Abstract
The prognostic significance of early diagnosis and therapeutic intervention in inflammatory rheumatic diseases has been well documented. However, a shortage of rheumatologists often impedes this approach in clinical practice. Therefore, it is of importance to identify those patients referred for diagnosis who would benefit most from a specialist’s care. We applied a telephone-based triage for appointment allocation during routine care. This retrospective, monocentric analysis evaluated the efficacy of our triage to identify patients with rheumatic disease with special regard to initial appointment category (elective, early arthritis clinic (EAC), or emergency appointment). Of the 1,782 patients assessed, 718 (40.3%) presented with an inflammatory rheumatic disease, and there were significant discrepancies between the appointment categories: elective 26.2%, EAC 49.2% (P < 0.001) and emergency appointment 56.6% (P < 0.001). We found that 61.2% of patients were allocated to the correct diagnostic category (inflammatory or noninflammatory) solely based on the telephone-based triage and 67.1% based on the combination of triage and C-reactive protein (CRP) count.
Keywords: triage, early arthritis clinic, referral, rheumatic disease
Introduction
An extensive body of evidence indicates that early diagnosis and therapeutic intervention with consistent maintenance of remission, known as treat to target or tight control, improves the prognosis of organ and/or joint damage and of concomitant or secondary diseases, such as cardiovascular complications, in cases of inflammatory rheumatic disease.1–10 The term window of opportunity was introduced in this context, and it was initially coined for rheumatoid arthritis (RA), but is similarly valid for other potentially destructive joint diseases, connective tissue diseases, and vasculitis.11,12
Several guidelines, including those of the German Rheumatology Association (DGRh), recommend that a rheumatologist should see every patient with at least two swollen joints and no other explanatory diagnosis no later than 6 weeks following the onset of symptoms, and treatment with disease-modifying anti-rheumatic drugs (DMARDs) should begin no later than 12 weeks after the onset of symptoms.6,9,13–16 However, data from the German national database centers show that patients with RA consult a rheumatologist only an average of 1.1 years after the onset of symptoms, which means that DMARD treatment commences only after a considerable delay in most cases.17
Various factors at patient level and at general practitioner and rheumatologist level delay the early initiation of specific immunomodulatory treatment.18–20 One particularly significant obstacle is that some patients may wait several months for an appointment with a rheumatologist in Germany, a symptom of inadequate capacity for rheumatologic diagnosis and treatment. The waiting time for first consultation with a rheumatologist in Germany was 5.74 ± 6.60 weeks (median 3.57; interquartile range (IQR) 1.72–7.81) in 2008.21 The shortage of rheumatologic treatment capacity affects the diagnostic work-up of suspected cases of inflammatory rheumatic systemic diseases and the ability to maintain constant remission (the treat-to-target or tight control approach) during the patient’s further care.22–25 This deficit is significantly intensified because patients with primary pain conditions or primary degenerative diseases of the musculoskeletal system often crowd the schedules of rheumatologists, who are then no longer available to diagnose and treat patients with inflammatory rheumatic systemic diseases.
Recent attempts to facilitate priority access to rheumatologic diagnostics and treatment were made for patients with strongly suspected inflammatory rheumatic disease by setting up early arthritis clinics (EACs). Various methods and approaches were used with varying degrees of success.26,27 The goal of this study was to develop a simple triage system that would enhance appropriate access of patients with rheumatic diseases to specialized medical care.
Materials and Methods
Our rheumatologic facility offers outpatient (>6,500 outpatient cases per annum) and inpatient care (academic teaching hospital) for adults, and it has a catchment area of approximately 100–150 km, which is mostly rural and corresponds to approximately 400,000 inhabitants. The digital patient files of 1,782 initial outpatient consultations between January 2015 and March 2016 were retrospectively analyzed. All patients were referred following consultation with a physician.
Appointments were scheduled after a simple telephone-based triage (Fig. 1) that was implemented as part of the clinical routine. There were three appointment categories to which the patients were allocated: elective initial appointment, EAC, and emergency appointment (Fig. 1A). Medical staff with several years of professional experience answered all telephone calls of patients referred for diagnosis. All patients had been previously seen by a doctor, usually a family doctor or an orthopedic specialist. On requesting an appointment, each patient was first asked during the appointment request whether he/she had already been diagnosed with an inflammatory rheumatic disease (list). If the answer was no, then he/she was asked about symptom duration (≤ or >6 months) and whether any abnormal laboratory findings specific to rheumatic disease were noted. If an appointment was requested by the referring practice itself, then an appointment in one of the three available categories was assigned solely based on the referring doctor’s assessment of the case’s urgency (Fig. 1B). Emergency appointments were given solely in response to an inquiry by a physician. The waiting time for an elective appointment was 12–16 weeks, 4–6 weeks for EAC, and no longer than 2 weeks for an emergency appointment. All patients were seen by a rheumatologist.
Figure 1.
Triage algorithm for calls by a patient (A) or doctor (B).
Diagnoses were categorized as follows: noninflammatory (eg, osteoarthritis, fibromyalgia), RA, axial spondyloarthritis, peripheral spondyloarthritis, arthritis of other form (eg, arthritis urica, Lofgren’s syndrome), inflammatory of other form (eg, polymyalgia rheumatica, autoinflammatory syndromes), connective tissue diseases, and vasculitis. Erythrocyte sedimentation rate (ESR) was ascertained using the Westergren method (normal value 6–11 mm during the first hour). Highly sensitive C-reactive protein (CRP) levels were measured using particle-enhanced immunonephelometry (F. Hoffmann-La Roche Diagnostics; normal value <0.5 mg/dL).
Data were assessed during routine care according to available recommendations and guidelines. No further inclusion or exclusion criteria were used. Data management and statistical analyses were performed for all data as appropriate using Microsoft Excel and SPSS, respectively.28,29 All performed inferential tests were two-tailed and considered statistically significant at P < 0.05. Pearson chi-square tests were used to compare the frequencies of categorical variables between patient subgroups. One-way analysis of variance (ANOVA) was performed to test for mean differences in continuous variables between independent patient subgroups. Post hoc analyses were performed where appropriate (Scheffé’s test for ANOVA models, standardized/z-transformed residuals for chi-square tests). Statistical analyses of prediction questions were performed using binary logistic regression procedures (“enter” option; dependent variable: observed dichotomized diagnosis; independent variables: eg, laboratory data, results of the telephone interview). The results of the binary logistic regression analyses are presented in classification tables along with additional summary statistics (eg, positive predictive value (ppv), negative predictive value (npv), percentage of correctly classified cases). This research was conducted in accordance with the principles of the Declaration of Helsinki.
Results
A total of 1,782 datasets were evaluated (Table 1). The percentage of inflammatory diagnoses in the entire collective was 40.3%. Inflammatory rheumatic disease was excluded in 59.7% of cases (Fig. 2).
Table 1.
Sociodemographic factors in total study sample.
| DEMOGRAPHIC FACTORS | PATIENTS WITH INFLAMMATORY DISEASE (n = 718) | PATIENTS WITH NONINFLAMMATORY DISEASE (n = 1,064) | TOTAL STUDY SAMPLE (n = 1,782) |
|---|---|---|---|
| Age (years) | 57.8 ± 16.8 | 52.3 ± 15.1 | 54.5 ±16.0 |
| Range | (7–92) | (6–92) | (6–92) |
| Gender | |||
| Women | 413 (57.5%) | 766 (72.0%) | 1,179 (66.2%) |
| Men | 305 (42.5%) | 298 (28.0%) | 603 (33.8%) |
| Triage | |||
| Elective appointment | 197 (27.4%) | 556 (52.3%) | 753 (42.3%) |
| Early arthritis clinic | 409 (57.0%) | 422 (39.7%) | 831 (46.6%) |
| Emergency appointment | 112 (15.6%) | 86 (8.1%) | 198 (11.1%) |
Figure 2.
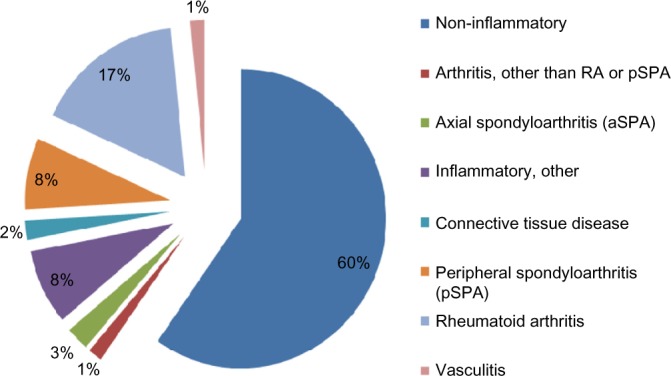
Diagnostic categories in the collective.
The percentage of inflammatory rheumatic diseases was 26.2% in the elective group, 49.2% in the EAC group, and 56.6% in the emergency group (Fig. 3). Significantly more patients with inflammatory rheumatic diagnoses (P < 0.001) were seen at the EAC and emergency appointments than in the elective group. The frequency of inflammatory diagnoses in the emergency group (56.6%) was significantly (P < 0.001) greater than at the EAC. RA in particular was diagnosed in 12.5% of the assessed patients in the elective group, 20.3% in the EAC group, and 17.2% in the emergency group without reaching statistical significance between appointment categories.
Figure 3.
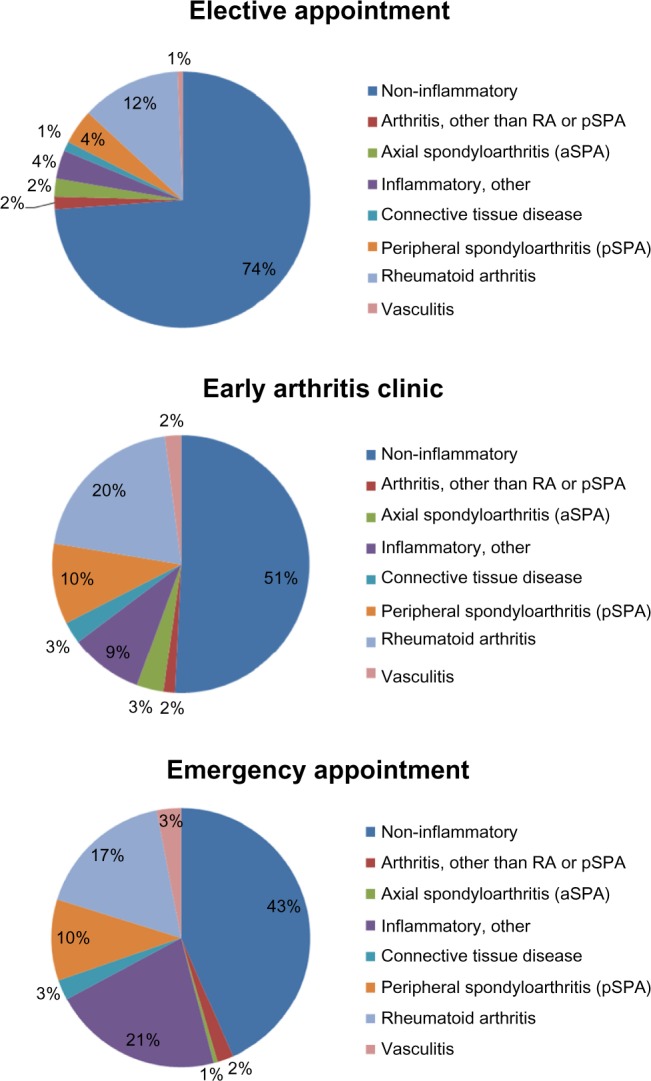
Percentage of inflammatory vs. noninflammatory diagnoses in each appointment category (*P < 0.001).
The percentage of cases in the diagnostic category “Inflammatory, other” in the emergency group (21.2%) was significantly higher than in the other two appointment categories, ie, EAC and elective. Of the emergency patients in this diagnostic category, 140 were diagnosed with polymyalgia rheumatica. RA was the most common inflammatory rheumatic diagnosis in the other two appointment groups. The higher percentage of inflammatory diagnoses in the EAC and emergency groups was reflected in the higher percentage of patients receiving specific treatment. Significantly more patients in the emergency group were treated with glucocorticoids than in the elective appointment group (P < 0.001). The percentage of patients receiving DMARDs was significantly higher in the emergency and EAC groups than in the elective appointment group (P < 0.001; Fig. 4).
Figure 4.
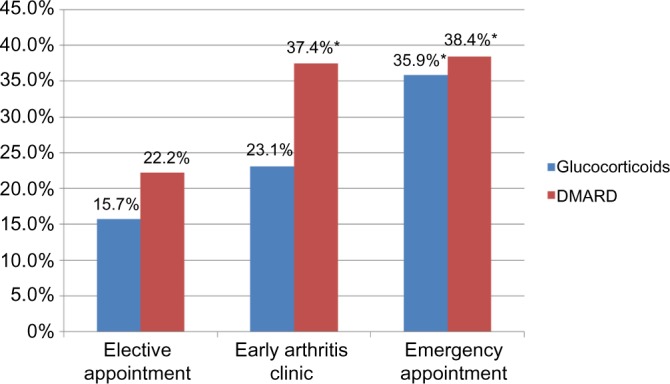
Percentage of patients receiving specific treatment in each appointment category (*P < 0.001).
Of the patients with an inflammatory diagnosis, 56.5% exhibited a normal CRP count. However, the percentage of pathological CRP values was significantly higher in the emergency group than in the EAC and elective appointment groups (P < 0.001). ESR was more often accelerated in the emergency group than in EAC and elective appointment groups, with this difference also being significant (P < 0.01). There was no significant difference in the percentages of patients with elevated CRP or accelerated ESR between the EAC and elective appointment groups (Fig. 5).
Figure 5.
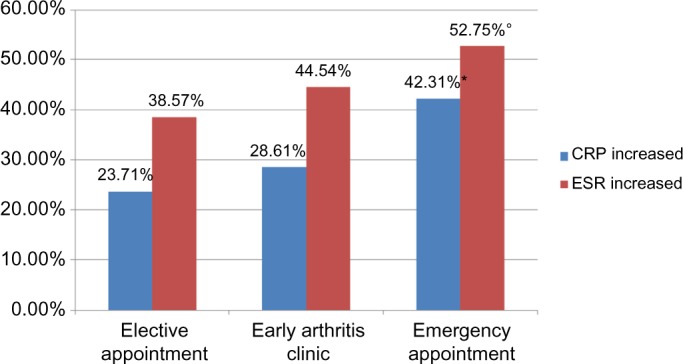
Percentage of patients with elevated CRP (*P < 0.001) or accelerated ESR (°P < 0.01) in each appointment category.
Binary logistic regression analysis revealed that 61.2% of all patients interviewed by triage were allocated to the correct (dichotomized) diagnostic category, ie, inflammatory or noninflammatory. The probability of a noninflammatory diagnosis being correctly predicted on the basis of telephone triage was 61.74% npv. The ppv was 56.56%. A total of 66.8% of patients were allocated to the correct category (inflammatory or noninflammatory) on the basis of CRP values alone, and 62.4% of patients were correctly allocated based on ESR values. A simultaneous inclusion of telephone-based triage and CRP values in the mathematical regression model resulted in 67.1% of patients being correctly allocated to the inflammatory or noninflammatory group (npv 68.0%, ppv 64.2%).
Discussion
Rheumatologic care in Germany is marked by a demand that far exceeds the diagnostic and therapeutic capacities available in this field. This increased demand of patients and referring physicians is the result of the considerable attention devoted to inflammatory rheumatic diseases by the media, the publicity work performed by patient associations, and the increased efforts by scientific organizations to educate referring physicians. However, data published by DGRh shows that the number of new consultants in the field of internal medicine specializing in rheumatology in Germany between 1991 and 2014 remained largely stagnant at between 35 and 71 per annum, which just compensates for physicians leaving the profession on demographic grounds. This means that the number of rheumatologists (including those specializing in orthopedic rheumatology) has essentially remained the same, with 1.56 rheumatologists per 100,000 inhabitants serving Germany’s population in 2012 compared to 1.45 rheumatologists per 100,000 inhabitants in 2002.30 This situation clearly undermines treatment concepts, such as the early diagnosis and initiation of treatment within three months (window of opportunity) and the consistent maintenance of remission (tight control, treat to target), despite the large body of scientific evidence supporting their effectiveness. These shortcomings support the need for strategies for the selection of patients referred for diagnosis to identify those patients who would benefit most from early diagnosis and therapeutic intervention.
The average time that elapses between the onset of symptoms and diagnosis in RA is currently 1.1 years, but this period has been shortened in recent years.17,26,31 In 2008, 198 internal-rheumatologic treatment facilities were questioned as part of a study conducted by the German Rheumatism Research Center, Berlin (DRFZ), and the Society of German Rheumatologists (BDRh); 19,908 patients (average age 55 ± 16 years, 67% women) were documented.21 Family doctors referred the majority (79%) of these patients, and only 23% managed to see a rheumatologist within three months of their symptoms onset. A Belgian study has found that diagnosis was delayed an average of 10 weeks at the patient level, 4 weeks at the family doctor level, and 7 weeks at the rheumatologist level.32 Only a minority of patients (21.6%) in that study were diagnosed within 12 weeks of the onset of symptoms. Various, mostly nonselective, approaches were used to shorten the time between symptom onset and the first visit to the family doctor. Concepts based purely on screening programs for the general population (eg, simple hand function tests, a Rheumatism Bus, and similar methods) or information on the Internet yielded a reliable diagnosis of RA only in 0.2%–1.5% of cases.33,34 Notably, a study conducted by Deane et al yielded a diagnosis of RA in only 1.5% of the 601 patients surveyed, and the percentage of subjects who tested positive for rheumatoid factor (RF) or anti-citrullinated peptide antibodies was 6.1%. A questionnaire-based screening of patients with a reliable diagnosis of psoriasis vulgaris, ie, a collective with a comparatively high pretest probability of psoriatic arthritis, led to an actual diagnosis of psoriatic arthritis in 10.1% of cases.35 This study demonstrated that preselection at this level significantly increased the likelihood of the diagnosis of an inflammatory rheumatic disease. The next level refers to the period that elapsed between the patient contacting his family doctor and consultation with a rheumatologist. Many studies report that this period is particularly critical. Approximately 22% of patients consult their family doctors because of pain, and 50% of these patients have musculoskeletal complaints.36 Therefore, it is initially understandable that not every patient is or must be referred to a rheumatologist immediately, and these patients generally see their family doctors up to four times before being referred.37 Two studies explored the usefulness of medical information on the family doctor’s referral slip in helping the rheumatologic institution stratify appointments. These studies used three categories of urgency, similar to our study. The ppv of inflammatory joint disease in patients referred by their family doctor as especially urgent cases was between 56.5% and 75%, which is comparable with our results.38,39 The percentage of inflammatory diagnoses in our collective data also conforms with current results from the EACs in Leiden and Groningen, which were calculated at 42% and 49%, respectively.40 Although the referral system and the number of available rheumatologists certainly differ between countries and rheumatology units, the results seem to be comparable.
The rate of inflammatory diagnoses at our emergency clinic (56.6%) was significantly higher (P < 0.001) than at the EAC, which emphasizes the importance of the referrer in the preselection process. Hazlewood et al.27 also recently showed the importance of the referrer in the preselection process in combination with a Central Referral And Triage in Rheumatology (CReATe Rheum) program. However, the low absolute increase of merely 7.4 points compared to the EAC in our cohort is surprising because appointments were scheduled solely in response to requests by physicians and these cases were presumably urgent. The percentage of inflammatory rheumatic diseases in our cohort was only 26.2% in the elective group. Thus, the greatest strength of the triage algorithm may be in its npv regarding inflammatory diagnosis and thus in its capacity to exclude inflammatory rheumatic diseases. There is no documentation to indicate whether the acuteness of the symptoms or other parameters, eg, laboratory test results, were the reasons for the patient referrals for emergency appointments. The higher percentage of inflammatory rheumatic diagnoses in our analysis was also reflected by the significantly higher percentage of patients in the EAC and emergency cohorts who were receiving immunomodulatory treatment compared to the percentage in the elective referral cohorts. This result supports the value of correct patient allocation to each appointment category for therapeutic consistency. The high proportion of cases with polymyalgia rheumatica may explain why the percentage of patients treated with glucocorticoids was significantly higher in the emergency category than in the EAC category. Similarly, ESR was more often accelerated in the emergency group than in the EAC and elective appointment groups. These differences may be due to the significantly higher percentage of patients with polymyalgia rheumatica in the emergency group.
Graydon and Thompson38 reported that 17% of the patients examined during routine care by a rheumatologist should have received earlier referrals. One major reason for the erroneous initial assessment of the urgency of these cases was incomplete information on existing diagnoses, symptom duration, and joint involvement in the referral document in more than 30% of cases. This finding confirms our own experience with the information provided in referral documents, which is frequently incomplete and varies considerably in quality. A total of 26.2% of patients in our elective referral cohorts also exhibited inflammatory rheumatic disease that would have qualified them for early appointments.
More complex appointment stratification systems comprise up to eight parameters, including family history, clinical findings, laboratory results (ESR, RF), and conventional X-ray findings.40 The ppv of inflammatory rheumatic disease was just 49% with ≥3 of 8 abnormal parameters, and any added value is negated by the increased outlay compared to a ppv of 64.2% based on our triage and CRP count. The delegation of such a comprehensive procedure to the family doctor seems to be impracticable because of the limited time and financial resources available and the limited experience of most primary care physicians in evaluations of earlier undifferentiated stages of these diseases. The diversity of inflammatory rheumatic diseases precludes the delineation of a single set of parameters that could be easily implemented by family doctors to provide an equally good representation of all rheumatic diseases.41
The so-called rapid access services use a completely different approach. These services involve a short, direct, symptom-based screening of all patients by an experienced rheumatologist without any preselection. If an inflammatory rheumatic disease is suspected, then another appointment is made to clarify the case in detail, or the patient is advised to consult other medical disciplines. The waiting time to see a rheumatologist for the first time was considerably shortened by this approach.41 Puchner et al.42 described a modification of the rapid access service model in which the patient’s progress was checked six months later. The percentage of patients who experienced symptoms for less than 3 months was 43%. Suspected RA was subsequently confirmed in 93% of cases.
Unfortunately, we cannot make any statements on the value for correct appointment stratification of individual parts of the three-part triage (reliable diagnosis of inflammatory rheumatic disease already made, symptom duration, and unusual laboratory findings) because there was no separate differentiation of the criteria used to allocate the appointments. The value of the laboratory findings in detecting early stages of inflammatory rheumatic disease may be especially interesting in future studies. Notably, elevated CRP was found in 23.71%, 28.61%, and 42.31%, and accelerated ESR was found in 38.57%, 44.54%, and 52.75% of the elective referral, EAC, and emergency groups, respectively. Logistic regression analysis demonstrated that elevated CRP was even superior to the triage described here as a criterion for the allocating of patients to the inflammatory or noninflammatory groups. However, a high percentage (56.5%) of patients with a proven diagnosis of inflammatory rheumatic disease did not have elevated CRP values, which suggests that elevated CRP is unsuitable as a sole marker for rheumatologic consultations. Therefore, the combination of a triage, such as the method described here, and an elevated CRP count is likely the most effective criterion for allocation.
Patients with noninflammatory diseases, of course, should also receive a thorough investigation by a specialist and advice on the best possible care available. However, the rheumatologic care that is available cannot be expanded at will, and balancing this entitlement with the need for the earliest possible diagnosis and treatment of inflammatory rheumatic disease means that a triage is indispensable in the future. The concept presented in this work shows that three simple, delegable, standardized decision pathways to allocate appointments on the basis of a referral by a primary care physician may significantly increase the percentage of inflammatory rheumatic diagnoses in first-time patients and facilitate the implementation of current diagnostic and treatment recommendations.
Conclusion
Rheumatologic care in Germany and many other countries is marked by a demand that by far exceeds the diagnostic and therapeutic capacities available in this field. This shortage clearly undermines treatment concepts such as early diagnosis and initiation of treatment and consistent maintenance of remission. In the collective of referrals to our outpatient rheumatology clinic under routine conditions, only a total of 718 (40.3%) of the 1,782 patients assessed were diagnosed with an inflammatory rheumatic disease, and there were significant differences between the appointment categories in this regard. The applied telephone-based triage concept for appointment stratification helped to significantly increase the percentage of referred patients who were diagnosed with inflammatory disease and the percentage of referred patients who began a specific immunomodulatory treatment. According to our analyses, the combination of a triage and an elevated CRP count yields the highest predictive power regarding total correct group allocation.
Acknowledgments
We thank Nadine Kaltenhauser for data entry.
Footnotes
ACADEMIC EDITOR: Chuanju Liu, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 2042 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: MF has served as a speaker, consultant, and an advisory board member for MSD, Roche, Abbvie, Chugai, Pfizer, and UCB, outside of the submitted work. AS has served as a speaker, consultant, and an advisory board member for Novo Nordisk, outside of the submitted work. APN has served as a speaker, consultant, and an advisory board member for MSD, Roche, Abbvie, Chugai, Pfizer, UCB, Janssen-Cilag, and Celgene, outside of the submitted work. MRK has served as a speaker, consultant and an advisory board member for MSD, Abbvie, Roche, Gilead, and Janssen-Cilag, outside of the submitted work.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: MF, APN. Analyzed the data: MF, AS. Wrote the first draft of the manuscript: MF. Contributed to the writing of the manuscript: MF, APN, MRK, AS. Agreed with manuscript results and conclusions: MF, APN, MRK, AS. Jointly developed the structure and arguments for the paper: MF, APN, MRK, AS. Made critical revisions and approved the final version: MF, APN, MRK, AS. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Abasolo L, Ivorra-Cortes J, Leon L, Jover JA, Fernandez-Gutierrez B, Rodriguez-Rodriguez L. Influence of demographic and clinical factors on the mortality rate of a rheumatoid arthritis cohort: a 20-year survival study. Semin Arthritis Rheum. 2016;45(5):533–8. doi: 10.1016/j.semarthrit.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Dougados M. Comorbidities in rheumatoid arthritis. Curr Opin Rheumatol. 2016;28(3):282–8. doi: 10.1097/BOR.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 3.Haugeberg G, Bøyesen P, Helgetveit K, Prøven A. Clinical and radiographic outcomes in patients diagnosed with early rheumatoid arthritis in the first years of the biologic treatment era: a 10-year prospective observational study. J Rheumatol. 2015;42(12):2279–87. doi: 10.3899/jrheum.150384. [DOI] [PubMed] [Google Scholar]
- 4.Ikdahl E, Rollefstad S, Olsen IC, et al. EULAR task force recommendations on annual cardiovascular risk assessment for patients with rheumatoid arthritis: an audit of the success of implementation in a rheumatology outpatient clinic. Biomed Res Int. 2015:515280. doi: 10.1155/2015/515280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monti S, Montecucco C, Bugatti S, Caporali R. Rheumatoid arthritis treatment: the earlier the better to prevent joint damage. RMD Open. 2015;1(Suppl 1):e000057. doi: 10.1136/rmdopen-2015-000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nell VP, Machold KP, Eberl G, Stamm TA, Uffmann M, Smolen JS. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology (Oxford) 2004;43(7):906–14. doi: 10.1093/rheumatology/keh199. [DOI] [PubMed] [Google Scholar]
- 7.Nikiphorou E, Norton S, Young A, et al. Association between rheumatoid arthritis disease activity, progression of functional limitation and long-term risk of orthopaedic surgery: combined analysis of two prospective cohorts supports EULAR treat to target DAS thresholds. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2015-208669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rannio T, Asikainen J, Kokko A, Hannonen P, Sokka T. Early remission is a realistic target in a majority of patients with DMARD-naive rheumatoid arthritis. J Rheumatol. 2016;43(4):699–706. doi: 10.3899/jrheum.141480. [DOI] [PubMed] [Google Scholar]
- 9.Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75(1):3–15. doi: 10.1136/annrheumdis-2015-207524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zha AM, Di Napoli M, Behrouz R. Prevention of stroke in rheumatoid arthritis. Curr Neurol Neurosci Rep. 2015;15(12):77. doi: 10.1007/s11910-015-0600-y. [DOI] [PubMed] [Google Scholar]
- 11.Raza K, Filer A. The therapeutic window of opportunity in rheumatoid arthritis: does it ever close? Ann Rheum Dis. 2015;74(5):793–4. doi: 10.1136/annrheumdis-2014-206993. [DOI] [PubMed] [Google Scholar]
- 12.van Nies JA, Tsonaka R, Gaujoux-Viala C, Fautrel B, van der Helm-van Mil AH. Evaluating relationships between symptom duration and persistence of rheumatoid arthritis: does a window of opportunity exist? Results on the Leiden early arthritis clinic and ESPOIR cohorts. Ann Rheum Dis. 2015;74(5):806–12. doi: 10.1136/annrheumdis-2014-206047. [DOI] [PubMed] [Google Scholar]
- 13.Kruger K, Wollenhaupt J, Albrecht K, et al. German 2012 guidelines for the sequential medical treatment of rheumatoid arthritis. Adapted EULAR recommendations and updated treatment algorithm. Z Rheumatol. 2012;71(7):592–603. doi: 10.1007/s00393-012-1038-0. [DOI] [PubMed] [Google Scholar]
- 14.Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 15.Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emery P, Breedveld FC, Dougados M, Kalden JR, Schiff MH, Smolen JS. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence based development of a clinical guide. Ann Rheum Dis. 2002;61(4):290–7. doi: 10.1136/ard.61.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zink A, Huscher D, Schneider M. How closely does rheumatology treatment follow the guidelines?: ambition and reality. Z Rheumatol. 2010;69(4):318–26. doi: 10.1007/s00393-009-0522-7. [DOI] [PubMed] [Google Scholar]
- 18.Kumar K, Daley E, Carruthers DM, et al. Delay in presentation to primary care physicians is the main reason why patients with rheumatoid arthritis are seen late by rheumatologists. Rheumatology (Oxford) 2007;46(9):1438–40. doi: 10.1093/rheumatology/kem130. [DOI] [PubMed] [Google Scholar]
- 19.Raza K, Stack R, Kumar K, et al. Delays in assessment of patients with rheumatoid arthritis: variations across Europe. Ann Rheum Dis. 2011;70(10):1822–5. doi: 10.1136/ard.2011.151902. [DOI] [PubMed] [Google Scholar]
- 20.Stack RJ, Shaw K, Mallen C, Herron-Marx S, Horne R, Raza K. Delays in help seeking at the onset of the symptoms of rheumatoid arthritis: a systematic synthesis of qualitative literature. Ann Rheum Dis. 2012;71(4):493–7. doi: 10.1136/ard.2011.155416. [DOI] [PubMed] [Google Scholar]
- 21.Westhoff G, Edelmann E, Kekow J, Zink A. Diagnostic spectrum, treatment indication and symptom duration in initial referrals to the rheumatologist. Z Rheumatol. 2010;69(10):910–8. doi: 10.1007/s00393-010-0715-0. [DOI] [PubMed] [Google Scholar]
- 22.Criswell LA, Such CL, Yelin EH. Differences in the use of second-line agents and prednisone for treatment of rheumatoid arthritis by rheumatologists and non-rheumatologists. J Rheumatol. 1997;24(12):2283–90. [PubMed] [Google Scholar]
- 23.Lacaille D, Anis AH, Guh DP, Esdaile JM. Gaps in care for rheumatoid arthritis: a population study. Arthritis Rheum. 2005;53(2):241–8. doi: 10.1002/art.21077. [DOI] [PubMed] [Google Scholar]
- 24.Rat AC, Henegariu V, Boissier MC. Do primary care physicians have a place in the management of rheumatoid arthritis? Joint Bone Spine. 2004;71(3):190–7. doi: 10.1016/j.jbspin.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Solomon DH, Bates DW, Panush RS, Katz JN. Costs, outcomes, and patient satisfaction by provider type for patients with rheumatic and musculoskeletal conditions: a critical review of the literature and proposed methodologic standards. Ann Intern Med. 1997;127(1):52–60. doi: 10.7326/0003-4819-127-1-199707010-00009. [DOI] [PubMed] [Google Scholar]
- 26.Villeneuve E, Nam JL, Bell MJ, et al. A systematic literature review of strategies promoting early referral and reducing delays in the diagnosis and management of inflammatory arthritis. Ann Rheum Dis. 2013;72(1):13–22. doi: 10.1136/annrheumdis-2011-201063. [DOI] [PubMed] [Google Scholar]
- 27.Hazlewood GS, Barr SG, Lopatina E, et al. Improving appropriate access to care with central referral and triage in rheumatology. Arthritis Care Res (Hoboken) 2016 doi: 10.1002/acr.22845. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Office Professional2010 [Program] Redmond, Washington: Microsoft Corporation; 2010. [Google Scholar]
- 29.SPSS for Windows Release 17.0 [Program] Armonk, NY: IBM; 2008. [Google Scholar]
- 30.Rautenstrauch J, Muller-Ladner U. The “JOINT specialist training scholarship” of the German Society for Rheumatology foundation: a success story. Z Rheumatol. 2015;74(9):760–2. doi: 10.1007/s00393-015-1694-y. [DOI] [PubMed] [Google Scholar]
- 31.Sorensen J, Hetland ML. All departments of rheumatology in Denmark. Diagnostic delay in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis: results from the Danish nationwide DANBIO registry. Ann Rheum Dis. 2015;74(3):e12. doi: 10.1136/annrheumdis-2013-204867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Cock D, Meyfroidt S, Joly J, et al. A detailed analysis of treatment delay from the onset of symptoms in early rheumatoid arthritis patients. Scand J Rheumatol. 2014;43(1):1–8. doi: 10.3109/03009742.2013.805242. [DOI] [PubMed] [Google Scholar]
- 33.Deane KD, Striebich CC, Goldstein BL, et al. Identification of undiagnosed inflammatory arthritis in a community health fair screen. Arthritis Rheum. 2009;61(12):1642–9. doi: 10.1002/art.24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eberhardt K, Recht L, Wollheim F, Lithman T, Pettersson H, Schersten B. Detection of suspected inflammatory joint disease with a new simple self-administered hand test. Br J Rheumatol. 1988;27(6):457–61. doi: 10.1093/rheumatology/27.6.457. [DOI] [PubMed] [Google Scholar]
- 35.Coates LC, Savage L, Waxman R, et al. Comparison of screening questionnaires to identify psoriatic arthritis in a primary care population: a cross sectional study. Br J Dermatol. 2016 doi: 10.1111/bjd.14604. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Frolund F, Frolund C. Pain in general practice. Pain as a cause of patient-doctor contact. Scand J Prim Health Care. 1986;4(2):97–100. doi: 10.3109/02813438609014810. [DOI] [PubMed] [Google Scholar]
- 37.van der Linden MP, le Cessie S, Raza K, et al. Long-term impact of delay in assessment of patients with early arthritis. Arthritis Rheum. 2010;62(12):3537–46. doi: 10.1002/art.27692. [DOI] [PubMed] [Google Scholar]
- 38.Graydon SL, Thompson AE. Triage of referrals to an outpatient rheumatology clinic: analysis of referral information and triage. J Rheumatol. 2008;35(7):1378–83. [PubMed] [Google Scholar]
- 39.Sathi N, Whitehead E, Grennan D. Can a rheumatologist accurately prioritize patients on the basis of information in the general practitioner referral letter? Rheumatology (Oxford) 2003;42(10):1270–1. doi: 10.1093/rheumatology/keg336. [DOI] [PubMed] [Google Scholar]
- 40.van Nies JA, Brouwer E, van Gaalen FA, et al. Improved early identification of arthritis: evaluating the efficacy of early arthritis recognition clinics. Ann Rheum Dis. 2013;72(8):1295–301. doi: 10.1136/annrheumdis-2012-202289. [DOI] [PubMed] [Google Scholar]
- 41.Gormley GJ, Steele WK, Gilliland A, et al. Can diagnostic triage by general practitioners or rheumatology nurses improve the positive predictive value of referrals to early arthritis clinics? Rheumatology (Oxford) 2003;42(6):763–8. doi: 10.1093/rheumatology/keg213. [DOI] [PubMed] [Google Scholar]
- 42.Puchner R, Janetschko R, Kaiser W, et al. Efficacy and outcome of rapid access rheumatology consultation: an office-based pilot cohort study. J Rheumatol. 2016;43(6):1130–5. doi: 10.3899/jrheum.151210. [DOI] [PubMed] [Google Scholar]



