Abstract
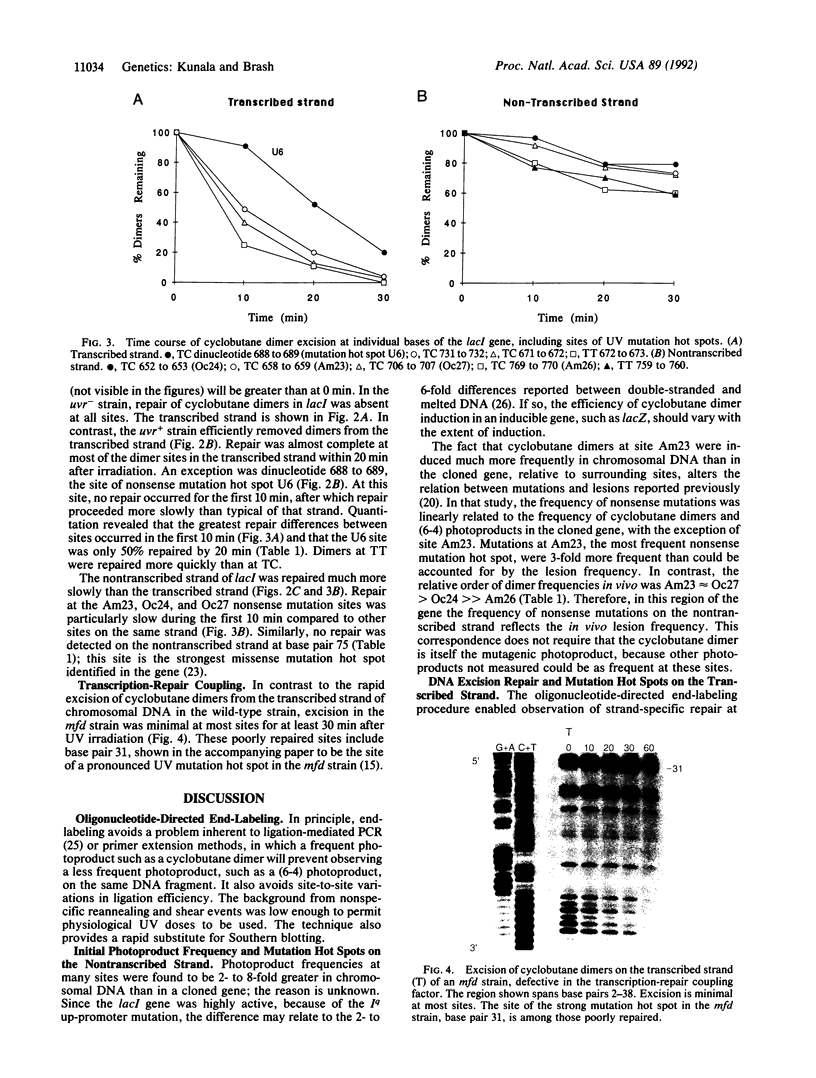
To determine whether base-to-base variations in the rate of excision repair influence the distribution of mutations, we have developed a method to measure UV photoproducts at individual nucleotides in the Escherichia coli chromosome. Specific gene fragments are 3' end-labeled using a sequence-specific oligonucleotide to direct the site of labeling, and photoproducts are identified by enzymatic incision. On the nontranscribed strand of the E. coli lacI gene, the cyclobutane pyrimidine dimer frequency was 2- to 8-fold higher in chromosomal DNA than in a cloned DNA fragment. The chromosomal lesion frequency corresponded to the frequency of UV-induced mutations at mutation hot spots reported in the literature. Only 0-30% of cyclobutane dimers at various sites on this strand were excised in 20 min. In contrast, repair on the transcribed strand was 80-90% complete in 20 min. However, the transcribed strand contained an excision repair "slow spot" at the site of its single mutation hot spot: At this site, no repair occurred for the first 10 min, after which repair proceeded more slowly than typical of that strand. In an mfd strain, deficient in a factor that couples repair to transcription in cell extracts, the excision rate at individual nucleotides on the transcribed strand was minimal at most sites for at least 30 min. Wild-type E. coli's bias for producing mutations at photoproducts on the nontranscribed strand, reported to require the mfd gene, therefore appears to be due to an excision repair system specific for the transcribed strand of chromosomal DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Becker M. M., Wang Z. Origin of ultraviolet damage in DNA. J Mol Biol. 1989 Dec 5;210(3):429–438. doi: 10.1016/0022-2836(89)90120-4. [DOI] [PubMed] [Google Scholar]
- Bockrath R. C., Palmer J. E. Differential repair of premutational UV-lesions at tRNA genes in E. coli. Mol Gen Genet. 1977 Nov 14;156(2):133–140. doi: 10.1007/BF00283485. [DOI] [PubMed] [Google Scholar]
- Bohr V. A. Gene specific DNA repair. Carcinogenesis. 1991 Nov;12(11):1983–1992. doi: 10.1093/carcin/12.11.1983. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Haseltine W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature. 1982 Jul 8;298(5870):189–192. doi: 10.1038/298189a0. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Rudolph J. A., Simon J. A., Lin A., McKenna G. J., Baden H. P., Halperin A. J., Pontén J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. III. Additional correlation of mutational sites with specific amino acid residues. J Mol Biol. 1977 Dec 15;117(3):525–567. doi: 10.1016/0022-2836(77)90056-0. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Franklin W. A., Haseltine W. A. Removal of UV light-induced pyrimidine-pyrimidone(6-4) products from Escherichia coli DNA requires the uvrA, uvrB, and urvC gene products. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3821–3824. doi: 10.1073/pnas.81.12.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D. L., Witkin E. M. Ultraviolet light-induced responses of an mfd mutant of Escherichia coli B/r having a slow rate of dimer excision. Mutat Res. 1975 Jun;28(3):347–354. doi: 10.1016/0027-5107(75)90229-8. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Koehler D. R., Awadallah S. S., Glickman B. W. Sites of preferential induction of cyclobutane pyrimidine dimers in the nontranscribed strand of lacI correspond with sites of UV-induced mutation in Escherichia coli. J Biol Chem. 1991 Jun 25;266(18):11766–11773. [PubMed] [Google Scholar]
- Kohne D. E., Levison S. A., Byers M. J. Room temperature method for increasing the rate of DNA reassociation by many thousandfold: the phenol emulsion reassociation technique. Biochemistry. 1977 Nov 29;16(24):5329–5341. doi: 10.1021/bi00643a026. [DOI] [PubMed] [Google Scholar]
- McGregor W. G., Chen R. H., Lukash L., Maher V. M., McCormick J. J. Cell cycle-dependent strand bias for UV-induced mutations in the transcribed strand of excision repair-proficient human fibroblasts but not in repair-deficient cells. Mol Cell Biol. 1991 Apr;11(4):1927–1934. doi: 10.1128/mcb.11.4.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Hanawalt P. C. Induction of the Escherichia coli lactose operon selectively increases repair of its transcribed DNA strand. Nature. 1989 Nov 2;342(6245):95–98. doi: 10.1038/342095a0. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Ganem D., Lu P., Schmitz A. Genetic studies of the lac repressor. I. Correlation of mutational sites with specific amino acid residues: construction of a colinear gene-protein map. J Mol Biol. 1977 Jan 15;109(2):275–298. doi: 10.1016/s0022-2836(77)80034-x. [DOI] [PubMed] [Google Scholar]
- Munn M. M., Rupp W. D. Interaction of the UvrABC endonuclease with DNA containing a psoralen monoadduct or cross-link. Differential effects of superhelical density and comparison of preincision complexes. J Biol Chem. 1991 Dec 25;266(36):24748–24756. [PubMed] [Google Scholar]
- Nishioka H., Doudney C. O. Different modes of loss of photoreversibility of mutation and lethal damage in ultraviolet-light resistant and sensitive bacteria. Mutat Res. 1969 Sep-Oct;8(2):215–228. doi: 10.1016/0027-5107(69)90001-3. [DOI] [PubMed] [Google Scholar]
- Nishioka H., Doudney C. O. Different modes of loss of photoreversibility of ultraviolet light-induced true and suppressor mutations to tryptophan independence in an auxotrophic strain of Escherichia coli. Mutat Res. 1970 Apr;9(4):349–358. doi: 10.1016/0027-5107(70)90017-5. [DOI] [PubMed] [Google Scholar]
- Oller A. R., Fijalkowska I. J., Dunn R. L., Schaaper R. M. Transcription-repair coupling determines the strandedness of ultraviolet mutagenesis in Escherichia coli. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11036–11040. doi: 10.1073/pnas.89.22.11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer G. P., Drouin R., Riggs A. D., Holmquist G. P. In vivo mapping of a DNA adduct at nucleotide resolution: detection of pyrimidine (6-4) pyrimidone photoproducts by ligation-mediated polymerase chain reaction. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1374–1378. doi: 10.1073/pnas.88.4.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout W. M., 3rd, Coetzee G. A., Olumi A. F., Jones P. A. 5-Methylcytosine as an endogenous mutagen in the human LDL receptor and p53 genes. Science. 1990 Sep 14;249(4974):1288–1290. doi: 10.1126/science.1697983. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Dunn R. L., Glickman B. W. Mechanisms of ultraviolet-induced mutation. Mutational spectra in the Escherichia coli lacI gene for a wild-type and an excision-repair-deficient strain. J Mol Biol. 1987 Nov 20;198(2):187–202. doi: 10.1016/0022-2836(87)90305-6. [DOI] [PubMed] [Google Scholar]
- Selby C. P., Witkin E. M., Sancar A. Escherichia coli mfd mutant deficient in "mutation frequency decline" lacks strand-specific repair: in vitro complementation with purified coupling factor. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11574–11578. doi: 10.1073/pnas.88.24.11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd P. A., Glickman B. W. Mutational specificity of UV light in Escherichia coli: indications for a role of DNA secondary structure. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4123–4127. doi: 10.1073/pnas.79.13.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieling H., Van Rooijen M. L., Groen N. A., Zdzienicka M. Z., Simons J. W., Lohman P. H., van Zeeland A. A. DNA strand specificity for UV-induced mutations in mammalian cells. Mol Cell Biol. 1989 Mar;9(3):1277–1283. doi: 10.1128/mcb.9.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Radiation-induced mutations and their repair. Science. 1966 Jun 3;152(3727):1345–1353. doi: 10.1126/science.152.3727.1345. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]