Abstract
BACKGROUND
Despite the revolutionary success of introducing tyrosine kinase inhibitors (TKIs), such as imatinib mesylate (IM), for treating chronic myeloid leukemia (CML), a substantial proportion of patients’ treatments fail.
AIM
This study investigates the correlation between patient adherence and failure of TKIs’ treatment in a follow-up study.
METHODS
This is a follow-up study of a new cohort of CML patients. Adherence to IM is assessed using the Medication Event Monitoring System (MEMS 6 TrackCap, AARDEX Ltd). The 9-item Morisky Medication Adherence Scale, medication possession ratio (MPR) calculation, and the electronic medical records are used for identifying potential factors that influence adherence. Clinical outcomes are assessed according to the European Leukemia Net 2013 guidelines via reverse transcriptase quantitative polymerase chain reaction measurement of the level of BCR-ABL1 transcripts in peripheral blood. Response is classified at the hematological, cytogenetic, and molecular levels into optimal, suboptimal, or failure.
RESULTS
A total of 36 CML patients (5 citizens and 31 noncitizen residents) consented to participate in the study. The overall mean MEMS score was 89. Of the 36 patients, 22 (61%) were classified as adherent (mean: 95) and 14 (39%) were classified as nonadherent (mean: 80.2). Adherent patients were significantly more likely to obtain optimal response (95%) compared to the nonadherent group (14.3%; P < 0.0001). The rate of poor adherence was as high as 39% using MEMS, which correlates with 37% treatment failure rate. The survey results show that 97% of patients increased the IM dose by themselves when they felt unwell and 31% of them took the missing IM dose when they remembered. Other factors known to influence adherence show that half of patients developed one or more side effects, 65% of patients experienced lack of funds, 13% of patients declared unavailability of the drug in the NCCCR pharmacy, and 72% of patients believed that IM would cure the disease. The MPR results reveal that 16% of patients had poor access to treatment through the hospital pharmacy.
DISCUSSION AND CONCLUSION
This is the first prospective study to evaluate CML patients’ adherence and response to IM in Qatar. The high rate of treatment failure observed in Qatar is explained by poor adherence. An economic factor (unaffordable drug prices) is one of the main causes of nonadherence and efforts should be made locally to improve access to medication for cancer diseases. Other risk factors associated with poor adherence could be improved by close monitoring and dose adjustment. Monitoring risk factors for poor adherence and patient education that include direct communication between the health-care teams, doctors, nurses, pharmacists, and patients are essential components for maximizing the benefits of TKI therapy and could rectify this problem. The preliminary results show that patients’ response to treatment may be directly linked to patients’ adherence to treatment. However, further in-depth and specific analysis may be necessary in a larger cohort.
Keywords: imatinib mesylate, chronic myeloid leukemia, adherence, 9-item Morisky Medication Adherence Scale, medication possession ratio, Medication Event Monitoring System, treatment response BCR-ABL1, ABL1, reverse transcriptase quantitative polymerase chain reaction, BCR-ABL1 mutations
Background
Despite the revolutionary success of introducing tyrosine kinase inhibitors (TKIs), such as imatinib mesylate (IM), in treating chronic myeloid leukemia (CML), a substantial proportion of CML patients fail treatment with interruption and discontinuation of the drug, rapidly leading to reemergence from minimal residual disease into full-blown disease.1–11
Adherence to therapy and compliance with professional instructions are critical in the management of CML. Compliance is defined by the World Health Organization (WHO) report as “the extent to which a patients’ behavior taking medication corresponds with agreed recommendations from a health-care provider”.12
Several studies suggest that poor adherence to IM is frequent and leads to worse clinical outcomes. Noens et al.13 reported that patients with suboptimal response are significantly more likely to miss doses of their IM (23%) than those with optimal response (7%). Similarly, Marin et al.14 showed that patients with a complete cytogenetic response (CCyR) are also less likely to neglect their IM (9%) compared to those with an incomplete cytogenetic response (23%). They show that the six-year probability of achieving major molecular response rates is significantly higher for patients who score more than 90% levels of adherence, followed by no complete molecular response (adherence ≤90%) and then no molecular responses (MRs; adherence ≤80%).14
Furthermore, Ibrahim et al.15 showed that the adherence rates in patients who eventually fail IM therapy is significantly lower (78.1%) than those of patients who respond to therapy (97.8%). In their study, patients with an adherence rate ≤85% had significantly lower event-free survival (EFS; 54.4%) than adherent patients (EFS rate: 91.4%). In addition, Jonsson et al.16 showed that 97% of their patients achieved optimal response in correlation with optimal adherence. On the other hand, the results of a multicenter STop IMatinib Trial by Mahon et al.17 showed that 61% of patients relapsed after discontinuation of IM, and Yhim et al showed that 71% of patients relapsed within 9.5 months after discontinuation of IM.14–19
In the first study of our series, we uncovered the mechanisms of resistance in 78% of patients, where two patients had BCR-ABL1 kinase domain mutations, one patient had E459K (rs1064156), one patient had a unique insertion of three nucleotides, six patients had additional chromosomal abnormalities as an underlying mechanism of resistance, four patients had no identifiable cause of resistance, and two patients were intolerant to treatment. However, 22% of the resistant cases displayed no explanatory underlying mechanisms.20,21
Thus, the main aim of this follow-up study is to investigate the relationship between adherence and failure of IM treatment in Qatar. Several methods including Medication Event Monitoring System (MEMS 6), 9-item Morisky Medication Adherence Scale (9-MMAS), medication possession ratio (MPR), and electronic medical records (eMR) are used. This study gives an insight into whether adherence affects patients’ responses to treatment and thus helps deign suitable interventions to increase patients’ awareness. As far as the author is aware, this is the first study to be conducted among patients in the state of Qatar.
Design and Method
Patient recruitment
Patients aged 16–65 years (mean age: 42 years) who attended the NCCCR between January 2010 and December 2012 with Philadelphia chromosome positive (Ph+) CML for at least 12 months and received only IM treatment are included in this study. A total of 36 CML patients met the inclusion criteria and have consented and been recruited into the study.
Treatment regimens
Patients in chronic phase (CP) received 400 mg orally once a day as first-line treatment IM, while accelerated phase (AP) patients received 600 mg once a day.
Adherence measures
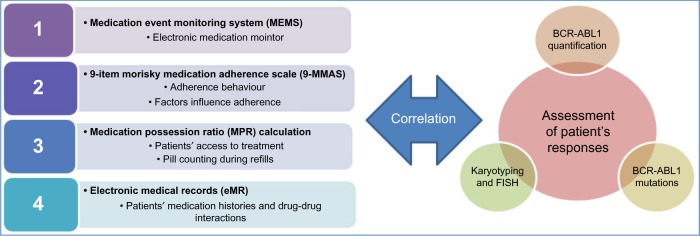
A holistic approach is taken to assess adherence of CML patients to treatment and correlate the result with response to treatment (Fig. 1).
Figure 1.
Assessment of patients’ adherence and response to IM and correlating with treatment response.
Notes: MEMS electronically monitors medication intake, 9 MMAS assesses adherence related behaviors and factors affecting it. On the other hand, MPR assesses the access of patient to treatment resources and counts the remaining pills at the time of refills. Finally, eMR examines medication history and drug–drug interactions.
Adherence to IM is assessed using the Medication Event Monitoring System (MEMS 6 TrackCap, AARDEX Ltd).
In addition, the 9-MMAS, MPR calculation, and eMR are used to identify potential factors that influence adherence. The presence or absence of nonadherent behaviors is measured by the percentage of time a patient has access to medication and by reviewing patients’ medication histories. There are four methods employed as follows.
Method 1: Medication Event Monitoring System (MEMS)
Patient adherence over a period of 6–12 months is monitored in real time. The MEMS medication bottles contain a microelectronic chip fitted into the cap of a normal-looking medication bottle that automatically records and registers the date and time of every bottle opening. Each bottle opening is assumed to represent an event of medication intake. The system does not monitor the actual dose received; however, MEMS is considered the gold standard for measuring adherence.22,23 A cutoff of less than or equal to 90% is considered nonadherence.14,22,23
Patients are not notified about the monitoring system in the bottle caps but are told that their adherence is going to be monitored by counting the number of IM tablets returned.
Method 2: adherence and factors that affect therapy
9-MMAS: Our questionnaire consists of two parts; the first part is adapted from the well-validated Morisky–Green test and the eight-item MMAS.24–28 The 9-MMAS includes nine items that measure the presence or absence of nonadherent behavior, rather than dose intake (see Table 1). Responses include either yes/no (questions 1–8) or five-point Likert responses (question 9). The summary score ranges from 1 to 13, where higher scores reflect better adherence. In this study, good adherence is defined by a Morisky score of 11 or higher (ie, ≥85%).
In the second part, patients are asked predefined follow-up questions to identify potential factors known to influence adherence to therapy. These questions revolve around the patient’s lifestyle, income, and knowledge related to IM factors (eg, degree of social support, knowledge of treatment, and the accessibility of the treating clinic). Patient characteristics and demographic data are also collected (see Table 2).25,26
Table 1.
The 9-item MMAS to measure and evaluate adherence to IM.
| NINE-ITEM MMAS | |
|---|---|
| 1. Do you sometimes forget to take medication? | Y/Na |
| 2. People sometimes miss taking their medications for reasons other than forgetting. Thinking over the past 2 weeks, were there any days when you did not take your medicine? | Y/N |
| 3. Have you ever cut back or stop taking your medication without telling your doctor, because you felt worse when you took it? | Y/N |
| 4. When you travel or leave home, do you sometimes forget to bring along your medicine? | Y/N |
| 5. Did you take your medicine yesterday? | Y/N |
| 6. Do you have a special routine or reminder system to help you take your medications? | Y/N |
| 7. Do you sometimes stop taking your medication if it feels like your disease is under control? | Y/N |
| 8. Taking medication every day is a real inconvenience for some people. Do you ever feel hassled about sticking to your treatment plan? | Y/N |
| 9. How often do you have difficulty remembering to take all of your medicines? | Scale 1–5b |
Note:
Questions 1–8 are answered by yes or no (Y/N). in questions 1–4, 7, and 8, the answer “yes” gives 0 point and “no” gives 1 point. In questions 5–6, the answer “yes” gives 1 point and “no” gives 0 point.
Question 9 is answered by “never/rarely” (5 points), “once in a while” (4 points), “sometimes” (3 points), “usually” (2 points), or “all the time” (1 point).
Table 2.
Potential factors that influence adherence to therapy.
| 1. Age | Years |
| 2. Year of diagnosis | Date |
| 3. Marital Status | MCQ |
| 4. Level of education | MCQ |
| 3. Year of starting Glivec (imatinib) treatment? | Date |
| 4. How much do you know about CML? | Scale 1–4 |
| 5. When did you receive the majority of this knowledge/information? | MCQ |
| 6. Who gave you most of this knowledge/information? | MCQ |
| 7. How much do you know about tour treatment? | Scale 1–4 |
| 8. When did you receive the majority of the knowledge/information about your treatment? | MCQ |
| 9. Who gave you the majority of this knowledge/information about your treatment? | MCQ |
Method 3: MPR calculation
MPR is employed to gain insight into patients’ access to treatment. The MPR is calculated by dividing the sum of supply days of medication by the number of days between the first fill and the last refill. An MPR ≥ 80% is considered high adherence and is the benchmark most commonly reported in the literature.29,30
Method 4: eMR
The eMR is reviewed to gain insight into the patients’ medication histories and drug–drug interactions using MICROMEDEX® 1.0 (Healthcare Series). Patient adherence is correlated with patient’s response treatment and response is assessed according to the 2013 European LeukemiaNet (ELN) guidelines.
Laboratory assessments/assessment of patients’ responses
Molecular investigation is carried out to assess the degree of response of the 36 patients and classify their response according to the ELN 2013. There are four methods employed as follows.
Method 1: absolute quantification of BCR-ABL1
Peripheral blood samples are collected and the level of BCR-ABL1 transcripts measured locally via reverse transcriptase quantitative polymerase chain reaction (RT-QPCR). Patients’ responses to treatment are assessed via serial RT-QPCR for absolute quantification of BCR-ABL1 to monitor the ratio of BCR-ABL1 to normal ABL1 transcripts. The measurement of BCR-ABL1 is optimized locally according to the international guidelines of the Europe against Cancer (EAC). The absolute quantification of BCR-ABL1 is done using two different kits and methods [(1) Ipsogen BCR-ABL1 Mbcr IS-MMR DX and (2) Xpert BCR-ABL Monitor™, Cepheid].
A fully automated cartridge-based assay (Xpert BCR-ABL Monitor™, Cepheid) combines sample preparation with real-time PCR. The results generated are converted to the IS using an assay-specific conversion factor determined by comparison to an IS reference assay.35–41
The MR is determined every third month by analysis of the BCR-ABL1 transcript level in peripheral blood (RT-QPCR) with ABL1 as a housekeeping gene. The BCR-ABL1 is reported in the IS37 as well as described elsewhere.20,21
Method 2: assessment of patients’ responses
The ELN 2013 recommendations for the management of CML is adopted and employed in this study to assess the response/resistance of patients to treatment. Responses are defined at the hematological, cytogenetic, and molecular levels. Patients’ responses are classified as optimal, suboptimal, or failure.42
Method 3: BCR-ABL1 mutations
Sequencing of tyrosine kinase domain of BCR-ABL1 is carried out to identify mutations as an underlying mechanism of resistance.
Sequencing reactions are performed in the forward and reverse directions separately using Big Dye chain terminator reagents on an ABI PRISM 3130 Genetic Analyzer, as described elsewhere.21
Method 4: karyotype analysis and fluorescence in situ hybridization (FISH)
Conventional karyotyping is carried out to identify ACAs as an underlying mechanism of resistance.
Chromosomes are identified and arranged according to the International System for Human Cytogenetic Nomenclature 2009. The number of cells investigated for each patient at each analysis ranges from 20 to 30 metaphases, and the cytogenetic response is assessed according to the ELN criteria. Cytogenetic analysis of metaphases of bone marrow samples as well as FISH for BCR-ABL on interphases of peripheral 100–200 white blood cells is done routinely as part of the clinical protocol, and the results are reported on the hospital’s health information system.
Ethics
Patients gave their written informed consent to participate in the study that was conducted in accordance with the principles of the Declaration of Helsinki and was approved by Hamad Medical Corporation Research Committee [HMC (GC-1013)]. All data and patients’ identities were stored anonymously, and only the principal investigator has access to the code key. Thus, individual results were not seen by the treating physician.
Statistical analysis
Descriptive statistics (means, ranges, frequencies, and percentages) were shown for each measure accordingly. Pearson correlation was used to investigate the relationship between the three adherence techniques (MMAS, MPR, and MEMS). In addition, Fisher’s exact test was performed to study patients’ adherence and responses and the factors that affect them. A significance level of P ≤ 0.05 (two tailed) was considered. GraphPad Prism 5 statistical package was used for analysis.
Results
Demographic features results
Of the 36 patients, 28 (78%) were male and 8 (22%) were female, with more than half (N: 21; 59%) having an educational level of secondary school or less. A total of 27 patients (75%) were married while 9 patients (25%) were single. Patients were from 12 different countries with only 5 Qatari patients (13%; Table 3).
Table 3.
Demographic features of the 36 patients (survey questionnaire).
| VARIABLE | VALUE (NUMBER) | PERCENTAGE % |
|---|---|---|
| Gender | ||
| Males | 28 | 78% |
| Females | 8 | 22% |
| Age, Years Average (Min-Max) | 42 (16–65) | |
| Marital status | ||
| Married | 27 | 75% |
| Single | 9 | 25% |
| Level of education | ||
| Primary | 11 | 31% |
| Secondary | 10 | 28% |
| Diploma | 2 | 5% |
| College | 11 | 31% |
| Master | 2 | 5% |
| Allergy | ||
| NA | 36 | 100% |
| Nationality | ||
| Qatar | 5 | 13% |
| Egypt | 7 | 19% |
| Philippines | 5 | 14% |
| India | 5 | 14% |
| Sudan | 4 | 11% |
| Pakistan | 3 | 8% |
| Nepal | 2 | 5% |
| Bangladesh | 2 | 5% |
| Sri Lanka | 1 | 3% |
| Palestine | 1 | 3% |
Note: Distribution of patients according to gender, age group, marital status, level of education, and their ethnic backgrounds.
The clinical presentation of the 36 CML patients at diagnosis was as follows: 89% (32 patients) CP, 9% (3 patients) AP, and 3% (1) blastic crisis phase (BCP). A total of 35 patients were alive and one patient had died (Table 4).
Table 4.
Clinical features/characteristics of the 36 patients.
| CHARACTERISTICS | TOTAL (%) |
|---|---|
| Clinical presentation at Diagnosis | |
| CP | 32 (89%) |
| AP | 3 (9%) |
| BCP | 1 (3%) |
| IM dose at diagnosis | |
| 400 mg | 32 |
| 600 mg | 4 |
| Switched to second line of treatment | |
| Dasatinib | 9 |
| Nilotinib | 2 |
| Dasatinib then Nilotinib | 1 |
| Alive | 35 (97%) |
| Dead | 1 (3%) |
A total of 32 patients were treated with standard dose IM 400 mg, while 4 patients were treated with 600 mg due to a history of either loss of complete cytogenetic response (CCyR; n = 1) or suboptimal MR (n = 3).
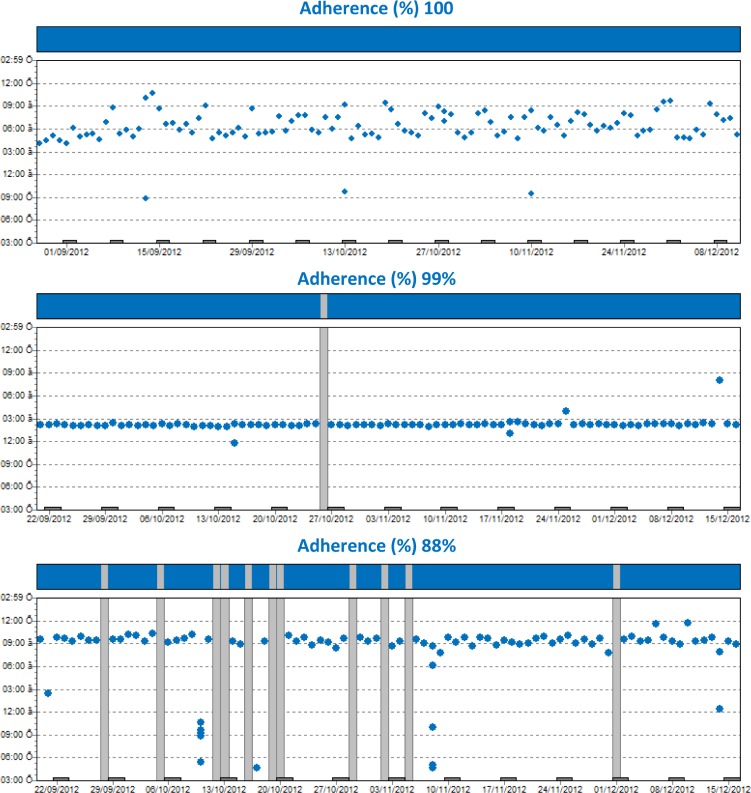
Method 1: MEMS results
The overall mean MEMS score was 89.0% (range: 40–100). Of the 36 patients, 22 (61%) were classified as adherent patients (mean: 95%; range: 91%–100%) and 14 (39%) were classified as nonadherent patients (mean: 80.2%; range: 40%–90%).
Adherent patients were significantly more likely to achieve optimal response [95% (21/22)] compared to the nonadherent group [14.3% (2/14); P < 0.0001; odds ratio (OR): 126, 95% confidence interval (CI): 10.3–1541; Table 5 and Fig. 2].
Table 5.
Patients’ response according to adherence status using the MEMS technique.
| TECHNIQUES | ADHERENCE | RESPONSE | TOTAL | P VALUE | ODDS RATIO (95% CONFIDENCE INTERVAL) | |
|---|---|---|---|---|---|---|
| OPTIMAL N (%) | FAILURE N (%) | |||||
| MEMS | Adherent | 21 (95%) | 1 (5%) | 22 | <0.0001 | 126.0 (10.30–1541) |
| Nonadherent | 2 (14.3%) | 12 (85.7%) | 14 | |||
Figure 2.
Patients’ adherence to IM using MEMS.
Method 2: adherence and factors that affect therapy
-
9-item MMAS results: The overall mean Morisky score of the 36 patients was 10.6 (range: 5–13). Of the 36 patients, 25 patients (69%) were classified as adherent (MMAS mean: 12; range: 11–13) and 11 patients (31%) were nonadherent (MMAS mean: 7.7; range: 5–10; Table 6).
The 9-MMAS revealed that 14 patients (38%) missed medication sometimes, 5 patients (14%) stopped taking their medication when they felt that the disease was under control or found sticking to treatment plan difficult. All patients (100%) had a special routine or reminder system to help them take medication on time, and 13 patients (36%) sometimes had difficulty remembering to take all their medicines.
Adherent patients were more likely to achieve optimal response (72%; 18/25) compared with the nonadherent group (45.5%; 5/11). However, this difference was not significant.
Patients’ behavior and knowledge about intake of IM results: A total of 32 patients (89%) took the IM after meals and 4 patients (11%) took the medication with grapefruit, while 4 patients (11%) took IM on an empty stomach. One patient (3%) increased the IM dose without consultation with their GP when they felt unwell. A total of 11 patients (31%) took the missing IM dose when they remembered, while 12 (33%) did nothing for missing dose (Table 7).
Table 6.
Patients’ response according to adherence status using the 9-MMAS technique.
| TECHNIQUES | ADHERENCE | RESPONSE | TOTAL | P VALUE | ODDS RATIO (95% CONFIDENCE INTERVAL) | |
|---|---|---|---|---|---|---|
| OPTIMAL N (%) | FAILURE N (%) | |||||
| 9-item MMAS | Adherent | 18 (72%) | 7 (28%) | 25 | 0.125 | 3.086 (0.70–13.47) |
| Nonadherent | 5 (45.5%) | 6 (54.5%) | 11 | |||
Table 7.
Patients behavior about IM intake and knowledge-based questions.
| VARIABLE | VALUE (NUMBER) | PERCENTAGE % |
|---|---|---|
| IM intake | ||
| After meal | 32 | 89 |
| Empty stomach | 4 | 11 |
| Grapefruit intake | ||
| Yes | 4 | 11 |
| No | 32 | 89 |
| Increasing dose of Imatinib | ||
| Yes | 1 | 3 |
| No | 35 | 97 |
| Action if missing dose | ||
| Nothing | 12 | 33 |
| Double the dose | 1 | 3 |
| Wait for next day | 12 | 33 |
| Take it same time when remembering | 11 | 31 |
A total of 18 patients (50%) developed one or more side effects such as fatigue, nausea, vomiting, headache, muscle pain, abdominal pain, skin rash/itching, orbital edema, leg edema, memory change, sunburn, weight gain, or infection (muscle pain and skin rashes accounted for 35% and 29% of the side effects, respectively).
Interestingly, 23 patients (74%) experienced lack of funds. However, these patients were offered the medicine completely free with the support of the hospital social services department, and five patients (13%) declared the unavailability of the drug in the NCCCR pharmacy. A total of 26 (72%) patients believed that IM would cure the disease (Table 8).
Table 8.
Side effects due to intake of IM.
| VARIABLE | VALUE (NUMBER) | PERCENTAGE % |
|---|---|---|
| Side effects | ||
| Yes | 18 | 50 |
| No | 18 | 50 |
| Side effects reported | ||
| Fatigue | 2 | 12 |
| Nausea | 2 | 12 |
| Vomiting | 3 | 18 |
| Headache | 2 | 12 |
| Muscle pain | 6 | 35 |
| Abdominal pain | 1 | 6 |
| Skin rash/itching | 5 | 29 |
| Orbital edema | 2 | 12 |
| Leg edema | 3 | 18 |
| Memory change | 1 | 6 |
| Sun burn | 1 | 6 |
| Weight gain | 1 | 6 |
| Infections | 2 | 12 |
| Thrombocytopenia | 1 | 6 |
| Lack of fund* | ||
| Yes | 23 | 74 |
| No | 8 | 26 |
| Non-availability of Imatinib | ||
| Yes | 5 | 13.9 |
| No | 31 | 86.1 |
| Education about Imatinib | ||
| Yes | 34 | 94.4 |
| No | 2 | 5.6 |
| By: | ||
| Physicians | 18 | |
| Physicians and Nurses | 2 | |
| Physicians and Online resources | 1 | |
| Physicians/Pharmacist/Nurse | 1 | |
| Physicians and Pharmacists | 10 | |
| Pharmacist | 2 | |
| None | 2 | |
| Believe in cure | ||
| Yes | 26 | 72 |
| No | 10 | 28 |
Note:
Excluding five Qatari patients as the IM is free of charge for citizens while non-citizen residents pay the remaining 10% of IM, which is uncovered by the hospital.
Table 9 shows how IM works, why and how to take IM, and information about the side effects of IM.
Table 9.
Quality of educational information provided to the patients.
| STATEMENT | NONE | SOME | GOOD | EXCELLENT |
|---|---|---|---|---|
| How Imatinib works | 7 (19.4%) | 2 (5.6%) | 18 (50.0%) | 9 (25.0%) |
| Why to take the medicine | 5 (13.9%) | 2 (5.6%) | 18 (50.0%) | 11 (30.6%) |
| How to take it | 6 (16.7%) | 1 (2.8%) | 14 (38.9%) | 15 (41.7%) |
| Side effects | 7 (19.4%) | 3 (8.3%) | 13 (36.1%) | 13 (36.1%) |
It is important to note that in-depth interviews with treating physicians revealed that two patients were not adherent to treatment, although it was reported in their survey questionnaire that they were. There was no significant difference between male and female patients (P = 0.17), level of education (illiterate, up to secondary school versus graduation and above; P = 0.30), married versus singles (P = 0.25), patients with or without side effects (P = 0.39), lack of funds (P = 1), or patients’ education, using the 9-item MMAS (P = 1; Table 10).
Table 10.
Comparison of categorical variables with adherence using the 9-item MMAS.
| VARIABLES | ADHERENCE CATEGORY | TOTAL | P VALUE | ||
|---|---|---|---|---|---|
| NONADHERENT N (%) | ADHERENT N (%) | ||||
| Sex | Female | 4 | 4 | 8 | 0.17 |
| Male | 7 | 21 | 28 | ||
| Level of education | Illiterate up to Secondary School | 7 | 12 | 19 | 0.3 |
| Graduation and above | 4 | 13 | 17 | ||
| Marital status | Married | 8 | 22 | 30 | 0.25 |
| Singles | 3 | 3 | 6 | ||
| Side effects | No | 4 | 12 | 16 | 0.39 |
| Yes | 7 | 13 | 20 | ||
| Lack of fund | No | 4 | 9 | 13 | 1 |
| Yes | 7 | 16 | 23 | ||
| Patients’ education | None and Some | 1 | 2 | 3 | 1 |
| Good and Excellent | 8 | 20 | 28 | ||
Method 3: MPR results
Of the 36 patients, 4 managed to obtain the IM at home. The mean of the overall MPR results for the 32 patients was 94% (range: 56.6–100).
Of the 32 patients, 27 (84%) were classified as adherent (MPR mean: 96%; range: 84.9%–100%) and 5 (16%) were classified as nonadherent (MPR mean: 57.8%; range: 56.6%–59.0%; Table 11).
Table 11.
Patients’ response according to adherence status using the MPR technique.
| TECHNIQUES | ADHERENCE | RESPONSE | TOTAL | P VALUE | ODDS RATIO (95% CONFIDENCE INTERVAL) | |
|---|---|---|---|---|---|---|
| OPTIMAL N (%) | FAILURE N (%) | |||||
| MPR* | Adherent | 20 (74%) | 7 (25%) | 27 | 0.0370 | 11.43 (1.1–120.4) |
| Nonadherent | 1 (20%) | 4 (80%) | 5 | |||
Note:
The total number of patients were assessed via MPR is 32 as 4 patients were excluded in the MPR analysis because their medications were obtained in their own countries.
The adherent patient group was significantly more likely to have optimal response (74%; 20/27) than the nonadherent patient group (20%; 1/5; P = 0.037; OR: 11.43, 95% CI: 1.1–120.4; Table 11).
Method 4: eMR results
No drug–drug interactions were identified using MICROMEDEX® 1.0 (Healthcare Series).
The relationship between the three techniques: Pearson correlation was performed for the three techniques (MMAS, MPR, and MEMS). There was a significant high positive correlation between MPR and MEMS (r = 0.74; P = 1.053373e−006), MMAS and MEMS (r = 0.66; P = 3.547150e−005), and MPR and MMAS (r = 0.5; P = 0.0036).
Patients’ response
Of the 36 patients, 23 (63%) responded optimally and 13 (37%) failed the treatment.
Those who responded optimally had complete hematological response, CCyR, and deep MR.
Of the 23 patients who responded optimally, 22 were from CP and 1 was from AP according to their initial diagnosis, and thus, patients’ initial disease phase (CP, AP, and BCP) could be attributed to the treatment outcomes.
It is important to note that achieving early-stage treatment targets is more likely to have better long-term outcomes. This study found that of the 23 patients who responded optimally, 22 achieved early-stage treatment and these patients continued to have optimal response.
On the other hand, in total, 13 patients failed treatment, 12 were resistant, and 1 (P25) showed intolerance. Of the 12 resistant cases, 1 patient (P14) showed both E459K mutation and ACAs, 3 patients (P6, P20, and P24) had ACAs, and all IM failed patients switched to the second line of treatment, either dasatinib or nioltinib.
Long-term disease progression or survival rates upon IM treatment showed that of the 36 patients, only 1 (P25) (2.8%) was deceased, and this patient was known to have primary resistance to all TKIs. None of the 36 patients progressed, and 35 (97.2%) survived (Supplementary Table 1).
Discussion and Conclusion
This is the first prospective study to evaluate CML patients’ adherence and response to IM in Qatar. The rate of treatment failure of CML patients treated with IM in Qatar has been previously reported to be high (54%).20,21 Due to this high rate of IM failure in Qatar, patient’s adherence to treatment was studied by observing a new cohort of 36 CML patients. One-third of the patients (N: 14, 39%) were classified as nonadherent and 13 patients (37%) failed treatment. Thus, poor adherence explains the high rate of treatment failure observed. Nonadherence to the treatment was one of the most common causes of IM failure in the patient cohort, documented in 39% of cases using the gold standard method (MEMS), which seems to be consistent with international data, indicating nonadherence rates between 25% and 50%.43 Economic factors (eg, unaffordable drug prices) were one of the main causes of nonadherence. Efforts should be made locally to improve access to medication for cancer diseases or through participation in patient assistance programmes such as Glivec International Patient Assistance Programme (GIPAP), which provides Glivec free of cost to eligible patients in developing countries who meet specific medical and socioeconomic guidelines. Briefly, GIPAP helps patients who are not insured, not reimbursed, cannot pay for treatment privately, and are in developing countries that have minimal reimbursement capabilities,44 or using generic IM that has been available in the market since February 2016 which could reduce the cost burden of treatment. Other risk factors associated with poor adherence can be improved by close monitoring and dose adjustment. Monitoring risk factors for poor adherence in combination with patient education that includes direct communication between the health-care teams (ie, doctors, nurses, and pharmacists) and patients are essential components for maximizing the benefits of TKIs and could rectify this problem. The preliminary results show that patients’ responses to treatment may be directly linked to patients’ adherence to the treatment. However, further in-depth and specific analysis may be necessary in a larger cohort.
In several studies, such as a Belgian study that included 169 patients, adherence to IM treatment was assessed via questionnaires, interviews, and pill counts. A total of 30% of the patients were shown to be nonadherent, and only 14% of the patients took the prescribed IM dose.13 In a study from the United Kingdom, 26% of the patients were shown to be nonadherent.14 In a follow-up study, the most common reason for intentional nonadherence was side effects, and the most common reason for unintentional nonadherence was forget fulness.45 In a Taiwanese study, Chen et al.19 showed that 26.9% of patients showed poor adherence to IM. In these three studies, in Belgium, Britain, and Taiwan, a relationship between nonadherence and treatment response was observed, which is consistent across the studies.14,19,45 Chen et al.46 also showed in another study that Taiwanese CML patients were adherent to IM and reported that adverse drug effects and associated polypharmacy were the main and key reasons and concerns influencing their adherence to long-term use of IM.
Interestingly, in a Swedish study, Jonsson et al.16 showed that 97% (37) of CML patients were classified as adherent and all patients optimally responded to treatment.
Nonadherence and treatment interruptions both lead to undesired clinical outcomes and appear to be more prevalent than previously believed or expected.
There are some limitations to the study. MMAS is a subjective method and is the least reliable of the methods. However, a questionnaire survey is able to identify individual patient’s concerns and subsequently tailor appropriate intervention.
Certainly, the disadvantages of such approaches should not be underestimated. Relatively poor sensitivity and specificity can occur due to false data input by patients, accidently or purposefully, or imperfect communication skills and questions constructed by interviewers or in the design of the survey.
Negativity in questions, suggesting blaming patients for not fulfilling their prescribed regime, may lead to bias. Patients’ psychological state can impact their answers to the questionnaire.
Physicians, nurses, and pharmacists need to educate patients and closely monitor their adherence to treatment. Improving adherence and limiting treatment interruptions would optimize clinical outcomes and reduce the burden of disease, and therefore should be assessed routinely as they are correlated with poor response to treatment.
Supplementary Material
Supplementary Table 1. Showing the 36 patients responses’ to IM treatment.
Footnotes
ACADEMIC EDITOR: William Chi-shing Cho, Editor in Chief
PEER REVIEW: Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1840 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by HMC Medical director’s grant Competition – Research Project (RP) Grant Competition (GC) no. 1013A. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author contributions
Designed the study and wrote the manuscript: NIA-D. Recruited patients into the study as well as provided clinical data: MAY. Dispensed IM in the MEMS bottles: RSG. Interviewed patients and collected survey questionnaires’ results: CCG, SKB, AJN. Carried out the molecular studies and assisted in data collection: MAI, AAA, MMA, RMA-J. Assisted in writing the initial draft of the manuscript and in statistical analysis: HMM, TIB-O, MMS. Assisted in designing the survey questionnaires and MPR calculation: RBK. In addition, all the authors provided critical revisions to the various manuscript drafts. All the authors read and approved the final manuscript.
REFERENCES
- 1.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–51. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chuah CT, Nakamae H, Shen ZX, Bradley-Garelik MB, Kim DW. Efficacy and safety of dasatinib versus imatinib in the East Asian subpopulation of the DASISION trial of newly diagnosed chronic myeloid leukemia in chronic phase. Leuk Lymphoma. 2014;55(9):2093–100. doi: 10.3109/10428194.2013.866663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujisawa S, Nakamae H, Ogura M, et al. Efficacy and safety of dasatinib versus imatinib in Japanese patients with newly diagnosed chronic-phase chronic myeloid leukemia (CML-CP): subset analysis of the DASISION trial with 2-year follow-up. Int J Hematol. 2014;99(2):141–53. doi: 10.1007/s12185-013-1470-1. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–70. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 5.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–9. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12(9):841–51. doi: 10.1016/S1470-2045(11)70201-7. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal M, Garg RJ, Cortes J, Quintas-Cardama A. Tyrosine kinase inhibitors: the first decade. Curr Hematol Malig Rep. 2010;5(2):70–80. doi: 10.1007/s11899-010-0045-y. [DOI] [PubMed] [Google Scholar]
- 8.Mauro MJ, Druker BJ, Maziarz RT. Divergent clinical outcome in two CML patients who discontinued imatinib therapy after achieving a molecular remission. Leuk Res. 2004;28(Suppl 1):S71–3. doi: 10.1016/j.leukres.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Rousselot P, Huguet F, Rea D, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109(1):58–60. doi: 10.1182/blood-2006-03-011239. [DOI] [PubMed] [Google Scholar]
- 10.Merante S, Orlandi E, Bernasconi P, Calatroni S, Boni M, Lazzarino M. Outcome of four patients with chronic myeloid leukemia after imatinib mesylate discontinuation. Haematologica. 2005;90(7):979–81. [PubMed] [Google Scholar]
- 11.Pagani IS, Spinelli O, Mattarucchi E, et al. Genomic quantitative real-time PCR proves residual disease positivity in more than 30% samples with negative mRNA-based qRT-PCR in Chronic Myeloid Leukemia. Oncoscience. 2014;1(7):510–21. doi: 10.18632/oncoscience.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Geest S, Sabate E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4):323. doi: 10.1016/S1474-5151(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 13.Noens L, van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113(22):5401–11. doi: 10.1182/blood-2008-12-196543. [DOI] [PubMed] [Google Scholar]
- 14.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381–8. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibrahim AR, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood. 2011;117(14):3733–6. doi: 10.1182/blood-2010-10-309807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonsson S, Olsson B, Soderberg J, Wadenvik H. Good adherence to imatinib therapy among patients with chronic myeloid leukemia – a single-center observational study. Ann Hematol. 2012;91(5):679–85. doi: 10.1007/s00277-011-1359-0. [DOI] [PubMed] [Google Scholar]
- 17.Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–35. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 18.Yhim HY, Lee NR, Song EK, et al. Imatinib mesylate discontinuation in patients with chronic myeloid leukemia who have received front-line imatinib mesylate therapy and achieved complete molecular response. Leuk Res. 2012;36(6):689–93. doi: 10.1016/j.leukres.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Chen TC, Chen LC, Huang YB, Chang CS. Imatinib adherence associated clinical outcomes of chronic myeloid leukaemia treatment in Taiwan. Int J Clin Pharm. 2014;36(1):172–81. doi: 10.1007/s11096-013-9876-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Dewik NI, Jewell AP, Yassin MA, El-Ayoubi HR, Morsi HM. Molecular monitoring of patients with chronic myeloid leukemia (CML) in the state of Qatar: optimization of techniques and response to imatinib. QScience Connect. 2014;2014(1):24. [Google Scholar]
- 21.Al-Dewik NI, Jewell AP, Yassin MA, El-Ayoubi HR, Morsi HM. Studying the impact of presence of point mutation, insertion mutation and additional chromosomal abnormalities in chronic myeloid leukemia patients treated with imatinib mesylate in the State of Qatar. QScience Connect. 2014;2014(1):13. [Google Scholar]
- 22.van den Boogaard J, Lyimo RA, Boeree MJ, Kibiki GS, Aarnoutse RE. Electronic monitoring of treatment adherence and validation of alternative adherence measures in tuberculosis patients: a pilot study. Bull World Health Organ. 2011;89(9):632–9. doi: 10.2471/BLT.11.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson CR, Simoni JM, Hoff P, Kurth AE, Martin DP. Assessing antiretroviral adherence via electronic drug monitoring and self-report: an examination of key methodological issues. AIDS Behav. 2007;11(2):161–73. doi: 10.1007/s10461-006-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Kjellgren KI, Ahlner J, Saljo R. Taking antihypertensive medication – controlling or co-operating with patients? Int J Cardiol. 1995;47(3):257–68. doi: 10.1016/0167-5273(94)02203-u. [DOI] [PubMed] [Google Scholar]
- 26.Kjellgren KI, Ahlner J, Dahlof B, Gill H, Hedner T, Saljo R. Patients’ and physicians’ assessment of risks associated with hypertension and benefits from treatment. J Cardiovasc Risk. 1998;5(3):161–6. [PubMed] [Google Scholar]
- 27.Sodergard B, Halvarsson M, Lindback S, Sonnerborg A, Tully MP, Lindblad AK. Differences in adherence and motivation to HIV therapy–two independent assessments in 1998 and 2002. Pharm World Sci. 2006;28(4):248–56. doi: 10.1007/s11096-006-9036-4. [DOI] [PubMed] [Google Scholar]
- 28.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10(5):348–54. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–74. doi: 10.1002/pds.1230. discussion 575–67. [DOI] [PubMed] [Google Scholar]
- 30.Nair KV, Belletti DA, Doyle JJ, et al. Understanding barriers to medication adherence in the hypertensive population by evaluating responses to a telephone survey. Patient Prefer Adherence. 2011;5:195–206. doi: 10.2147/PPA.S18481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Velden VH, Hochhaus A, Cazzaniga G, Szczepanski T, Gabert J, van Dongen JJ. Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia. 2003;17(6):1013–34. doi: 10.1038/sj.leu.2402922. [DOI] [PubMed] [Google Scholar]
- 32.Branford S, Cross NC, Hochhaus A, et al. Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia. 2006;20(11):1925–30. doi: 10.1038/sj.leu.2404388. [DOI] [PubMed] [Google Scholar]
- 33.Nakamae H, Yoshida C, Miyata Y, et al. A new diagnostic kit, ODK-1201, for the quantitation of low major BCR-ABL mRNA level in chronic myeloid leukemia: correlation of quantitation with major BCR-ABL mRNA kits. Int J Hematol. 2015;102(3):304–11. doi: 10.1007/s12185-015-1826-9. [DOI] [PubMed] [Google Scholar]
- 34.Ahn S, Lim YA, Lee WG, Jeong SH, Park JS, Cho SR. Comparison of an international scale method and a log reduction method for monitoring of early molecular response in chronic myeloid leukemia patients. Blood Res. 2016;51(1):58–61. doi: 10.5045/br.2016.51.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cayuela JM, Macintyre E, Darlington M, et al. Cartridge-based automated BCR-ABL1 mRNA quantification: solving the issues of standardization, at what cost? Haematologica. 2011;96(5):664–71. doi: 10.3324/haematol.2010.034389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabert J, Beillard E, van der Velden VH, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia – a Europe Against Cancer program. Leukemia. 2003;17(12):2318–57. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- 37.Branford S, Fletcher L, Cross NC, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008;112(8):3330–8. doi: 10.1182/blood-2008-04-150680. [DOI] [PubMed] [Google Scholar]
- 38.Kantarjian HM, Talpaz M, Cortes J, et al. Quantitative polymerase chain reaction monitoring of BCR-ABL during therapy with imatinib mesylate (STI571; gleevec) in chronic-phase chronic myelogenous leukemia. Clin Cancer Res. 2003;9(1):160–6. [PubMed] [Google Scholar]
- 39.Dufresne SD, Belloni DR, Levy NB, Tsongalis GJ. Quantitative assessment of the BCR-ABL transcript using the Cepheid Xpert BCR-ABL Monitor assay. Arch Pathol Lab Med. 2007;131(6):947–50. doi: 10.5858/2007-131-947-QAOTBT. [DOI] [PubMed] [Google Scholar]
- 40.Martinelli G, Montefusco V, Testoni N, et al. Clinical value of quantitative long-term assessment of bcr-abl chimeric transcript in chronic myelogenous leukemia patients after allogeneic bone marrow transplantation. Haematologica. 2000;85(6):653–8. [PubMed] [Google Scholar]
- 41.Winn-Deen ES, Helton B, Van Atta R, et al. Development of an integrated assay for detection of BCR-ABL RNA. Clin Chem. 2007;53(9):1593–600. doi: 10.1373/clinchem.2007.085472. [DOI] [PubMed] [Google Scholar]
- 42.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–84. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–9. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 44.Kanavos P, Vandoros S, Garcia-Gonzalez P. Benefits of global partnerships to facilitate access to medicines in developing countries: a multi-country analysis of patients and patient outcomes in GIPAP. Global Health. 2009;5:19. doi: 10.1186/1744-8603-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eliasson L, Clifford S, Barber N, Marin D. Exploring chronic myeloid leukemia patients’ reasons for not adhering to the oral anticancer drug imatinib as prescribed. Leuk Res. 2011;35(5):626–30. doi: 10.1016/j.leukres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 46.Chen LC, Chen TC, Huang YB, Chang CS. Disease acceptance and adherence to imatinib in Taiwanese chronic myeloid leukaemia outpatients. Int J Clin Pharm. 2014;36(1):120–7. doi: 10.1007/s11096-013-9867-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Showing the 36 patients responses’ to IM treatment.