Abstract
We identified Erythrocyte membrane protein band 4.1-like 5 (Epb41l5) as a substrate for the E3 ubiquitin ligase Mind bomb 1 (Mib1), which is essential for activation of Notch signaling. Although loss of Epb41l5 does not significantly alter the pattern of neural progenitor cells (NPCs) specified as neurons at the neural plate stage, it delays their delamination and differentiation after neurulation when NPCs normally acquire organized apical junctional complexes (AJCs) in the zebrafish hindbrain. Delays in differentiation are reduced by knocking down N-cadherin, a manipulation expected to help destabilize adherens junctions (AJs). This suggested that delays in neuronal differentiation in epb41l5-deficient embryos are related to a previously described role for Epb41l5 in facilitating disassembly of cadherin-dependent AJCs. Mib1 ubiquitylates Epb41l5 to promote its degradation. DeltaD can compete with Epb41l5 to reduce Mib1-dependent Epb41l5 degradation. In this context, increasing the number of NPCs specified to become neurons, i.e. cells expressing high levels of DeltaD, stabilizes Epb41l5 in the embryo. Together, these observations suggest that relatively high levels of Delta stabilize Epb41l5 in NPCs specified as neurons. This, we suggest, helps coordinate NPC specification with Epb41l5-dependent delamination and differentiation as neurons.
KEY WORDS: Notch signaling, Mind bomb, Zebrafish, Epb41l5, Neurogenesis, Neuronal differentiation, Epithelial morphogenesis
Highlighted article: The FERM domain protein Epb41l5 competes with the Notch ligand Delta as a substrate for Mib1 E3 ligase to coordinate neuronal specification and differentiation at the post-translational level.
INTRODUCTION
Neural progenitor cells (NPCs) in the neuroepithelium are selected to become neurons by the process of lateral inhibition mediated by Notch signaling (Artavanis-Tsakonas et al., 1999; Schweisguth, 2004: Louvi and Artavanis-Tsakonas, 2006; Fiuza and Arias, 2007). NPCs selected to become neurons begin to express relatively high levels of proneural transcription factors that facilitate differentiation of neurons (Blader et al., 1997; Bertrand et al., 2002). The proneural factors determine expression of the Notch ligand Delta, which activates Notch in neighboring NPCs and prevents them from being specified as neurons. Expression of high levels of proneural factors in NPCs is followed by their delamination and terminal differentiation into neurons, sometimes preceded by their migration away from where they were born (Pacary et al., 2012). The first morphological change in fate-determined NPCs is the disassembly of apical junctional complexes (AJCs) and the delamination of these cells from the neuroepithelium (Pacary et al., 2012; Itoh et al., 2013). AJCs, which are composed of tight junctions (TJs), adherens junctions (AJs) and desmosomes, are located at the apical end of the lateral membrane of epithelial and neuroepithelial cells and regulate cell adhesion and polarity (Shin et al., 2006; Meng and Takeichi, 2012; Desai et al., 2009; Spadaro et al., 2012). Although the precise mechanisms of AJC disassembly are not fully understood, studies have shown that downregulation of N-cadherin (Cadherin 2), a major component of neuroepithelial AJs (Redies and Takeichi, 1996; Gumbiner, 2005), is associated with the delamination and differentiation of fate-determined NPCs. n-cadherin expression is downregulated after fate determination (Hatta et al., 1987; Barami et al., 1994; Redies and Takeichi, 1996; Seki et al., 2007; Yagita et al., 2009; Kurusu et al., 2012; Paulson et al., 2014). Knockdown of n-cadherin disrupts AJs and facilitates apical detachment and differentiation of NPCs (Radice et al., 1997; Ganzler-Odenthal and Redies, 1998; Kadowaki et al., 2007; Zhang et al., 2010). Elimination of regulatory proteins of AJs results in similar phenotypes (Cappello et al., 2006). These results suggest that downregulation of N-cadherin is a prerequisite for delamination and may facilitate neuronal differentiation of fate-determined NPCs. However, the mechanisms that coordinate fate specification and downregulation of N-cadherin in fate-determined NPCs remain poorly understood.
Recently Rousso et al. showed that the Forkhead transcription factors FoxP2 and FoxP4 (FoxP2/4), expression of which is induced by the proneural transcription factors Ngn2 (Neurog3) and NeuroD4, directly downregulates expression of n-cadherin in fate-determined NPCs (Rousso et al., 2012; Pacary et al., 2012). As FoxP2/4 only reduces n-cadherin transcripts, existing N-cadherin protein at AJs in fate-determined NPCs also needs to be downregulated. However, the mechanisms that determine disassembly of existing AJs at the onset of neuronal differentiation remain poorly defined. In this study, we suggest that a membrane scaffold protein, Epb41l5, post-translationally facilitates disassembly of AJs in fate-determined NPCs and works as a post-translational regulator that contributes to coordination of fate specification and delamination.
We identified Epb41l5 as a substrate protein of Mib1 E3 ubiquitin ligase. Mib1 was originally identified as an E3 ubiquitin ligase for the Notch ligand Delta. Mib1 ubiquitylates and promotes endocytosis of Delta, which is essential for activation of Notch (Itoh et al., 2003; Chen and Casey Corliss, 2004; Lai et al., 2005; Le Borgne et al., 2005; Pitsouli and Delidakis, 2005; Matsuda and Chitnis, 2009). Epb41l5 belongs to a family of FERM proteins, which function as adaptors linking interacting cytoplasmic proteins to specific membrane compartments (Diakowski et al., 2006). Previous studies suggested diverse functions for Epb41l5 in the establishment of apical-basal polarity in neuroepithelial cells (Jensen et al., 2001; Jensen and Westerfield, 2004; Hsu et al., 2006; Laprise et al., 2006; Gosens et al., 2007), the promotion of epithelial-to-mesenchymal transition (EMT) (Lee et al., 2007; Hirano et al., 2008) and the regulation of apical membrane constriction of epithelial cells (Nakajima and Tanoue, 2010; Chu et al., 2013).
We show that in epb41l5-deficient embryos, although neurogenesis is normal at the neural plate stage, there is mislocalization of neurons and a delay in neuronal differentiation after neurulation when AJCs become organized in the hindbrain neuroepithelium. Overexpression of Epb41l5 promotes differentiation of neurons, suggesting that some function of Epb41l5 facilitates differentiation of NPCs as neurons. We show that Mib1 ubiquitylates Epb41l5 and facilitates its degradation. Furthermore, the efficacy of Mib1-mediated degradation of Epb41l5 and its potential to disassemble AJs can be regulated by the abundance of Delta protein in a cell, which dynamically changes during fate specification and differentiation of neurons. Taken together, our observations suggest a potential model for how Epb41l5 might coordinate cell fate specification and delamination of neuronal progenitors for subsequent neuronal differentiation.
RESULTS
Identification of Epb41l5 as a novel Mib1-interacting protein
We identified Epb41l5 as a novel interacting protein of Mib1 using a yeast two-hybrid screen. The screen identified six independent cDNA clones encoding a human FERM domain protein, erythrocyte membrane protein band 4.1 like 4B (EPB41L4; also known as EHM2), which is similar to Epb41l5 [also known as Mosaic eyes (Moe)] in zebrafish (Fig. 1B). Epb41l5 has a FERM domain and an FA (FERM-adjacent region) domain in its N terminus, which is well-conserved among the members of the band 4.1 superfamily (Baines, 2006; Tepass, 2009). The C-terminal domain of Epb41l5 lacks the actin-binding domain but retains a conserved PDZ-binding domain.
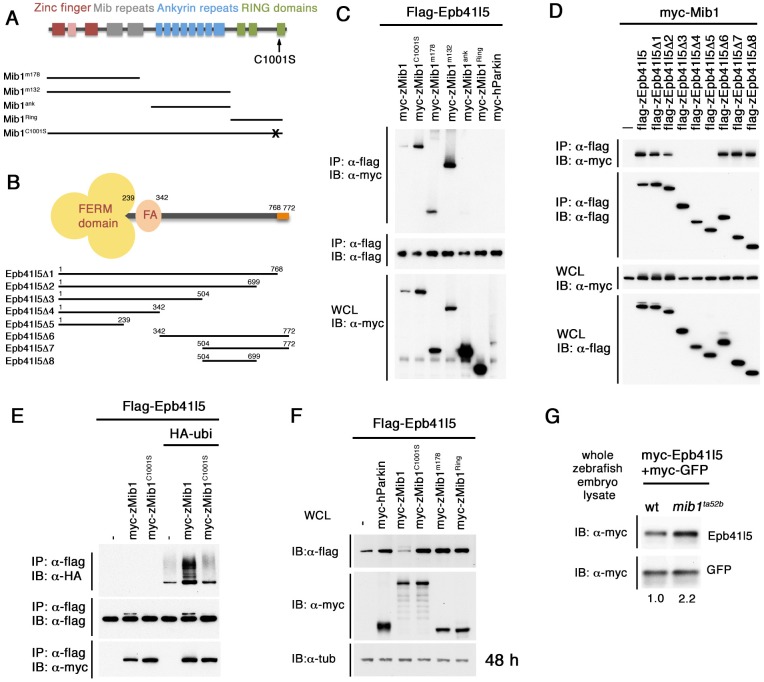
Fig. 1.
Epb41l5 is a Mib1-interacting protein that is ubiquitylated by Mib1. (A) Structure of Mib1 protein. Truncated forms of Mib1 are shown below. Mib1m132, which contains zinc finger and Mib repeats, was used for the yeast two-hybrid screen. C1001S, C to S mutation (shown as X) in the RING domain. (B) Structure of Epb41l5 protein, which contains a FERM domain and an FA (FERM-adjacent region) domain at the N terminus and PDZ-binding domain at the C terminus (orange). Truncated forms of Epb41l5 are shown below. (C) Epb41l5 interacts with the N-terminal domain of Mib1. Full-length Epb41l5 was co-expressed with various truncated forms of Mib1 in HEK293 cells. (D) Mib1 interacts with the C-terminal domain of Epb41l5. Full-length Mib1 was co-expressed with various forms of Epb41l5 in HEK293 cells. Mib1 was immunoprecipitated by Epb41l5 forms containing amino acids 504-609. (E) Epb41l5 is ubiquitylated by Mib1, but not by Mib1C1001S. (F) Mib1 facilitates degradation of Epb41l5. A functional RING domain is required for its degradation. (G) Epb41l5 is stabilized in zebrafish mib1ta52b mutants. In vitro-transcribed myc-epb41l5 mRNA was microinjected into one-cell-stage embryos together with myc-gfp mRNA as standardized control. Total embryo lysate was extracted from mib1ta52b mutant embryos and wild-type siblings at 24 hpf. Numbers below indicate relative band intensity of individual bands.
Interaction between Epb41l5 and Mib1 was confirmed by immunoprecipitation in HEK293 cells (Fig. 1C). We identified domains required for their interaction using various deletion constructs (Fig. 1A,B). A truncated N-terminal fragment of Mib1 (Mib1m178) was sufficient for the interaction with Epb41l5 (Fig. 1C). A part of the C-terminal domain of Epb41l5 containing aa 504-699 was required for the interaction with Mib1 (Fig. 1D).
Epb41l5 does not change Mib1 functions in Notch signaling
Next, we investigated the significance of the Mib1-Epb41l5 interaction. One possibility was that Epb41l5, which is typically localized on the cell surface (Fig. S1), interacts with Mib1 to promote its surface localization. This could facilitate interaction of Mib1 with Delta ligands and their subsequent endocytosis. Although Epb41l5 did promote association of Mib1 with the plasma membrane in vitro (Fig. S1), DeltaD was primarily localized in intracellular puncta in epb41l5-deficient moeb476 mutants and epb41l5 morphants, as in wild-type embryos, and did not accumulate on the cell surface, as in mib1m178 mutants (Fig. S2G-K). This suggests that Epb41l5 is not required for Mib1-mediated DeltaD endocytosis, which is essential for effective Notch activation.
Notch-mediated lateral inhibition limits the number of NPCs that are allowed to differentiate as neurons and its failure increases neurogenesis. In epb41l5-deficient embryos, however, there were no significant changes in expression of neurogenin 1 (ngn1; neurog1), a basic helix-loop-helix (bHLH) transcription factor that gives cells the potential to differentiate as neurons (Blader et al., 1997; Bertrand et al., 2002); deltaA, expression of which is determined by such proneural bHLH factors (Haddon et al., 1998); or her4, expression of which is determined by Notch signaling (Takke et al., 1999; Yeo et al., 2007) (Fig. S2A-F). This suggests that the number of NPCs specified as neurons is not increased and Notch signaling is not reduced in epb41l5-deficient embryos.
It should be noted that some cell death was observed in the hindbrains of epb41l5-deficient embryos (data not shown). p53 (Tp53)-dependent off-target effects of some morpholinos results in similar patterns of cell death (Robu et al., 2007; Schulte-Merker and Stainier, 2014). However, it is unlikely that the cell death observed in epb41l5 morphants simply reflects off-target effects of the epb41l5 morpholinos, as moeb476 mutants have a similar pattern of increased cell death and co-injection of p53 morpholino reduces cell death in both epb41l5 mutants and morphants. To avoid confusion that could arise from changes associated with cell death, p53 morpholino was co-injected in all epb41l5 mutants and morphants and our analysis focused on changes that were not affected by p53-dependent cell death.
Although some reduction in her4 expression was observed in epb41l5-deficient embryos not co-injected with the p53 morpholino (Ohata et al., 2011), co-injection of the p53 morpholino restored her4 expression (Fig. S4A,B). This suggested that the reduction in her4 is specifically associated with p53-dependent cell death and does not reflect a general loss of Notch signaling in epb41l5-deficient embryos. This conclusion was supported by the observations that there was no obvious increase in neurogenesis marked by expression of ngn1 or deltaA (Fig. S2A-F) and that epb41l5-deficient embryos did not show any problems with formation of somites in the caudal part of the trunk or the tail (data not shown), characteristic features of mutants with loss of Notch signaling (Lewis et al., 2009).
Mib1 ubiquitylates Epb41l5 and facilitates its degradation
As analysis of epb41l5-deficient embryos did not appear to suggest an essential role for Epb41l5 in determining Mib1-dependent Notch signaling, we asked if instead Mib1 interacts with Epb41l5 to regulate functions of Epb41l5 through its ubiquitylation as with some other substrates of Mib1 (Cajanek et al., 2015; Kwon et al., 2013; Villumsen et al., 2013). When Epb41l5 was co-expressed with Mib1, two bands of Epb41l5 were observed (Fig. 1E). The upper band was not observed when a mutant form of Mib1 with no E3 Ub ligase activity (Mib1C1000S) was co-expressed (Fig. 1E). Co-expression of HA-Ubiquitin confirmed that the upper band is the ubiquitylated form of Epb41l5. Mib1 co-expression facilitated degradation of Epb41l5 in HEK293 cells (Fig. 1F) and exogenously expressed Epb41l5 was stabilized in mib1ta52b mutants (Fig. 1G). These results suggest that Mib1 determines ubiquitylation-dependent degradation of Epb41l5 and regulates its function by determining its stability. Interestingly, degradation of Epb41l5 did not occur when the PDZ-binding domain at the C terminus of Epb41l5 was deleted (Fig. S3), suggesting a requirement of the PDZ-binding domain for Mib1-mediated degradation of Epb41l5.
Mislocalization of neurons in epb41l5-deficient embryos
To understand the functional significance of the regulation of Epb41l5 stability in neurogenesis, we looked for changes in the developing neural tube that might be related to previously described roles for Epb41l5 in epithelial morphogenesis. Previous studies showed that Epb41l5 interacts with the apical determinant Crb, restricts subcellular distribution of Crb to the ventricular surface of the neuroepithelium and organizes apico-basal polarity in neuroepithelium (Jensen et al., 2001; Jensen and Westerfield, 2004; Hsu et al., 2006; Laprise et al., 2006; Gosens et al., 2007). In zebrafish, epb41l5 was originally identified as mosaic eyes (moe) and Crb2a protein was more diffusely distributed in the neuroepithelium in moeb476 mutants (Hsu et al., 2006; Laprise et al., 2006) and in epb41l5-deficient embryos (Fig. 2A,B). This change was accompanied by poorly organized neuroepithelial AJs during the progression of neural tube formation (Fig. 2D-I), resulting in a failure of AJC organization and brain ventricle formation (Fig. 2B,N,O; Jensen et al., 2001; Hsu et al., 2006).
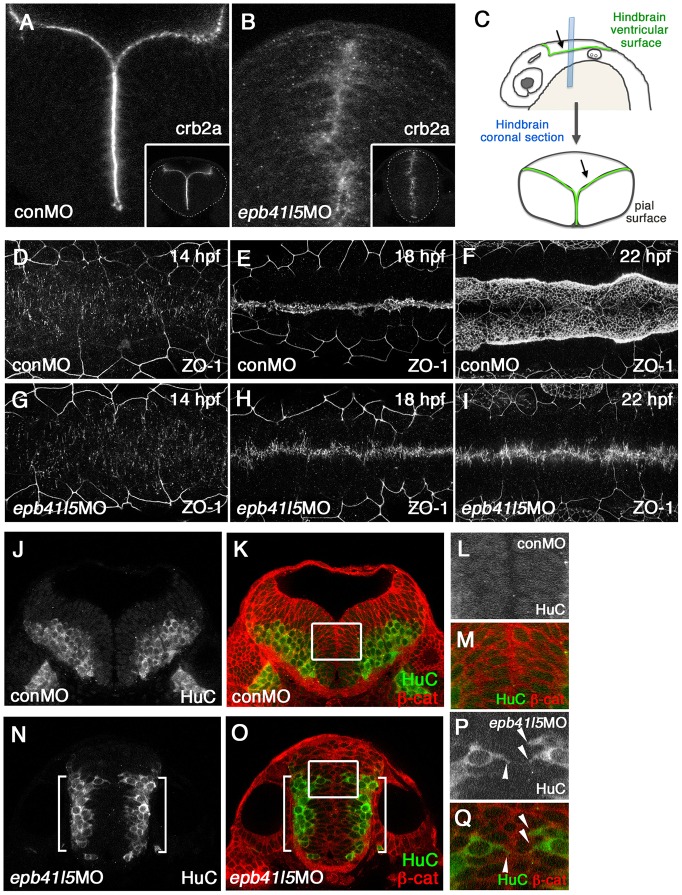
Fig. 2.
Epb41l5 is required for the formation of the hindbrain ventricle and the proper localization of neurons. (A-C) Coronal sections showing mislocalization of Crb2a in the hindbrain of epb41l5 morphants. Whereas Crb2a is restricted to a well-defined apical/ventricular surface in wild-type embryos injected with control morpholino (MO) (conMO), Crb2a is diffusely expressed in epb41l5 morphants (epb41l5MO). Lower-magnification images are shown in insets. Diagram in C illustrates approximate positions of coronal sections in the hindbrain. (D-I) Failure of organization of ZO-1-positive AJs and formation of the hindbrain ventricle in epb41l5 morphants. Confocal images were taken from the dorsal side of embryos. The anterior is to the left and the posterior is to the right. In control MO embryos, accumulation of ZO-1+ puncta at 14 hpf is gradually aligned to the midline of the hindbrain and forms a continuous line at 18 hpf. The hindbrain ventricle inflates at 22 hpf. In epb41l5 morphants, ZO-1 accumulation at 14 hpf is gradually aligned to the midline of the hindbrain but fails to form a continuous line at 18 hpf. The hindbrain ventricle is not formed at 22 hpf. (J-Q) Coronal sections showing mislocalization of HuC-expressing neurons in epb41l5 morphants. In control MO embryos, HuC-positive neurons are localized at the pial region of the hindbrain and do not have contacts with the ventricular surface. In epb41l5 morphants, a fraction of HuC-positive cells were located near the midline of the hindbrain and maintain contacts with the prospective ‘ventricular’ surface. Boxed areas in K and O are shown at higher magnification in L,M and P,Q, respectively. Brackets indicate HuC-positive neurons which are still localized near the basal/pial surface of the hindbrain. Arrowheads indicate HuC-positive cells which are localized near the midline.
Along with the failure of brain ventricle formation, we noticed mislocalization of neurons in the hindbrain of epb41l5-deficient embryos. After NPCs are selected for becoming neurons, they detach from the apical ventricular surface and translocate to the basal/pial side of the neural tube where they express markers of differentiation such as HuC (Elavl3) (Kim et al., 1996). Those cells are no longer attached to the ventricular surface (Fig. 2J-M). In epb41l5-deficient embryos, however, some HuC-positive cells were still localized near the midline of the hindbrain where the apical ventricular surface is normally formed in wild-type embryos (Fig. 2P,Q arrowheads). This observation is similar to the mislocalization of Isl1-positive motor neurons in moerw306 mutants (Ohata et al., 2011). Another proposed function of Epb415 is to facilitate disassembly of epithelial AJs and promote EMT (Lee et al., 2007; Hirano et al., 2008). Because delamination of fate-determined NPCs requires disassembly of neuroepithelial AJs, we hypothesized that Epb41l5 might facilitate disassembly of neuroepithelial AJs and that the aberrant localization of HuC-positive cells might reflect their less effective disassembly in epb41l5-deficient embryos.
A delay in AJ disassembly and neuronal differentiation in epb41l5-deficient embryos
Although some HuC-positive neurons were mislocalized in epb41l5-deficient embryos, most HuC-positive neurons were still properly localized near the basal/pial surface of the hindbrain (Fig. 2N,O, brackets). This suggested that although Epb41l5 might be required for efficient disassembly of neuroepithelial AJs, it is not an absolute requirement. To demonstrate more clearly that Epb41l5 facilitates disassembly of neuroepithelial AJs, we challenged the underlying mechanisms by increasing the number of NPCs undergoing differentiation. We treated embryo with DAPT, a γ-secretase inhibitor (Geling et al., 2002), to block Notch signaling. This forced a larger fraction of NPCs to be specified as neurons and consequently increased the number of delaminating NPCs.
Following 5 h of exposure to DAPT, highly organized AJCs in wild-type embryos disappeared from the ventricular surface of the hindbrain (Fig. 3A-D′,I-J′) and the hindbrain was filled by HuC-positive neurons (Fig. 3E-H′,M-N′). In epb41l5 morphants, by contrast, there was a delay in the loss of ZO-1-positive AJs (Fig. 3K-L′,O-Q). It should be noted that AJC formation failed in epb41l5-deficient embryos: ZO-1-associated AJs were not properly organized at the midline along the prospective ventricular surface and the hindbrain ventricle did not inflate. These observations are related to aberrant apico-basal polarity and mislocalization of ion pumps, which prevents effective inflation of the ventricles (Lowery and Sive, 2005). Despite the disorganization of AJCs, it was clear that a significant number of AJs remained after 5 h of DAPT treatment (Fig. 3K-L′,Q). This suggested that neuroepithelial AJs are not effectively disassembled in epb41l5-deficient embryos.
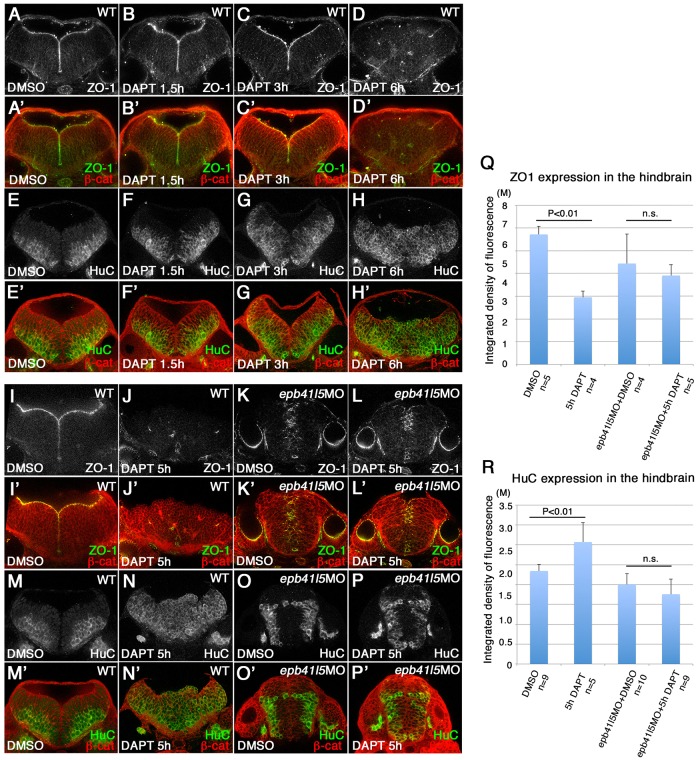
Fig. 3.
Delays in disassembly of AJs and neuronal differentiation in epb41l5 morphants. (A-H′) Timeline of AJ disassembly (A-D′) and differentiation of neurons (E-H′) during DAPT treatment in wild-type embryos. Embryos were treated with 50 μM DAPT for 1.5, 3 or 6 h and immunostained at 32 hpf. Loss of Notch signaling by DAPT treatment results in loss of AJs and premature differentiation of NPCs into neurons. (I-P′) Delays in AJ disassembly and differentiation of neurons in epb41l5 embryos. Embryos were treated with DAPT for 5 h. In control embryos (WT), 5 h DAPT treatment eliminates ZO-1 at the apical/ventricular surface, accompanied by a corresponding increase in HuC. In epb41l5 morphants, there is a much smaller reduction in ZO-1 immunostaining and no obvious increase in HuC immunostaining. (Q,R) Quantitative analyses of ZO-1 and HuC expression. Total fluorescence intensities of ZO-1 and HuC in the hindbrain were measured in individual confocal images using ImageJ. Error bars represent s.d. n.s., not significant. M, million integrated pixel intensity.
The persistence of AJs in epb41l5-deficient embryos was coupled with a small increase in HuC-positive neurons (Fig. 3M-P′,R), suggesting a delay in neuronal differentiation. It remained possible that the number of HuC-positive cells was reduced because DAPT had not blocked Notch signaling in epb41l5-deficient embryos as effectively as in wild-type embryos. However, reduced expression of her4 confirmed that DAPT had effectively inhibited Notch signaling in epb41l5 morphants (Fig. S4). Furthermore, DAPT treatment dramatically increased DeltaD expression in the hindbrain (Fig. 4J,J′,P,P′), consistent with the failure of Notch-mediated lateral inhibition allowing many more NPCs to be specified as neurons in epb41l5 morphants.
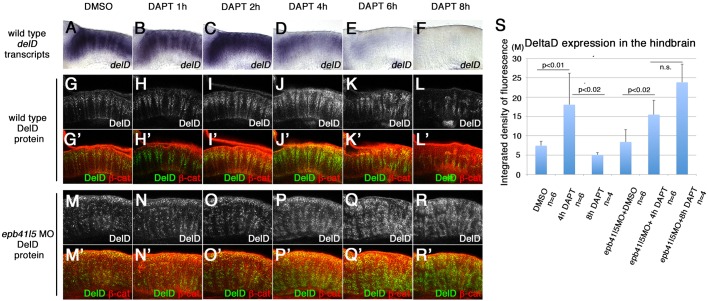
Fig. 4.
‘Intermediate’ neuronal progenitor population expressing DeltaD is increased in epb41l5-deficient embryos. (A-L′) Timeline of disappearance of DeltaD in progenitors during neuronal differentiation in wild-type embryos. Two hours of DAPT treatment increases expression of deltaD transcripts, followed by increased expression of DeltaD protein at 4 h. Expression of deltaD and DeltaD diminishes after 6 and 8 h of DAPT treatment. (M-R′) Continued expression of DeltaD in epb41l5 morphants. Increased DeltaD expression persists after 8 h of DAPT treatment. (S) Quantitative analysis of DeltaD expression. Total fluorescence intensity of DeltaD in the hindbrain was measured in individual confocal slices using ImageJ. Error bars represent s.d. n.s., not significant. M, million integrated pixel intensity.
Intermediate progenitor population is increased in epb41l5-deficient embryos
Although expression of deltaD and DeltaD is upregulated in NPCs selected to become neurons, the upregulation is normally short-lived. In wild-type embryos, the expression of deltaD transcripts increased after 2 h of DAPT treatment but decreased after 4 h (Fig. 4A-F). Similarly, an initial increase in DeltaD protein decreased after 6 h (Fig. 4G-L′). The timing of this reduced expression of DeltaD coincided with a robust increase in the expression of HuC (Fig. 3H,H′). This suggests that NPCs selected to become neurons transiently express high levels of DeltaD but downregulate DeltaD once their differentiation begins.
In epb41l5-deficient embryos, deltaD/DeltaD expression was increased following exposure to DAPT. However, DeltaD expression remained high in epb41l5 morphants after 8 h of DAPT treatment (Fig. 4R,R′,S) when it had already decreased in wild-type embryos (Fig. 4L,L′,S). This suggests that although inhibition of Notch signaling had effectively increased NPCs specified to become neurons, their differentiation was delayed and they remained in an ‘intermediate’ state for an extended period in epb41l5-deficient embryos. Taken together, these results suggest that the delay in AJ disassembly observed in epb41l5-deficient embryos is linked to a delay in differentiation.
Neuronal differentiation is delayed in epb41l5-deficient NPCs
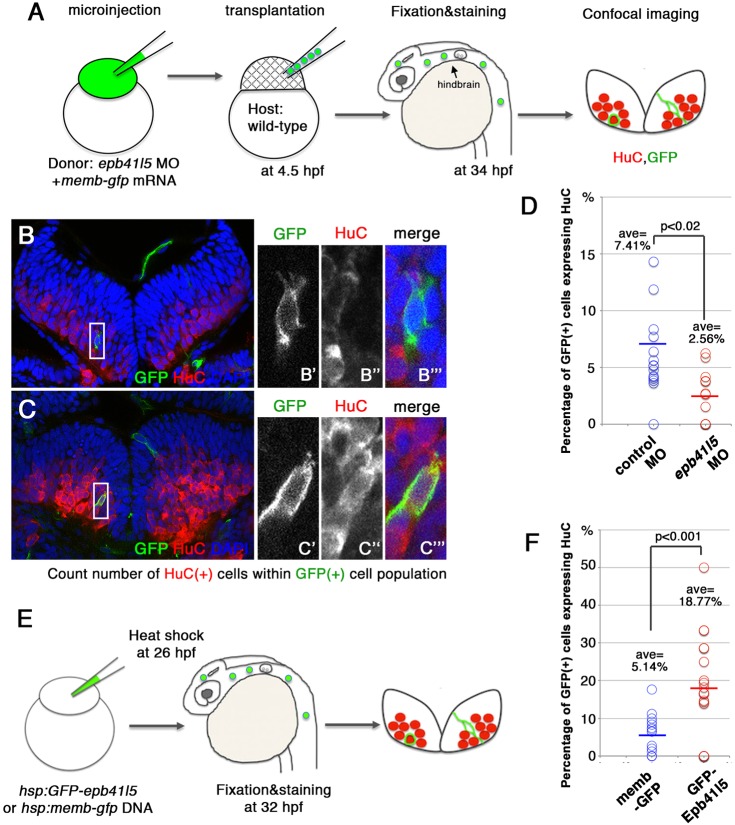
The disorganization of AJs and the failure to inflate the hindbrain ventricle in epb41l5 embryos raised the possibility that the delays in AJ disassembly and differentiation might be secondary effects of the disorganized neuroepithelium. It was important to determine whether reduced Epb41l5 function contributes to the same problems in a context in which there is no broad disorganization of AJCs in the neuroepithelium. We examined the fate of epb41l5-deficient cells transplanted into wild-type embryos with otherwise normal morphology and apicobasal polarity. mRNA encoding myc-membrane-tethered GFP (myc-membGFP) was co-injected into donor embryos to identify transplanted cells. Host embryos were then immunostained at 34 hours post-fertilization (hpf) to determine what fraction of transplanted cells had begun differentiation as neurons (Fig. 5A-C). In a total of 428 cells transplanted from control embryos, 7.41% cells expressed HuC (Fig. 5D). By contrast, from a total of 221 cells transplanted from epb41l5-deficient embryos, only 2.56% cells expressed HuC (Fig. 5D). These results confirm that loss of epb41l5 in individual cells suppresses their differentiation as neurons in the absence of broader disorganization of AJCs, and the delay in neuronal differentiation is not a secondary effect of the disorganized hindbrain structure in epb41l5-deficient embryos.
Fig. 5.
Epb41l5 facilitates differentiation of neurons. (A) Schematic of the experiment. epb41l5 morpholino was injected into one-cell-stage embryos with memb-gfp mRNA. Twenty to thirty cells were transplanted to wild-type embryos. Ratios of HuC-expressing cells within total GFP-expressing transplanted cells were analyzed in individual confocal images. (B,C) Representative images of HuC-positive neurons in the hindbrain. Boxed areas in B and C are enlarged in the right-hand panels. The cell in B does not express HuC, suggesting an undifferentiated NPC. The cell in C does express HuC, suggesting a differentiated neuron. (D) Reduced expression of epb41l5 attenuates differentiation of neurons. Out of the 428 control transplanted cells in 16 embryos that were analyzed, 7.41% cells expressed HuC. Out of the 221 epb41l5-deficient transplanted cells in 12 embryos that were analyzed, 2.56% cells expressed HuC. ave, average. (E) Schematic of the experiment. Plasmid DNAs encoding membGFP or GFP-Epb41l5 were microinjected. At 6 h after heat-shock treatment, HuC expression in GFP-expressing cells was analyzed at 32 hpf. (F) GFP-Epb41l5 facilitated differentiation of neurons. Out of the 623 membGFP-expressing cells in eight embryos that were analyzed, 5.14% cells expressed HuC. Out of the 277 cells overexpressing GFP-Epb41l5 in six embryos that were analyzed, 18.77% cells expressed HuC.
Neuronal differentiation is facilitated in NPCs with increased Epb41l5 expression
Next, we examined whether increased expression of Epb41l5 facilitates delamination and differentiation of neurons. We injected one-cell-stage embryos with plasmids encoding gfp-epb41l5 or myc-membgfp downstream of a heat shock promoter (Fig. 5E). In this context, GPF-Epb41l5 or myc-membGFP were expressed in a mosaic pattern following induction of expression with heat shock. We optimized the amount of injected DNA so that expression of GFP was induced in less than 5% of cells. Six hours after heat-shock treatment, we examined what fraction of GFP-positive cells had begun differentiation as neurons. We counted a total of 623 cells expressing myc-membGFP and 277 cells expressing GFP-Epb41l5. Of those cells expressing GFP-Epb41l5 18.77% expressed HuC, whereas only 5.14% of cells expressing myc-membGFP expressed HuC (Fig. 5F). These results are consistent with Epb41l5 expression promoting differentiation of NPCs as neurons.
A partial knockdown of n-cadherin facilitates neuronal differentiation in epb41l5-deficient embryos
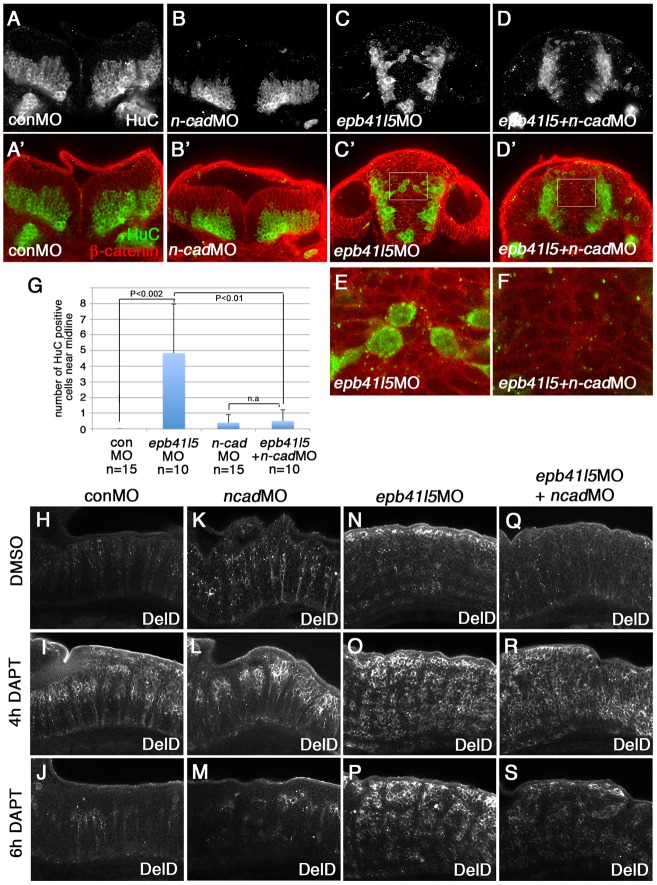
Previous studies showed that downregulation of N-cadherin is required for delamination and differentiation of NPCs (Hatta et al., 1987; Barami et al., 1994; Radice et al., 1997; Ganzler-Odenthal and Redies, 1998; Redies and Takeichi, 1996; Kadowaki et al., 2007; Seki et al., 2007; Yagita et al., 2009; Zhang et al., 2010; Kurusu et al., 2012; Paulson et al., 2014). Because Epb41l5 promotes EMT by modulating E-cadherin (Cadherin 1), a major component of epithelial AJs (Lee et al., 2007; Hirano et al., 2008), we hypothesized that Epb41l5 might reduce the stability of N-cadherin and help disassembly of existing neuroepithelial AJs, complementing transcriptional mechanisms that reduce n-cadherin expression (Rousso et al., 2012). In this context, N-cadherin might be more stable at AJs in epb41l5-deficient NPCs. We hypothesized that a partial knockdown of n-cadherin might reduce N-cadherin and facilitate AJ disassembly to restore timely differentiation of NPCs in epb41l5-deficient embryos.
A complete loss of N-cadherin function disorganizes the structure and integrity of the hindbrain (Pujic and Malicki, 2001; Lele et al., 2002; Erdmann et al., 2003; Malicki et al., 2003; Masai et al., 2003). We used 0.1 ng of n-cadherin morpholino for microinjection to minimize the effects of n-cadherin knockdown on the overall morphology and distribution of HuC-positive neurons in wild-type embryos (Fig. 6B,B′). This partial knockdown of n-cadherin nevertheless reduced mislocalization of HuC-positive neurons in the hindbrain of epb41l5-deficient embryos (Fig. 6D-G), suggesting that partial reduction of n-cadherin facilitated delamination of neurons in epb41l5-deficient embryos. Partial knockdown of n-cadherin also reduced the persistent DeltaD expression following 6 h of DAPT treatment (Fig. 6N-S). These results support the hypothesis that slower disassembly of AJs in epb41l5-deficient embryos delays differentiation of fate-determined NPCs that express high levels of DeltaD. Taken together, these results suggest that Epb41l5 facilitates neuroepithelial AJ disassembly for the timely differentiation of fate-determined NPCs.
Fig. 6.
Reduced expression of n-cadherin rescues a delay of delamination and differentiation of neurons in epb41l5-deficient embryos. (A-F) Coronal sections showing localization of HuC-expressing neurons in the hindbrain. HuC-positive cells are only localized at the pial side of the hindbrain in control MO- or 0.1 ng n-cadherin MO-injected embryos. A fraction of HuC-positive cells are mislocalized near the midline in epb41l5 morphants. The mislocalization of HuC-positive cells is rescued by partial knockdown of n-cadherin. The boxed areas in C′ and D′ are enlarged in E and F, respectively. (G) Statistical analysis of distribution of HuC-positive cells in the hindbrain. Error bars represent s.d. n.s., not significant. (H-S) DeltaD expression in the hindbrain. Lateral views. After 4 h of DAPT treatment DeltaD expression is increased. After 8 h of DAPT treatment, whereas DeltaD expression is decreased in control embryos and n-cadherin morphants, DeltaD expression persists in epb41l5 morphants. The persistent expression of DeltaD in epb41l5 morphants is rescued by partial knockdown of n-cadherin.
Epb41l5 protein is stabilized in NPCs specified as neurons
We identified Epb41l5 as a Mib1-interacting protein and showed that Mib1 ubiquitylates Epb41l5 to promote its degradation (Fig. 1E-G). In addition, our observations suggested that Epb41l5 facilitates disassembly of AJs in NPCs (Figs 3, 4). Based on these observations, we hypothesized that Mib1-mediated degradation of Epb41l5 might be selectively reduced in NPCs specified as neurons; the resulting stabilization of Epb41l5 may promote disassembly of AJs, facilitating effective delamination and subsequent differentiation as neurons.
If Mib1-mediated degradation of Epb41l5 is reduced in NPCs specified as neurons, overall stability of Epb41l5 should be increased in embryos in which inhibition of Notch signaling allows a larger number of NPCs to become neurons. We did not examine the stability of endogenous Epb41l5 because inhibition of Notch signaling alters the number of cells expressing epb41l5 (Fig. S6) and, hence, Epb41l5. Instead, we microinjected mRNAs encoding Myc-Epb41l5 and HA-RFP, and examined the effect of Notch inhibition on their stability. The stability of Myc-Epb41l5 was increased in DAPT-treated embryos, whereas the stability of HA-RFP was not affected (Fig. 7A). These observations are consistent with increased stability of Epb41l5 protein in embryos in which a larger number of NPCs are selected to become neurons.
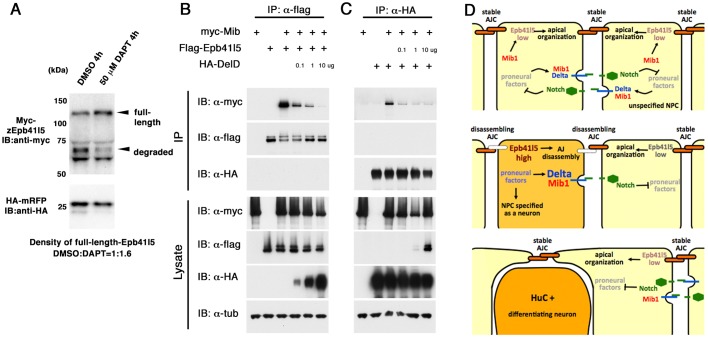
Fig. 7.
Epb41l5 and DeltaD compete for Mib1 binding. (A) Epb41l5 protein is stabilized in DAPT-treated embryos. mRNA encoding Myc-Epb41l5 and HA-mRFP was co-injected into wild-type embryos. Embryos were treated with DAPT for 4 h. HA-mRFP was used for standardization. (B) Co-expression with DeltaD inhibits Epb41l5-Mib1 interaction in HEK293 cells. When HA-Delta is co-expressed, a smaller amount of myc-Mib1 is co-immunoprecipitated with Flag-Epb41l5 in a dose-dependent manner. No HA-Delta is co-immunoprecipitated by Flag-Epb41l5. The destabilization of Flag-Epb41l5 by Mib1 is rescued by co-expression of HA-DeltaD. (C) Co-expression of Flag-Epb41l5 reduces DeltaD-Mib1 interaction. When Flag-Epb41l5 is co-expressed, a smaller amount of Myc-Mib1 is co-immunoprecipitated with HA-DeltaD. No Flag-Epb415 is co-immunoprecipitated by HA-DeltaD. Mib1 or Epb41l5 does not change the stability of HA-DeltaD. (D) A model for how Epb41l5 might coordinate specification and differentiation of NPCs selected to become neurons. In unspecified NPCs (yellow), with relatively low levels of proneural factors and Delta, more Mib1 is available for ubiquitylation and degradation of Epb41l5. The resulting relatively low level of Epb41l5 might keep AJCs stable. In NPCs specified to become neurons (orange), with high levels of proneural factors and Delta, a greater proportion of Mib1 interacts with Delta and less with Epb41l5. This might reduce Mib1-dependent Epb41l5 degradation, allowing Epb41l5 to accumulate and facilitate disassembly of AJCs and differentiation of NPCs.
Epb41l5 and DeltaD compete for Mib1 binding to determine the stability of Epb41l5
Next, we explored the mechanisms underlying Epb41l5 stabilization in NPCs selected to become neurons. Previous studies showed that the N-terminal domain of Mib1 binds to Delta (Itoh et al., 2003; Chen and Casey Corliss, 2004; Palardy and Chitnis, 2015). We showed that the same N-terminal domain interacts with Epb41l5 (Fig. 1C). This raised the possibility that Epb41l5 and Delta might compete for Mib1 binding. Delta expression dynamically changes during neurogenesis; Delta expression is relatively high in NPCs selected to become neurons and low in neighboring NPCs. If Delta and Epb41l5 compete for Mib1 binding, the amount of Delta in a cell could determine what fraction of Mib1 interacts with and degrades Epb415. This could determine the relative stability of Epb41l5 and its potential to disassemble AJs in NPCs.
To test this hypothesis, we carried out a biochemical competition assay in HEK293 cells. We examined whether co-expression of DeltaD changes the amount of Epb41l5 interacting with Mib1 or the stability of Epb41l5. Myc-Mib1 was co-immunoprecipitated with Flag-Epb41l5 and destabilized Flag-Epb41l5 (Fig. 7B, lanes 2,3). When HA-Delta was co-expressed, however, a smaller amount of Myc-Mib1 was co-immunoprecipitated with Flag-Epb41l5 and Flag-Epb41l5 was stabilized (Fig. 7B, lanes 3-6). These results suggest that increasing DeltaD expression can reduce the amount of Mib1 available to determine Epb41l5 degradation in a cell. The DeltaD-Mib1 interaction was also decreased when Epb41l5 was co-expressed (Fig. 7C), supporting our competition model. Taken together, these results suggest that Epb41l5 and DeltaD can compete for Mib1 binding and that the abundance of Delta can regulate the stability of Epb41l5 in vitro. Increasing expression of Delta in NPCs specified as neurons would be expected to capture increasing proportions of Mib1, leaving less Mib1 available for ubiquitylation and degradation of Epb41l5. Because of technical limitations, we were not able to show in vivo that increasing Delta expression stabilizes endogenous Epb41l5 in individual NPCs specified to become neurons. However, consistent with this possibility, we showed that increasing the number of Delta-expressing NPCs specified to become neurons stabilizes exogenously provided Epb41l5 (Fig. 7A).
DISCUSSION
We have shown that Mib1 ubiquitylates Epb41l5, promoting its degradation. We have also shown that Epb41l5 and DeltaD compete to interact with Mib1, and that this competition can regulate Mib1-dependent degradation of Epb41l5. These observations and previous studies that have demonstrated a role for Epb41l5 in disassembly of AJs in epithelial cells indicate a relatively simple post-translational mechanism for helping the coordination of fate specification, delamination and differentiation of neurons. We suggest that the competition between Epb41l5 and Delta for Mib1 binding could determine the stability of Epb41l5 and hence its potential to disassemble AJs in NPCs (Fig. 7D). NPCs selected to become neurons express high levels of proneural factors and Delta. In these cells, we suggest, a greater proportion of Mib1 interacts with Delta and less with Epb41l5. This could reduce Mib1-dependent Epb41l5 degradation, allowing Epb41l5 to accumulate and facilitate disassembly of AJs and differentiation of NPCs. NPCs that have not been selected to become neurons express relatively low levels of proneural factors and Delta. In these cells, Mib1 will be more likely to ubiquitylate and degrade Epb41l5. The resulting relatively low level of Epb41l5 could keep AJs stable, maintaining their epithelial integrity in the neuroepithelium. Such a post-translational mechanism would complement transcriptional mechanisms previously described (Rousso et al., 2012) and contribute to the effective coupling of specification of NPC as neurons to disassembly of their neuroepithelial AJs.
epb41l5-deficient embryos were characterized by the mislocalization of differentiating neurons (Fig. 2N-Q). NPCs specified as neurons remained for an extended period in an intermediate state, whereby they continued to express DeltaD (Fig. 4) and their differentiation as neurons was delayed (Fig. 3). We believe that this problem is related to the previously described role of Epb41l5 in the disassembly of E-cadherin-based AJs in epithelial cells at the onset of EMT (Lee et al., 2007; Hirano et al., 2008). Support for this interpretation comes from the observation that a partial knockdown of n-cadherin reduced the delay in neuronal differentiation in epb41l5-deficient embryos (Fig. 6). Why effective disengagement of fate-determined NPCs from their neighbors facilitates their eventual differentiation as neurons remains unclear at this time.
Under normal circumstances, the ectopic expression of Epb41l5 (Fig. 5) or the inhibition of N-cadherin function in an NPC (Zhang et al., 2010: Rousso et al., 2012) promotes its differentiation as a neuron. In this context, disengagement of NPCs from their neighbors is likely to promote their neuronal specification, at least in part, because the reduction of AJCs is expected to impair Delta-Notch interactions at AJCs and make lateral inhibition from adjacent NPCs less effective (Hatakeyama et al., 2014). Conversely, less efficient disassembly of AJs of fate-specified NPCs in epb41l5-deficient embryos could permit persistent lateral inhibition from neighboring cells, which might further delay neuronal differentiation. However, this is unlikely because neuronal differentiation was delayed in embryos in which lateral inhibition had been already blocked (Figs 3, 4). Hence, the delay in neuronal differentiation in epb41l5-deficient embryos is likely to be related to some additional signaling interaction that persists when AJs are not effectively disassembled.
Although our study has focused on changes related to less effective disassembly of AJs, we recognize that some changes in epb41l5-deficient embryos might be related to other functions of Epb41l5. A characteristic phenotype of epb41l5-deficient embryos is the aberrant morphology of the hindbrain, which is associated with the failure of the organization of AJCs lining the putative ventricular surface and subsequent failure in brain ventricle inflation. These defects are believed to be associated with functions of Epb41l5 in limiting the distribution of Crb to the apical/ventricular surface of the neural tube (Hsu et al., 2006; Laprise et al., 2006; Gosens et al., 2007). These observations raised the possibility that the disorganization of the neuroepithelium and neural tube morphogenesis might contribute to the delays in delamination and differentiation of neurons in epb41l5-deficient embryos. However, transplanted epb41l5-deficient cells in the wild-type hindbrain showed a delay in neuronal differentiation (Fig. 5D). This suggests that the delays in neuronal differentiation in epb41l5-deficient cells are cell-autonomous and the broader disorganization of the hindbrain morphology is not the primary cause of the delays. Although differentiation of epb41l5-deficient cells was delayed, it is not entirely clear that the delay in differentiation is only determined by the delay in delamination. At least in some cases, differentiation was also delayed in epb41l5-deficient cells that had apparently delaminated (Fig. 5B). This raises the possibility that although a Epb41l5-dependent delay in delamination may contribute to a delay in neuronal differentiation, other Epb41l5-dependent mechanisms may also contribute to this delay.
We have analyzed embryos that have a broad loss of Epb41l5 functions and have described changes that are best understood in the context of the specific role of Epb41l5 in delamination and differentiation of NPCs. Although our observations did not suggest a clear role for Epb41l5 in Notch signaling, previous analysis of the moerw306 allele, which specifically lacks the ability to interact with Crb, led to a different conclusion (Ohata et al., 2011). They suggested that Crb directly interacts with Notch and inhibits its activation, whereas Epb41l5 reverses this inhibition by binding to Crb. In this manner, Epb41l5 facilitates Notch signaling. This conclusion was supported with the observation that her4 expression was reduced in moerw306 mutants. However, we did not focus on reduced expression of her4 as it was associated with cell death. Furthermore, there were no other changes in either neurogenesis or somitogenesis that were consistent with failure of Notch signaling. Therefore, we could not conclude that changes we had described were related to potential roles of Epb41l5 in Notch signaling.
Can we reconcile these apparently contradictory conclusions? Our study focuses on the potential role of Epb41l5 in facilitating delamination and differentiation of NPCs that have been selected to become neurons. We suggest that NPCs, with relatively low levels of Notch activation, selected to become neurons, express relatively high levels of Epb41l5. One possibility is that Epb41l5 facilitates their differentiation as neurons by facilitating delamination. However, Epb41l5 might have additional roles in NPCs that have not been selected to become neurons. In these NPCs, Epb41l5 might interact with Crb and organize apicobasal polarity. Furthermore, Epb41l5 might interact with Crb in these NPCs to facilitate Notch signaling by preventing interactions with Crb, as suggested by (Ohata et al., 2011).
There is, however, an alternative explanation for why our analysis of epb41l5 morphants might have come to a different conclusion about a potential role of Epb41l5 in Notch signaling. Ohata et al. have examined changes in the moerw306 allele in which Epb41l5 lacks a domain required for its interaction with Crb (Ohata et al., 2011). Hence, functions of Epb41l5 that require Crb interactions might be specifically impaired in moerw306 mutants. In moerw306 mutants, Crb is aberrantly expressed on the cell surface, which could inhibit Notch signaling. However, although the Epb41l5rw306 mutant protein still binds to Mib1, we found that it is not effectively ubiquitylated or degraded by Mib1 (data not shown). Therefore, it is possible that some changes observed in moerw306 mutants might be the result of increased expression of a stabilized version of Epb41l5. One consequence of this could be increased AJs disassembly and an accompanying reduction of Notch signaling. In this context, it would be interesting to determine whether knockdown of epb41l5 in moerw306 mutants leads to a recovery of Notch signaling. Although we avoided analysis of phenotypes associated with cell death, cell death is a real consequence of reduced Epb41l5 function and previous studies have also shown an increase in p53-dependent apoptosis when Crb function is reduced (Yamaguchi et al., 2004). Understanding the link between Epb41l5 and Crb, p53-dependent apoptosis and changes in Notch signaling will require further investigation.
MATERIALS AND METHODS
Plasmids
Zebrafish Epb41l5 was purchased from Open Biosystems (BC066560). Epb41l5 full-length and truncated versions were PCR amplified and cloned into pCS2-Flag expression vector. HA-Ubiquitin and Myc-Mib1 plasmids were previously described (Itoh et al., 2003).
Yeast two-hybrid screen
The Ras Recruitment System (Broder et al., 1998) and human Fetal Brain cDNA Library (Stratagene) were used.
Fish maintenance and lines
Fish lines were maintained under standard conditions (Kimmel et al., 1995). The use of animals was approved by the Institutional Animal Care and Use Committee at Rutgers and NIH. AB wild-type fish were obtained from ZIRC. moeb476 mutant line was obtained from Dr Westerfield (Jensen and Westerfield, 2004). mib1ta52b and mib1m178 mutants were described previously (Itoh et al., 2003). Male and female fish were used.
Morpholino antisense oligonucleotide micro injection
Morpholino oligonucleotides (Table S1) were purchased from Gene Tools. p53 morpholino was co-injected to prevent p53-dependent cell death and associated off-target effects of morpholinos (Robu et al., 2007).
Whole-mount in situ hybridization
Probes for in situ hybridization were labeled by digoxigenin-UTP (Roche). In situ hybridization was performed as described previously (Matsuda and Chitnis, 2009). BCIP/NBT substrate kit (Vector Laboratories) was used for the coloration.
Whole-mount immunocytochemistry
Embryos were fixed with either 4% paraformaldehyde (PFA) in PBS overnight (anti-Crb2a antibody) or with 10% trichloroacetic acid for 30 min on ice (anti-DeltaD and anti-ZO-1 antibodies). Embryos were incubated with primary antibodies (Table S2) overnight at 4°C. Embryos were then incubated with Alexa488- or Cy3-conjugated secondary antibodies (715-165-151, 711-165-152, 715-545-151, 711-545-152; Jackson ImmunoResearch) at 1:500. An LSM510 confocal microscope (Zeiss) or an A1R confocal microscope system (Nikon) was used for imaging.
DAPT treatment
DAPT (50 μM; Calbiochem) was applied to embryos for the appropriate durations as described in Results to block Notch signaling (Geling et al., 2002). Control embryos were treated with DMSO.
Immunostaining of cultured cells
HEK293 and MCDK cells were purchased from ATCC. Cells were transfected with FuGENE6 (Roche) following the manufacturer's instructions. Cells were fixed for 15 min with 4% PFA. Cells were incubated with primary antibodies (Table S2) for 2 h and then incubated with fluorescent secondary antibodies (715-165-151, 711-165-152, 715-545-151, 711-545-152; Jackson ImmunoResearch) for 45 min.
Immunoprecipitation and western blotting
HEK293 cells were harvested 24-48 h after transfection. Cell lysates were incubated with primary antibodies (Table S2) and with protein G sepharose. Beads were washed and boiled in SDS sample buffer for western blotting. Chemiluminescent signals were quantified using a Chemidoc Bioimager (Bio-Rad).
In vitro transcription and mRNA preparation for microinjection
pCS2-myc-Epb141l5 and pCS2-myc-membGFP plasmids were linearized by NotI. Capped mRNAs were in vitro synthesized using mMessage mMachine SP6 kit (Ambion). After purification using G-25 spin columns (GE BioHealth), mRNAs were microinjected into one-cell-stage embryos.
DNA microinjection and heat-shock treatment
GFP-Epb41l5 and myc-membGFP were inserted downstream of the heat shock protein 70 promoter. Plasmid DNAs were microinjected into one-cell-stage embryos. At 24 hpf, embryos were heat-shock treated by being placed in a 42°C water bath for 30 min. After a 6 h incubation, embryos were immunostained with anti-HuC and anti-myc antibodies (Table S2).
Transplantation
Control morpholino or epb41l5 morpholino was microinjected into one-cell-stage embryos together with p53 morpholino and mRNA encoding Myc-membGFP. Twenty to thirty cells were transplanted from donor embryos into host wild-type embryos at 4.5 hpf. Embryos at 32 hpf were immunostained with anti-HuC and anti-myc antibodies (Table S2).
Quantification and statistical analyses
Quantification of fluorescence signal in whole-mount immunostained embryos was performed by analyzing individual single-plane images. The integrated fluorescence intensities of anti-ZO-1 and anti-HuC immunostaining in the hindbrain were measured using ImageJ software. The hindbrain regions were defined by anti-β-catenin immunostaining. The one-tailed t-test was performed for comparison of individual groups using GraphPad software.
Acknowledgements
We thank members of M.M. laboratory and A.B.C laboratory for technical assistant and comments on the manuscript. We thank Abbie Jensen and Jane McGlade for discussions and help during an early phase of this project.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: M.M., A.B.C.; Methodology: M.M., A.B.C.; Formal analysis: M.M.; Investigation: M.M., K.R., G.P., N.S., H.I., D.D.N., M.I.; Writing – original draft preparation: M.M.; Writing – review and editing: M.M., D.D.N., A.B.C.; Funding acquisition: M.M., A.B.C.
Funding
This research was supported by the National Institute of Child Health and Human Development (NICHD, NIH) [HD062561 to M.M] and NICHD intramural program [HD001012 to A.B.C.]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.138743.supplemental
References
- Artavanis-Tsakonas S., Rand M. D. and Lake R. J. (1999). Notch signaling: cell fate control and signal integration in development. Science 284, 770-776. 10.1126/science.284.5415.770 [DOI] [PubMed] [Google Scholar]
- Baines A. J. (2006). A FERM-adjacent (FA) region defines a subset of the 4.1 superfamily and is a potential regulator of FERM domain function. BMC Genomics 7, 85 10.1186/1471-2164-7-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barami K., Kirschenbaum B., Lemmon V. and Goldman S. A. (1994). N-cadherin and Ng-CAM/8D9 are involved serially in the migration of newly generated neurons into the adult songbird brain. Neuron 13, 567-582. 10.1016/0896-6273(94)90026-4 [DOI] [PubMed] [Google Scholar]
- Bertrand N., Castro D. S. and Guillemot F. (2002). Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 3, 517-530. 10.1038/nrn874 [DOI] [PubMed] [Google Scholar]
- Blader P., Fischer N., Gradwohl G., Guillemot F. and Strahle U. (1997). The activity of neurogenin1 is controlled by local cues in the zebrafish embryo. Development 124, 4557-4569. [DOI] [PubMed] [Google Scholar]
- Broder Y. C., Katz S. and Aronheim A. (1998). The ras recruitment system, a novel approach to the study of protein-protein interactions. Curr. Biol. 8, 1121-1130. 10.1016/S0960-9822(98)70467-1 [DOI] [PubMed] [Google Scholar]
- Cajanek L., Glatter T. and Nigg E. A. (2015). The E3 ubiquitin ligase Mib1 regulates Plk4 and centriole biogenesis. J. Cell Sci. 128, 1674-1682. 10.1242/jcs.166496 [DOI] [PubMed] [Google Scholar]
- Cappello S., Attardo A., Wu X., Iwasato T., Itohara S., Wilsch-Brauninger M., Eilken H. M., Rieger M. A., Schroeder T. T., Huttner W. B. et al. (2006). The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat. Neurosci. 9, 1099-1107. 10.1038/nn1744 [DOI] [PubMed] [Google Scholar]
- Chen W. and Casey Corliss D. (2004). Three modules of zebrafish Mind bomb work cooperatively to promote Delta ubiquitination and endocytosis. Dev. Biol. 267, 361-373. 10.1016/j.ydbio.2003.11.010 [DOI] [PubMed] [Google Scholar]
- Chu C.-W., Gerstenzang E., Ossipova O. and Sokol S. Y. (2013). Lulu regulates Shroom-induced apical constriction during neural tube closure. PLoS ONE 8, e81854 10.1371/journal.pone.0081854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai B. V., Harmon R. M. and Green K. J. (2009). Desmosomes at a glance. J. Cell Sci. 122, 4401-4407. 10.1242/jcs.037457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakowski W., Grzybek M. and Sikorski A. F. (2006). Protein 4.1, a component of the erythrocyte membrane skeleton and its related homologue proteins forming the protein 4.1/FERM superfamily. Folia Histochem. Cytobiol. 44, 231-248. [PubMed] [Google Scholar]
- Erdmann B., Kirsch F.-P., Rathjen F. G. and More M. I. (2003). N-cadherin is essential for retinal lamination in the zebrafish. Dev. Dyn. 226, 570-577. 10.1002/dvdy.10266 [DOI] [PubMed] [Google Scholar]
- Fiuza U.-M. and Arias A. M. (2007). Cell and molecular biology of Notch. J. Endocrinol. 194, 459-474. 10.1677/JOE-07-0242 [DOI] [PubMed] [Google Scholar]
- Ganzler-Odenthal S. I. and Redies C. (1998). Blocking N-cadherin function disrupts the epithelial structure of differentiating neural tissue in the embryonic chicken brain. J. Neurosci. 18, 5415-5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geling A., Steiner H., Willem M., Bally-Cuif L. and Haass C. (2002). A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 3, 688-694. 10.1093/embo-reports/kvf124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosens I., Sessa A., den Hollander A. I., Letteboer S. J. F., Belloni V., Arends M. L., Le Bivic A., Cremers F. P. M., Broccoli V. and Roepman R. (2007). FERM protein EPB41L5 is a novel member of the mammalian CRB-MPP5 polarity complex. Exp. Cell Res. 313, 3959-3970. 10.1016/j.yexcr.2007.08.025 [DOI] [PubMed] [Google Scholar]
- Gumbiner B. M. (2005). Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6, 622-634. 10.1038/nrm1699 [DOI] [PubMed] [Google Scholar]
- Haddon C., Smithers L., Schneider-Maunoury S., Coche T., Henrique D. and Lewis J. (1998). Multiple delta genes and lateral inhibition in zebrafish primary neurogenesis. Development 125, 359-370. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J., Wakamatsu Y., Nagafuchi A., Kageyama R., Shigemoto R. and Shimamura K. (2014). Cadherin-based adhesions in the apical endfoot are required for active Notch signaling to control neurogenesis in vertebrates. Development 141, 1671-1682. 10.1242/dev.102988 [DOI] [PubMed] [Google Scholar]
- Hatta K., Takagi S., Fujisawa H. and Takeichi M. (1987). Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev. Biol. 120, 215-227. 10.1016/0012-1606(87)90119-9 [DOI] [PubMed] [Google Scholar]
- Hirano M., Hashimoto S., Yonemura S., Sabe H. and Aizawa S. (2008). EPB41L5 functions to post-transcriptionally regulate cadherin and integrin during epithelial-mesenchymal transition. J. Cell Biol. 182, 1217-1230. 10.1083/jcb.200712086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y.-C., Willoughby J. J., Christensen A. K. and Jensen A. M. (2006). Mosaic Eyes is a novel component of the Crumbs complex and negatively regulates photoreceptor apical size. Development 133, 4849-4859. 10.1242/dev.02685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Kim C.-H., Palardy G., Oda T., Jiang Y.-J., Maust D., Yeo S.-Y., Lorick K., Wright G. J., Ariza-McNaughton L. et al. (2003). Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell 4, 67-82. 10.1016/S1534-5807(02)00409-4 [DOI] [PubMed] [Google Scholar]
- Itoh Y., Tyssowski K. and Gotoh Y. (2013). Transcriptional coupling of neuronal fate commitment and the onset of migration. Curr. Opin. Neurobiol. 23, 957-964. 10.1016/j.conb.2013.08.003 [DOI] [PubMed] [Google Scholar]
- Jensen A. M. and Westerfield M. (2004). Zebrafish mosaic eyes is a novel FERM protein required for retinal lamination and retinal pigmented epithelial tight junction formation. Curr. Biol. 14, 711-717. 10.1016/j.cub.2004.04.006 [DOI] [PubMed] [Google Scholar]
- Jensen A. M., Walker C. and Westerfield M. (2001). mosaic eyes: a zebrafish gene required in pigmented epithelium for apical localization of retinal cell division and lamination. Development 128, 95-105. [DOI] [PubMed] [Google Scholar]
- Kadowaki M., Nakamura S., Machon O., Krauss S., Radice G. L. and Takeichi M. (2007). N-cadherin mediates cortical organization in the mouse brain. Dev. Biol. 304, 22-33. 10.1016/j.ydbio.2006.12.014 [DOI] [PubMed] [Google Scholar]
- Kim C.-H., Ueshima E., Muraoka O., Tanaka H., Yeo S.-Y., Huh T.-L. and Miki N. (1996). Zebrafish elav/HuC homologue as a very early neuronal marker. Neurosci. Lett. 216, 109-112. 10.1016/0304-3940(96)13021-4 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B. and Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310. 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- Kurusu M., Katsuki T., Zinn K. and Suzuki E. (2012). Developmental changes in expression, subcellular distribution, and function of Drosophila N-cadherin, guided by a cell-intrinsic program during neuronal differentiation. Dev. Biol. 366, 204-217. 10.1016/j.ydbio.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon D. Y., Dimitriadi M., Terzic B., Cable C., Hart A. C., Chitnis A., Fischbeck K. H. and Burnett B. G. (2013). The E3 ubiquitin ligase mind bomb 1 ubiquitinates and promotes the degradation of survival of motor neuron protein. Mol. Biol. Cell 24, 1863-1871. 10.1091/mbc.E13-01-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E. C., Roegiers F., Qin X., Jan Y. N. and Rubin G. M. (2005). The ubiquitin ligase Drosophila Mind bomb promotes Notch signaling by regulating the localization and activity of Serrate and Delta. Development 132, 2319-2332. 10.1242/dev.01825 [DOI] [PubMed] [Google Scholar]
- Laprise P., Beronja S., Silva-Gagliardi N. F., Pellikka M., Jensen A. M., McGlade C. J. and Tepass U. (2006). The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev. Cell 11, 363-374. 10.1016/j.devcel.2006.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R., Remaud S., Hamel S. and Schweisguth F. (2005). Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS Biol. 3, e96 10.1371/journal.pbio.0030096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. D., Silva-Gagliardi N. F., Tepass U., McGlade C. J. and Anderson K. V. (2007). The FERM protein Epb4.1l5 is required for organization of the neural plate and for the epithelial-mesenchymal transition at the primitive streak of the mouse embryo. Development 134, 2007-2016. 10.1242/dev.000885 [DOI] [PubMed] [Google Scholar]
- Lele Z., Folchert A., Concha M., Rauch G. J., Geisler R., Rosa F., Wilson S. W., Hammerschmidt M. and Bally-Cuif L. (2002). parachute/n-cadherin is required for morphogenesis and maintained integrity of the zebrafish neural tube. Development 129, 3281-3294. [DOI] [PubMed] [Google Scholar]
- Lewis J., Hanisch A. and Holder M. (2009). Notch signaling, the segmentation clock, and the patterning of vertebrate somites. J. Biol. 8, 44 10.1186/jbiol145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvi A. and Artavanis-Tsakonas S. (2006). Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 7, 93-102. 10.1038/nrn1847 [DOI] [PubMed] [Google Scholar]
- Lowery A. and Sive H. (2005). Initial formation of zebrafish brain ventricles occurs independently of circulation and requires the nagie oko and snakehead/atp1a1a.1 gene products. Development 132, 2057-2067. 10.1242/dev.01791 [DOI] [PubMed] [Google Scholar]
- Malicki J., Jo H. and Pujic Z. (2003). Zebrafish N-cadherin, encoded by the glass onion locus, plays an essential role in retinal patterning. Dev. Biol. 259, 95-108. 10.1016/S0012-1606(03)00181-7 [DOI] [PubMed] [Google Scholar]
- Masai I., Lele Z., Yamaguchi M., Komori A., Nakata A., Nishiwaki Y., Wada H., Tanaka H., Nojima Y., Hammerschmidt M. et al. (2003). N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development 130, 2479-2494. 10.1242/dev.00465 [DOI] [PubMed] [Google Scholar]
- Matsuda M. and Chitnis A. B. (2009). Interaction with Notch determines endocytosis of specific Delta ligands in zebrafish neural tissue. Development 136, 197-206. 10.1242/dev.027938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W. and Takeichi M. (2012). Adherens junction: molecular architecture and regulation. Cold Spring Har. Perspect. Biol. 1, a002899 10.1101/cshperspect.a002899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H. and Tanoue T. (2010). Epithelial cell shape is regulated by Lulu proteins via myosin-II. J. Cell Sci. 123, 555-566. 10.1242/jcs.057752 [DOI] [PubMed] [Google Scholar]
- Ohata S., Aoki R., Kinoshita S., Yamaguchi M., Tsuruoka-Kinoshita S., Tanaka H., Wada H., Watabe S., Tsuboi T., Masai I. et al. (2011). Dual roles of Notch in regulation of apically restricted mitosis and apicobasal polarity of neuroepithelial cells. Neuron 69, 215-230. 10.1016/j.neuron.2010.12.026 [DOI] [PubMed] [Google Scholar]
- Pacary E., Martynoga B. and Guillemot F. (2012). Crucial first steps: the transcriptional control of neuron delamination. Neuron 74, 209-211. 10.1016/j.neuron.2012.04.002 [DOI] [PubMed] [Google Scholar]
- Palardy G. and Chitnis A. B. (2015). Identification of the Mind Bomb1 interaction domain in zebrafish DeltaD. PLoS ONE 10, e0127864 10.1371/journal.pone.0127864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson A. F., Prasad M. S., Thuringer A. H. and Manzerra P. (2014). Regulation of cadherin expression in nervous system development. Cell Adhes. Migr. 8, 19-28. 10.4161/cam.27839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsouli C. and Delidakis C. (2005). The interplay between DSL proteins and ubiquitin ligases in Notch signaling. Development 132, 4041-4050. 10.1242/dev.01979 [DOI] [PubMed] [Google Scholar]
- Pujic Z. and Malicki J. (2001). Mutation of the zebrafish glass onion locus causes early cell-nonautonomous loss of neuroepithelial integrity followed by severe neuronal patterning defects in the retina. Dev. Biol. 234, 454-469. 10.1006/dbio.2001.0251 [DOI] [PubMed] [Google Scholar]
- Radice G. L., Rayburn H., Matsunami H., Knudsen K. A., Takeichi M. and Hynes R. O. (1997). Developmental defects in mouse embryos lacking N-cadherin. Dev. Biol. 181, 64-78. 10.1006/dbio.1996.8443 [DOI] [PubMed] [Google Scholar]
- Redies C. and Takeichi M. (1996). Cadherins in the developing central nervous system: an adhesive code for segmental and functional subdivisions. Dev. Biol. 180, 413-423. 10.1006/dbio.1996.0315 [DOI] [PubMed] [Google Scholar]
- Robu M. E., Larson J. D., Nasevicius A., Beiraghi S., Brenner C., Farber S. A. and Ekker S. C. (2007). p53 activation by knockdown technologies. PLoS Genet. 3, e78 10.1371/journal.pgen.0030078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousso D. L., Pearson C. A., Gaber Z. B., Miquelajauregui A., Li S., Portera-Cailliau C., Morrisey E. E. and Novitch B. G. (2012). Foxp-mediated suppression of N-cadherin regulates neuroepithelial character and progenitor maintenance in the CNS. Neuron 74, 314-330. 10.1016/j.neuron.2012.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Merker S. and Stainier D. Y. (2014). Out with the old, in with the new: reassessing morpholino knockdowns in light of genome editing technology. Development 141, 3103-3104. 10.1242/dev.112003 [DOI] [PubMed] [Google Scholar]
- Schweisguth F. (2004). Regulation of notch signaling activity. Curr. Biol. 14, R129-R138. 10.1016/j.cub.2004.01.023 [DOI] [PubMed] [Google Scholar]
- Seki T., Namba T., Mochizuki H. and Onodera M. (2007). Clustering, migration, and neurite formation of neural precursor cells in the adult rat hippocampus. J. Comp. Neurol. 502, 275-290. 10.1002/cne.21301 [DOI] [PubMed] [Google Scholar]
- Shin K., Fogg V. C. and Margolis B. (2006). Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 22, 207-235. 10.1146/annurev.cellbio.22.010305.104219 [DOI] [PubMed] [Google Scholar]
- Spadaro D., Tapia R., Pulimeno P. and Citi S. (2012). The control of gene expression and cell proliferation by the epithelial apical junctional complex. Essays Biochem. 53, 83-93. 10.1042/bse0530083 [DOI] [PubMed] [Google Scholar]
- Takke C., Dornseifer P. V., Weizsacker E. and Campos-Ortega J. A. (1999). her4, a zebrafish homologue of the Drosophila neurogenic gene E(spl), is a target of NOTCH signalling. Development 126, 1811-1821. [DOI] [PubMed] [Google Scholar]
- Tepass U. (2009). FERM proteins in animal morphogenesis. Curr. Opin. Genet. Dev. 19, 357-367. 10.1016/j.gde.2009.05.006 [DOI] [PubMed] [Google Scholar]
- Villumsen B. H., Danielsen J. R., Povlsen L., Sylvestersen K. B., Merdes A., Beli P., Yang Y.-G., Choudhary C., Nielsen M. L., Mailand N. et al. (2013). A new cellular stress response that triggers centriolar satellite reorganization and ciliogenesis. EMBO J. 32, 3029-3040. 10.1038/emboj.2013.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita Y., Sakurai T., Tanaka H., Kitagawa K., Colman D. R. and Shan W. (2009). N-cadherin mediates interaction between precursor cells in the subventricular zone and regulates further differentiation. J. Neurosci. Res. 87, 3331-3342. 10.1002/jnr.22044 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Hirose F., Inoue Y. H., Ohno K., Yoshida H., Hayashi Y., Deak P. and Matsukage A. (2004). Genetic link between p53 and genes required for formation of the zonula adherens junction. Cancer Sci. 95, 436-441. 10.1111/j.1349-7006.2004.tb03228.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo S.-Y., Kim M., Kim H.-S., Huh T.-L. and Chitnis A. B. (2007). Fluorescent protein expression driven by her4 regulatory elements reveals the spatiotemporal pattern of Notch signaling in the nervous system of zebrafish embryos. Dev. Biol. 301, 555-567. 10.1016/j.ydbio.2006.10.020 [DOI] [PubMed] [Google Scholar]
- Zhang J., Woodhead G. J., Swaminathan S. K., Noles S. R., McQuinn E. R., Pisarek A. J., Stocker A. M., Mutch C. A., Funatsu N. and Chenn A. (2010). Cortical neural precursors inhibit their own differentiation via N-cadherin maintenance of beta-catenin signaling. Dev. Cell 18, 472-479. 10.1016/j.devcel.2009.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]