flow, oxygenation, and metabolism in women with fibromyalgia. Arthritis Res Ther 14: R236 10.1186/ar4079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elert J, Aspegren Kendall S, Larsson B, Månsson B, Gerdle B (2001) Chronic pain and difficulty in relaxing postural muscles in patients with fibromylgia and chronic whiplash associated disorders. J Rheumatol 28: 1361–1368. [PubMed] [Google Scholar]
- 94.Gerdle B, Grönlund C, Karlsson S, Holtermann A, Roeleveld K (2010) Altered neuromuscular control mechanisms of the trapezius muscle in fibromyalgia. BMC Musculoskeletal Disorders 11: 42 10.1186/1471-2474-1111-1142v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bazzichi L, Dini M, Rossi A, Corbianco S, De Feo F, Giacomelli C, et al. (2009) Muscle modifications in fibromyalgic patients revealed by surface electromyography (SEMG) analysis. BMC Musculoskelet Disord 10: 36 10.1186/1471-2474-10-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Falla D, Andersen H, Danneskiold-Samsøe B, Arendt-Nielsen L, Farina D (2010) Adaptations of upper trapezius muscle activity during sustained contractions in women with fibromyalgia. J Electromyogr Kinesiol 20: 457–464. 10.1016/j.jelekin.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 97.de Paoli FV, Ortenblad N, Pedersen TH, Jorgensen R, Nielsen OB (2010) Lactate per se improves the excitability of depolarized rat skeletal muscle by reducing the Cl- conductance. J Physiol 588: 4785–4794. 10.1113/jphysiol.2010.196568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Hall G (2010) Lactate kinetics in human tissues at rest and during exercise. Acta Physiol (Oxf) 199: 499–508. 10.1111/j.1748-1716.2010.02122.x [DOI] [PubMed] [Google Scholar]
- 99.Lanza IR, Nair KS (2009) Muscle mitochondrial changes with aging and exercise. Am J Clin Nutr 89: 467S–471S. 10.3945/ajcn.2008.26717D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, et al. (2008) Endurance exercise as a countermeasure for aging. Diabetes 57: 2933–2942. 10.2337/db08-0349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bengtsson A (2002) Editorial, The muscle in fibromyalgia. Rheumatol 41: 721–724. [DOI] [PubMed] [Google Scholar]
- 102.Bengtsson A, Henriksson KG, Larsson J (1986) Muscle biopsy in primary fibromyalgia. Light-microscopical and histochemical findings. Scand J Rheumatol 15: 1–6. 10.3109/03009748609092661 [DOI] [PubMed] [Google Scholar]
- 103.Schmidt-Wilcke T, Clauw DJ (2011) Fibromyalgia: from pathophysiology to therapy. Nat Rev Rheumatol 7: 518–527. 10.1038/nrrheum.2011.98 [DOI] [PubMed] [Google Scholar]
- 104.Gerdle B, Forsgren MF, Bengtsson A, Leinhard OD, Soren B, Karlsson A, et al. (2013) Decreased muscle concentrations of ATP and PCR in the quadriceps muscle of fibromyalgia patients—a 31P-MRS study. Eur J Pain 17: 1205–1215. 10.1002/j.1532-2149.2013.00284.x [DOI] [PubMed] [Google Scholar]
- 105.Park JH, Phothimat P, Oates CT, Hernanz-Schulman M, Olsen NJ (1998) Use of P-31 magnetic resonance spectroscopy to detect metabolic abnormalities in muscles of patients with fibromyalgia. Arthritis Rheum 41: 406–413. [DOI] [PubMed] [Google Scholar]
- 106.Norregaard J, Bulow PM, Danneskiold-Samsoe B (1994) Muscle strength, voluntary activation, twitch properties, and endurance in patients with fibromyalgia. J Neurol Neurosurg Psychiatry 57: 1106–1111. 10.1136/jnnp.57.9.1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ren G, Eiskjaer S, Kaspersen J, Christensen FB, Rasmussen S (2009) Microdialysis of paraspinal muscle in healthy volunteers and patients underwent posterior lumbar fusion surgery. Eur Spine J 18: 1604–1609. 10.1007/s00586-009-1021-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mantyselka P, Miettola J, Niskanen L, Kumpusalo E (2008) Chronic pain, impaired glucose tolerance and diabetes: a community-based study. Pain 137: 34–40. 10.1016/j.pain.2007.08.007 [DOI] [PubMed] [Google Scholar]
- 109.Mantyselka P, Miettola J, Niskanen L, Kumpusalo E (2008) Glucose regulation and chronic pain at multiple sites. Rheumatology (Oxford) 47: 1235–1238. 10.1093/rheumatology/ken220 [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Zhang S, Gao Y, Tan A, Yang X, Zhang H, et al. (2013) Factors associated with the pressure pain threshold in healthy Chinese men. Pain Med 14: 1291–1300. 10.1111/pme.12158 [DOI] [PubMed] [Google Scholar]
- 111.Sjogaard G, Rosendal L, Kristiansen J, Blangsted AK, Skotte J, Larsson B, et al. (2010) Muscle oxygenation and glycolysis in females with trapezius myalgia during stress and repetitive work using microdialysis and NIRS. Eur J Appl Physiol 108: 657–669. 10.1007/s00421-009-1268-2 [DOI] [PubMed] [Google Scholar]
- 112.Hannibal KE, Bishop MD (2014) Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther 94: 1816–1825. 10.2522/ptj.20130597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chalaye P, Goffaux P, Bourgault P, Lafrenaye S, Devroede G, Watier A, et al. (2012) Comparing pain modulation and autonomic responses in fibromyalgia and irritable bowel syndrome patients. Clin J Pain 28: 519–526. 10.1097/AJP.0b013e31823ae69e [DOI] [PubMed] [Google Scholar]
- 114.Kulshreshtha P, Deepak KK (2013) Autonomic nervous system profile in fibromyalgia patients and its modulation by exercise: a mini review. Clin Physiol Funct Imaging 33: 83–91. 10.1111/cpf.12000 [DOI] [PubMed] [Google Scholar]
- 115.Hurley BF, Hanson ED, Sheaff AK (2011) Strength training as a countermeasure to aging muscle and chronic disease. Sports Med 41: 289–306. 10.2165/11585920-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 116.Ghafouri N, Ghafouri B, Fowler CJ, Larsson B, Turkina MV, Karlsson L, et al. (2014) Effects of two different specific neck exercise interventions on palmitoylethanolamide and stearoylethanolamide concentrations in the interstitium of the trapezius muscle in women with chronic neck shoulder pain. Pain Med 15: 1379–1389. 10.1111/pme.12486 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
In this appendix are MD results reported more in detail i.e. at the five time points during the MD sessions before and after the intervention in the two group of subjects.
(PDF)
Interstitial concentration of glutamate (mean ± SEM; μmol l-1) before and after the 15-week exercise intervention in FMS (upper panel) and CON (lower panel) at the time points 140 (baseline), 160 (immediately after 20-min work period), 180 (recovery), 200 (recovery), and 220 (recovery) min. * denotes significant difference in interstitial concentration of glutamate at that time point between before and after the exercise intervention.
(PDF)
Interstitial concentration of lactate (mean ± SEM; mmol l-1) before and after the 15-week exercise intervention in FMS (upper panel) and CON (lower panel) at the time points 140 (baseline), 160 (immediately after 20-min work period), 180 (recovery), 200 (recovery), and 220 (recovery) min. * denotes significant difference interstitial concentration of lactate at that time point between before and after the exercise intervention.
(PDF)
Interstitial concentration of pyruvate (mean ± SEM; μmol l-1) before and after the 15-week exercise intervention in FMS (upper panel) and CON (lower panel) at the time points 140 (baseline), 160 (immediately after 20-min work period), 180 (recovery), 200 (recovery), and 220 (recovery) min. * denotes significant difference in interstitial concentration of pyruvate at that time point between before and after the exercise intervention.
(PDF)
Interstitial concentration of glycerol (mean ± SEM; mmol l-1) before and after the 15-week exercise intervention in FMS (upper panel) and CON (lower panel) at the time points 140 (baseline), 160 (immediately after 20-min work period), 180 (recovery), 200 (recovery), and 220 (recovery) min. * denotes significant difference in concentration of glycerol at that time point between before and after the exercise intervention.
(PDF)
Interstitial concentration of glucose (mean ± SEM; mmol l-1) before and after the 15-week exercise intervention in FMS (upper panel) and CON (lower panel) at the time points 140 (baseline), 160 (immediately after 20-min work period), 180 (recovery), 200 (recovery), and 220 (recovery) min. * denotes significant difference in concentration of glucose at that time point between before and after the exercise intervention.
(PDF)
Blood flow of the trapezius (mean ± SEM; arbitrary units) before and after the 15-week exercise intervention in FMS (upper panel) and CON (lower panel) at the time points 140 (baseline), 160 (immediately after 20-min work period), 180 (recovery), 200 (recovery), and 220 (recovery) min. * denotes significant difference in blood flow at that time point between before and after the exercise intervention.
(PDF)
(PDF)
Data Availability Statement
Data can be found in the Public repository of Linköping University: http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-111796.