Abstract
We developed a thermoswitchable polymeric photosensitizer (T-PPS) by conjugating PS (Pheophorbide-a, PPb-a) to a temperature-responsive polymer backbone of biocompatible hydroxypropyl cellulose. Self-quenched PS molecules linked in close proximity by π–π stacking in T-PPS were easily transited to an active monomeric state by the temperature-induced phase transition of polymer backbones. The temperature-responsive intermolecular interaction changes of PS molecules in T-PPS were demonstrated in synchrotron small-angle X-ray scattering and UV–vis spectrophotometer analysis. The T-PPS allowed switchable activation and synergistically enhanced cancer cell killing effect at the hyperthermia temperature (45 °C). Our developed T-PPS has the considerable potential not only as a new class of photomedicine in clinics but also as a biosensor based on temperature responsiveness.
Current clinically practiced photodynamic therapy (PDT) has been considered as a promising ablative therapeutic option of malignant tissues.1 PDT utilizes reactive species generated through redox processes of photosensitizer (PS) upon light irradiation at an appropriate wavelength.2 PS is a critical and fundamental component of PDT for inducing irreversible damage of cancer cells and has been applied topically or systemically in clinical practices.3 However, the use of PS has been limited by several major issues including (1) poor solubility in blood plasma, (2) fast renal clearance, (3) inadequate selectivity, and (4) the uncontrollable photo-activity.1 Many types of PS and PS-nanocarriers have been developed to overcome those limitations.4 Integration with other oncologic interventions such as thermal therapies has been considered for synergistic photo cancer therapies.5
Despite the developments, nonspecific activation of PS is another barrier toward clinical PDT therapy. Administered PS in patients will be collected and stays in the cells for several weeks.6 If the patients are exposed to sunlight or other forms of environmental light during this period, the exposed tissues can quickly be damaged in only a few minutes.7 Thus, patients treated with PDT are often advised to take precautions for at least 30 days.8 In this regard, it is urgent to develop a switchable PS that can be selectively activated at particular condition beside lights.
Stimuli-responsive PS is one of the most promising approaches for the switchable PS to potently and specifically destruct cancer cells with a specific environment.4d,9 Various polymeric PS responding to internal and external stimuli such as pH,10 enzymes,11 and nucleic acid12 have been actively studied. To this goal, conjugation of PS with thermosensitive polymers is an attractive strategy since it can improve PS solubility, control photoactivity, and enable combining PDT with thermal therapies. Thermoresponsiveness of the polymer is represented by intermolecular or intramolecular structural change along with environment temperature variation.13 The structural changes of the polymers switch the photoactivity of their pendant PS upon an external temperature change. Ideally, thermoswitchable PS should be inactivated at body temperature (“OFF” state), but rapidly restore their photoactivity in a locally heated disease site (“ON” state). This approach can be well integrated with thermal cancer therapies for enhancing cancer therapeutic outcomes.
Thermo-switchable PS, however, has rarely been studied to our best knowledge. Although several researchers reported the temperature-driven fluorescent or oxygenation rate control technology based on thermosensitive poly(N-isopropylacrylamide) (PNIPAM),14 PNIPAM may not be appropriate for clinical applications since the polymer is not biocompatible and its monomer is suspected to be carcinogenic.15 Therefore, it is crucial to develop a thermoswitchable PS having a good bioavailability.
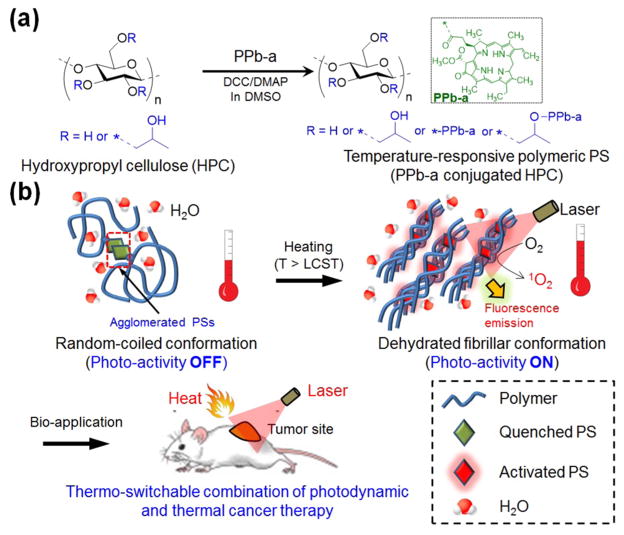
In these regards, we developed a thermoswitchable biopolymeric PS (T-PPS) and studied switching of its photoactivity and synergistic combination with thermal cancer therapy. T-PPS was synthesized by conjugating PS (pheophorbide-a, PPb-a) to a temperature-responsive polymer backbone of hydroxypropyl cellulose (HPC) (Scheme 1a). Biocompatible HPC, a natural cellulose approved by the U.S. FDA,16 was chosen as the polymer backbone. It has a lower critical solution temperature (LCST) in water (~40–45 °C) and undergoes a phase transition in an aqueous environment from hydrophilic random-coil conformation to hydrophobic-collapsed form as temperature increases above the LCST.17 The transition would cause dehydration of the polymer above the LCST due to both of increased hydrophobic interactions among polymer chains and decreased intermolecular hydrogen (H)-bonding interactions between ester groups in HPC and water molecules.
Scheme 1. Schematic Illustration of Temperature-Responsive Polymeric PSa.
a(a) Chemical synthetic route of T-PPS (PPb-a conjugated HPC). (b) Temperature-dependent change in structure and photoactivity of T-PPS for bioapplications.
As depicted in Scheme 1b, we hypothesized that our T-PPS would be inactive to light at temperatures below the polymer’s LCST because in this state, the PS molecules conjugated on polymer chains can be aggregated in aqueous environment, resulting in quenching of the photoactivity of PS below LCST including body temperature region (~37 °C). As, however, temperature increases above the LCST (at temperature for hyperthermia cancer therapy, ~45 °C), the polymer becomes dehydrated and so does PS. The dehydration would create nonpolar microenvironment between the T-PPS molecules, which led to a monomeric state of the PS. As a result, the photoactivity of PS would be significantly enhanced in that condition.
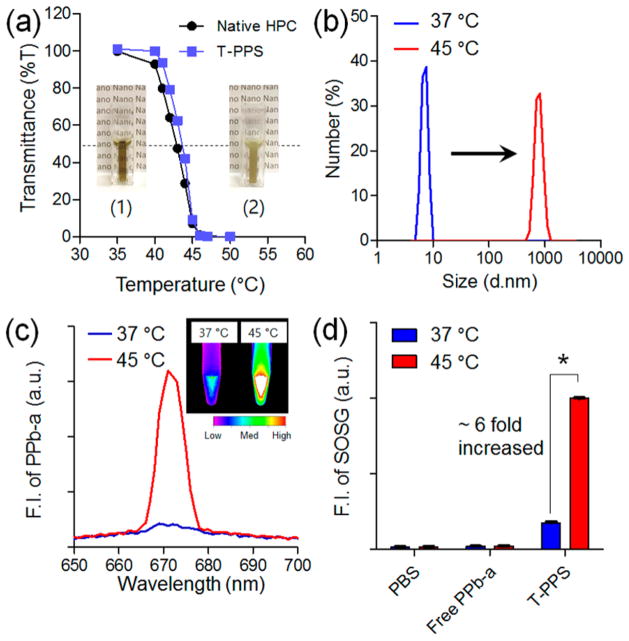
To prove our hypothesis, T-PPS was synthesized by facile conventional carbodiimide reaction with assistance of dicyclo-hexylcarbodiimide (DCC) and 4-(dimethylamino)pyridine (DMAP) (Scheme 1a). An optimal degree of substitution (DS) of PPb-a on the T-PPS was chosen with one to avoid self-quenching of PSs (Figure S1). The fed PPb-a could be randomly reacted with the hydroxyl groups of HPC. Successful synthesis of T-PPS was confirmed using 1H NMR and UV–vis spectrophotometry analysis (Figure S2 and Table S1). Figure 1a depicts the temperature-dependent change of the transmittance of T-PPS aqueous solution at 500 nm. The synthesized T-PPS exhibited phase transition at a temperature higher than 40 °C. The difference between LCST value of native HPC (42.8 ± 0.7) and T-PPS (43.4 ± 0.6) was negligible. The phase transition behavior of T-PPS was reversible when cooling to room temperature (Figure S3). As shown in Figure 1b, the hydrodynamic size of T-PPS measures ~8 nm in average diameter at 37 °C, the physiological temperature (below LCST). The size of T-PPS was significantly increased to ~820 nm at 45 °C (above LCST). To investigate influence of the temperature-sensitive changes on the photoactivity of T-PPS, fluorescent emission and singlet-oxygen generation (SOG) of T-PPS were measured at both 37 and 45 °C. The fluorescence emission of T-PPS was much higher above LCST than below LCST (Figure 1c). The fluorescent emission change was shown as reversible manner along with temperature variation (Figure S4). Temperature-dependent SOG of T-PPS was then quantified with singlet oxygen sensor green (SOSG), chemical SOG probe, under laser irradiation (670 nm).18 Consistent with fluorescence emission data, the T-PPS exhibited significantly increased SOG at 45 °C than at 37 °C, while control groups (PBS or Free PPb-a) did not show any meaningful change (Figure 1d). These results prove our hypothesis, indicating that increasing temperature over the LCST of T-PPS significantly enhances the photo-activity in both fluorescence emission and SOG.
Figure 1.
Physicochemical characterization of T-PPS in different temperature conditions. Temperature-dependent change in (a) transmittance of T-PPS and native HPC (inset: photograph of T-PPS aqueous solution (10 mg/mL in water) at T < LCST and T > LCST), (b) particle size distribution, (c) fluorescent intensity (inset: fluorescent image of T-PPS aqueous solution (10 mg/mL in water) at 37 and 45 °C), and (d) singlet oxygen generation of T-PPS in aqueous solution (n = 3, *p < 0.001).
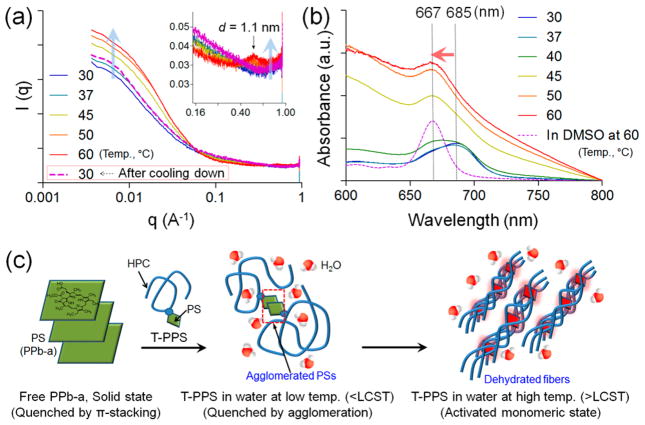
To further understand the temperature-dependent photo-activity change by the intra/intermolecular interaction transition of the T-PPS along with external temperature, we studied the structure changes of T-PPS at different temperature using small-angle X-ray scattering (SAXS). SAXS was performed for T-PPS aqueous solution at different temperature conditions (Figure 2a). The scattering intensity at low q values increased with increasing the temperature and decreased back when the sample was cooled down. Increase of the low q scattering indicates the formation of a larger structure, of which size corresponds to the q region (d-spacing = 2π/q).19 In a polymer solution system, the increase of the low q scattering has been attributed to a phase separation of polymer chain with forming aggregates, of which structure can be fibrillary (Figure S5).20 For example, similar low q behaviors have been observed in the studies of thermoreversible hydration and dehydration of methyl cellulose, where the polymer chains disperse well when hydrated and form fibers when dehydrated.21 Native HPC has also been investigated with scattering techniques (Figure S6) and reported to present an increase of hydrodynamic radius, indicating the aggregation of polymer chains due to the dehydration.22 In addition, we found that the diffraction peak at q = 0.5688 Å−1, corresponding to d-spacing of 1.1 nm, disappeared at low temperature and reappeared as temperature increased (inset in the Figure 2a). In fact, the peak that is also present in the diffraction pattern of a pure HPC in a solid state (Figure S7) is 001 reflection of the HPC.23 On the other hand, the diffraction peaks of the powder PPb-a shown in Figure S7 were not observed at any temperature, which indicates PPb-a molecules do not form their own domain because they are bound to the polymer backbone. To supplement the SAXS results, the intermolecular interaction of PS molecules in T-PPS in various environmental conditions was investigated using a UV–vis spectrophotometer. Figure S8 shows the UV–vis absorption spectra of T-PPS in the different solvent. The spectrum of T-PPS in water displays the broaden Qy-band at 685 nm, which is characteristic for the PPb-a dimer.24 However, monomeric characteristic Qy-band at 667 nm was observed in the organic solvent (DMSO).24,25 In Figure 2b, when the temperature of T-PPS in water was increased, a clear blue shift of the Qy-band (685 → 667 nm) was observed, whereas there is no change in the organic solvent (Figure S9). Interestingly, the intensity of Qy-band of T-PPS at the temperature above LCST was significantly increased, and the increasing intensity of Qy-band of T-PPS showed the same trend in various concentrations range (Figure S10). As a comparison, free PPb-a was also tested at same conditions (Figure S11). These results indicate that the agglomerated PPb-a molecules on the HPC polymer at the temperatures below LCST become a monomeric state at the increased temperatures over LCST.
Figure 2.
SAXS analysis of T-PPS at different temperature conditions. (a) SAXS curve for T-PPS in aqueous solution (10 mg/mL) at different temperature (from 30 to 60 °C) and at 30 °C after cooled down. (b) Light absorption spectra for T-PPS in aqueous solution (10 mg/mL) at different temperature (from 30 to 60 °C). (c) Schematic representation for proposed structural transition of T-PPS in different temperature conditions.
Taken all together, we proposed the structural transition of T-PPS-induced photoactivity changes by temperature stimuli (Figure 2c). The conjugated PPb-a was inactive at temperatures below the LCST since strong hydrophobic PPb-a molecules can be agglomerated in aqueous solution. However, at the temperature above the LCST, the H-bonding between HPC and water started to break, and the polysaccharide chains were dehydrated and formed fibril structure.26 As a result, the PS molecules could undergo intermolecular rearrangement into a monomeric state due to molecular separation by the nonpolar and dehydrated rigid polymer chains (Figure 2c). Monomerization of PSs was critical to activate/increase photoactivity because the packed PS by intermolecular π-interaction, as seen by the low activity of the free PS, would significantly quench the photoactivity through the nonradiative deactivation (Figure 1c and 1d).24,27 Therefore, the conjugation of PS to the HPC polymer enables thermoswitchable PS for PDT applications.
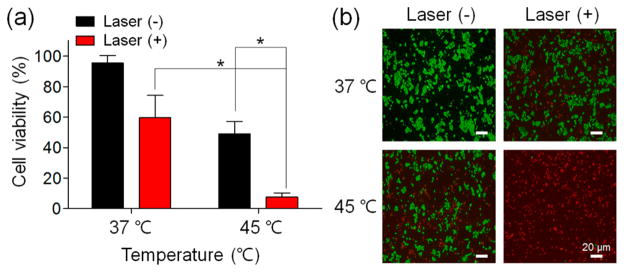
To verify the feasibility of T-PPS for the potential combination of PDT and thermal cancer therapy, in vitro cytotoxicity test was performed using CCK-8 and live/dead assay at 37 (physiological condition) and 45 °C (hyperthermia condition) with or without laser irradiation. Quantitative phototoxicity of T-PPS against PANC-2 (Figure 3a) was significantly enhanced at 45 °C compared to that at 37 °C (*p < 0.002). A similar trend of result was observed in the live (green)/dead (red) assay (Figure 3b). However, without laser irradiation, an average cell viability at 45 °C was decreased over 30% compared to that at 37 °C regardless of polymer existence with various concentrations (Figure S12), which suggests that the toxicity at 45 °C could mainly be attributed to the combinational hyperthermic cell killing effect.28 These results indicate that the temperature-induced photoactivation of the T-PPS remarkably enhanced the cancer cell killing effect.
Figure 3.
In vitro photocytotoxicity of T-PPS at various temperatures on PANC-2 cells. (a) CCK-8 and (b) live/dead assay for cell viability of PANC-2 cells incubated with T-PPS (concentration of PPb-a, 1 μg/mL) under laser irradiation (3.0 J/cm2) at 37 and 45 °C (n = 4, *p < 0.002).
Lastly, in vivo fluorescent imaging was performed in BALB/c nude mice, as a preliminary study to confirm the heat-induced activation of T-PPS in the physiological condition. As shown Figure S13, fluorescent signal of T-PPS was significantly increased under heat-treatment (45 °C) compared to nonheat-treated region, indicating the temperature-mediated photo-activity control of our T-PPS can also be worked in physiological condition.
In conclusion, we developed a thermoswitchable biopolymeric photosensitizer (T-PPS) for selective activation and effective combinational cancer therapy. T-PPS was successfully synthesized with conjugation of conventional PS and biocompatible HPC. Photoactivity of the synthesized T-PPS could be easily controlled by temperature modulation that resulted in thermoswitchable PS activity and enhanced cancer cell killing effect at the hyperthermia temperature (45 °C). SAXS and UV–vis spectrophotometric analysis demonstrated the temperature-responsive structural change of T-PPS inducing photoactivity switching of PS. We believe that developed T-PPS and characterized properties will be valuable for designing and developing new effective PS for cancer therapy applications. Further, our thermoswitchable PS has the considerable potential not only as a new class of photomedicine in clinics but also as a biosensor and drug delivery platform based on temperature responsiveness.
Supplementary Material
Acknowledgments
This work was supported by four grants R01CA141047, R21CA173491, R21EB017986, and R21CA185274 from the NCI and NIBIB. This research was also supported by the Basic Research Laboratory (BRL) Program (no. 2015R1A4A1042350), through the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP). B.L. was supported by MICCoM as part of the Computational Materials Sciences Program funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Materials Sciences and Engineering Division. This work used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
Footnotes
Notes
The authors declare no competing financial interest.
- Additional characterization data for T-PPS, SAXS analysis for free PPb-a and HPC powder, UV–vis absorption spectra of T-PPS and free PPb-a, and in vitro/in vivo test of T-PPS (PDF)
References
- 1.Dolmans DE, Fukumura D, Jain RK. Nat Rev Cancer. 2003;3:380. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 2.Henderson BW, Dougherty TJ. Photochem Photobiol. 1992;55:145. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 3.(a) Brown SB, Brown EA, Walker I. Lancet Oncol. 2004;5:497. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]; (b) Morton CA, Brown S, Collins S, Ibbotson S, Jenkinson H, Kurwa H, Langmack K, McKenna K, Moseley H, Pearse AD, Stringer M, Taylor DK, Wong G, Rhodes LE. Br J Dermatol. 2002;146:552. doi: 10.1046/j.1365-2133.2002.04719.x. [DOI] [PubMed] [Google Scholar]
- 4.(a) Dichtel WR, Serin JM, Edder C, Fréchet JM, Matuszewski M, Tan LS, Ohulchanskyy TY, Prasad PN. J Am Chem Soc. 2004;126:5380. doi: 10.1021/ja031647x. [DOI] [PubMed] [Google Scholar]; (b) Fan H, Yan G, Zhao Z, Hu X, Zhang W, Liu H, Fu X, Fu T, Zhang XB, Tan W. Angew Chem, Int Ed. 2016;128:5567. doi: 10.1002/anie.201510748. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kolemen S, Ozdemir T, Lee D, Kim GM, Karatas T, Yoon J, Akkaya EU. Angew Chem, Int Ed. 2016;55:3606. doi: 10.1002/anie.201510064. [DOI] [PubMed] [Google Scholar]; (d) Lucky SS, Soo KC, Zhang Y. Chem Rev. 2015;115:1990. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- 5.(a) Jang B, Park JY, Tung CH, Kim IH, Choi Y. ACS Nano. 2011;5:1086. doi: 10.1021/nn102722z. [DOI] [PubMed] [Google Scholar]; (b) Tian B, Wang C, Zhang S, Feng L, Liu Z. ACS Nano. 2011;5:7000. doi: 10.1021/nn201560b. [DOI] [PubMed] [Google Scholar]
- 6.(a) Divaris D, Kennedy J, Pottier R. Am J Pathol. 1990;136:891. [PMC free article] [PubMed] [Google Scholar]; (b) Moriwaki SI, Misawa J, Yoshinari Y, Yamada I, Takigawa M, Tokura Y. Photodermatol, Photoimmunol Photomed. 2001;17:241. doi: 10.1034/j.1600-0781.2001.170507.x. [DOI] [PubMed] [Google Scholar]
- 7.Hopper C. Lancet Oncol. 2000;1:212. doi: 10.1016/s1470-2045(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 8.Triesscheijn M, Baas P, Schellens JH, Stewart FA. Oncologist. 2006;11:1034. doi: 10.1634/theoncologist.11-9-1034. [DOI] [PubMed] [Google Scholar]
- 9.Lovell JF, Liu TW, Chen J, Zheng G. Chem Rev. 2010;110:2839. doi: 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- 10.Ling D, Park W, Park S-j, Lu Y, Kim KS, Hackett MJ, Kim BH, Yim H, Jeon YS, Na K, Hyeon T. J Am Chem Soc. 2014;136:5647. doi: 10.1021/ja4108287. [DOI] [PubMed] [Google Scholar]
- 11.Koide Y, Urano Y, Yatsushige A, Hanaoka K, Terai T, Nagano T. J Am Chem Soc. 2009;131:6058. doi: 10.1021/ja900443b. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Zhu G, You M, Song E, Shukoor MI, Zhang K, Altman MB, Chen Y, Zhu Z, Huang CZ, Tan W. ACS Nano. 2012;6:5070. doi: 10.1021/nn300694v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong B, Kim SW, Bae YH. Adv Drug Delivery Rev. 2002;54:37. doi: 10.1016/s0169-409x(01)00242-3. [DOI] [PubMed] [Google Scholar]
- 14.(a) Gota C, Okabe K, Funatsu T, Harada Y, Uchiyama S. J Am Chem Soc. 2009;131:2766. doi: 10.1021/ja807714j. [DOI] [PubMed] [Google Scholar]; (b) Koizumi H, Shiraishi Y, Tojo S, Fujitsuka M, Majima T, Hirai T. J Am Chem Soc. 2006;128:8751. doi: 10.1021/ja062949c. [DOI] [PubMed] [Google Scholar]; (c) Okabe K, Inada N, Gota C, Harada Y, Funatsu T, Uchiyama S. Nat Commun. 2012;3:705. doi: 10.1038/ncomms1714. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Shiraki T, Dawn A, Le TNL, Tsuchiya Y, Tamaru S-i, Shinkai S. Chem Commun. 2011;47:7065. doi: 10.1039/c1cc11288k. [DOI] [PubMed] [Google Scholar]
- 15.Pelton R. Adv Colloid Interface Sci. 2000;85:1. doi: 10.1016/s0001-8686(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee SS, Hughes P, Ross AD, Robinson MR. Pharm Res. 2010;27:2043. doi: 10.1007/s11095-010-0159-x. [DOI] [PubMed] [Google Scholar]
- 17.(a) Gao J, Haidar G, Lu X, Hu Z. Macromolecules. 2001;34:2242. [Google Scholar]; (b) Lu X, Hu Z, Schwartz J. Macromolecules. 2002;35:9164. [Google Scholar]
- 18.Flors C, Fryer MJ, Waring J, Reeder B, Bechtold U, Mullineaux PM, Nonell S, Wilson MT, Baker NR. J Exp Bot. 2006;57:1725. doi: 10.1093/jxb/erj181. [DOI] [PubMed] [Google Scholar]
- 19.Li T, Senesi AJ, Lee B. Chem Rev. 2016 doi: 10.1021/acs.chemrev.5b00690. [DOI] [PubMed] [Google Scholar]
- 20.Rubatat L, Gebel G, Diat O. Macromolecules. 2004;37:7772. [Google Scholar]
- 21.(a) Chatterjee T, Nakatani AI, Adden R, Brackhagen M, Redwine D, Shen H, Li Y, Wilson T, Sammler RL. Biomacromolecules. 2012;13:3355. doi: 10.1021/bm301123a. [DOI] [PubMed] [Google Scholar]; (b) Kobayashi K, Huang C-i, Lodge TP. Macromolecules. 1999;32:7070. [Google Scholar]; (c) Li T, Zan X, Winans RE, Wang Q, Lee B. Angew Chem, Int Ed. 2013;52:6638. doi: 10.1002/anie.201209299. [DOI] [PubMed] [Google Scholar]; (d) Lott JR, McAllister JW, Wasbrough M, Sammler RL, Bates FS, Lodge TP. Macromolecules. 2013;46:9760. [Google Scholar]
- 22.Lu X, Hu Z, Gao J. Macromolecules. 2000;33:8698. [Google Scholar]
- 23.(a) Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J. Chem Soc Rev. 2011;40:3941. doi: 10.1039/c0cs00108b. [DOI] [PubMed] [Google Scholar]; (b) Samuels RJ. J Polym Sci, Part A-2: Polym Phys. 1969;7:1197. [Google Scholar]
- 24.Eichwurzel I, Stiel H, Röder B. J Photochem Photobiol, B. 2000;54:194. doi: 10.1016/s1011-1344(00)00016-6. [DOI] [PubMed] [Google Scholar]
- 25.Knop K, Mingotaud AF, El-Akra N, Violleau F, Souchard JP. Photochem Photobiol Sci. 2009;8:396. doi: 10.1039/b811248g. [DOI] [PubMed] [Google Scholar]
- 26.Yan L, Lin W, Bangal PR. Macromol Biosci. 2006;6:532. doi: 10.1002/mabi.200600067. [DOI] [PubMed] [Google Scholar]
- 27.(a) Krasnovsky A, Neverov K, Egorov SY, Roeder B, Levald T. J Photochem Photobiol, B. 1990;5:245. doi: 10.1016/1011-1344(90)80009-m. [DOI] [PubMed] [Google Scholar]; (b) Tanielian C, Wolff C, Esch M. J Phys Chem. 1996;100:6555. [Google Scholar]
- 28.(a) Henle KJ, Leeper DB. Cancer Res. 1979;39:2665. [PubMed] [Google Scholar]; (b) Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Lancet Oncol. 2002;3:487. doi: 10.1016/s1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.