Abstract
We investigated the mechanism whereby cell contact injury stimulates the α-smooth muscle actin (SMA) promoter, a key process for epithelial–mesenchymal transition (EMT) during organ fibrosis. Contact disruption by low-Ca2+ medium (LCM) activated Rac, PAK and p38 MAPK, and triggered the nuclear accumulation of myocardin-related transcription factor (MRTF), an inducer of the SMA promoter. Dominant negative (DN) Rac, DN-PAK, DN-p38, or the p38 inhibitor SB203580 suppressed the LCM-induced nuclear accumulation of MRTF and the activation of the SMA promoter. These studies define novel pathway(s) involving Rac, PAK, and p38 in the regulation of MRTF and the contact-dependent induction of EMT.
Keywords: Epithelial–mesenchymal transition, MRTF, Epithelial injury, Cell adhesion
1. Introduction
Myocardin and myocardin-related transcription factors (MRTFs, also known as MALs, MKLs) are coactivators of serum response factor (SRF) a key regulator of cell growth, differentiation and muscle-specific gene expression [1–3]. The recent discovery of MRTFs greatly promoted our understanding of two old enigmas of muscle differentiation [4,5]. First, the regulation of MRTF explained how SRF, a ubiquitously expressed protein, is able to control growth in a variety of tissues, while it induces muscle-specific gene expression only in a narrow subset (muscle cells, fibroblasts). Second, it has been long known that the cytoskeleton, and particularly actin polymerization, regulates muscle gene expression [6], but only recent studies have identified MRTF as the cytoskeleton-dependent element in this process. In fibroblasts, under resting conditions monomeric (G) actin binds to the RPEL motif at the N-terminus of MRTF and this prevents the nuclear translocation of MRTF. When myogenic stimuli induce F-actin polymerization, G-actin dissociates from MRTF, which then translocates to the nucleus and activates SRF [7].
Epithelial–mesenchymal transition (EMT), a key process in organ development and cancer, has recently emerged as an important pathomechanism in organ fibrosis [8–10]. Exposed to fibrogenic stimuli, such as TGF-β1, a variety of epithelial cells transform into fibroblasts, and ultimately to myofibroblasts (MFs), which express smooth muscle α-actin (SMA), a hallmark of this phenotype. MFs play a crucial role in the progression of fibrosis [10]. Our recent studies have shown that in kidney tubular (LLC-PK1) cells TGF-β1 alone is insufficient to provoke this myogenic transformation; the other prerequisite is an injury or absence of intercellular junctions, which can be modeled by scratch wounding the monolayer or by disrupting the Ca2+-dependent contacts using low-Ca2+ medium [11,12]. Using this powerful model system, we have shown that cell contact disruption activates the SMA promoter by inducing the translocation of MRTF into the nucleus. Investigating the underlying mechanisms we found that the contact injury-induced activation of the Rho/Rho kinase pathway, and the ensuing myosin light chain (MLC) phosphorylation contributed to this effect [12]. Since, in addition to RhoA, Rac, another member of the Rho family, is also involved in the regulation of intercellular contacts [13], and it shares several common downstream effects with Rho on the cytoskeleton (e.g. actin polymerization, cofilin and MLC phosphorylation) [14], we asked whether this small GTPase also participates in the contact-dependent regulation of myogenic transformation. Our results show that Rac, its downstream effector PAK, and the activation of p38 MAP kinase are indispensable for efficient contact-dependent translocation of MRTF and the activation of the SMA promoter, and thus ultimately for EMT.
2. Materials and methods
2.1. Materials and reagents
All reagents were from Sigma (St. Louis, MO) if not otherwise stated. Jasplakinolide, Y-27632 and SB203580 were from Calbiochem (La Jolla, CA). The following antibodies were used: Rac (Upstate Biotechnology, Lake Placid, NY), Hsp27 (Stressgen, Victoria, BC), Myc (9E-10), Cdc42 and p38 (Santa Cruz Biotechnology, Santa Cruz, CA); phospho-cofilin, cofilin, monophospho-MLC, phospho-Hsp27(Ser82), phospho-p38(Thr180/Tyr182) and phospho-PAK1(Thr423)/ PAK2(Thr402) (Cell Signaling Technology, Danvers, MA); histones (Chemicon, Temecula, CA) and tubulin (Sigma). The polyclonal anti-BSAC raised against the mouse MKL1 protein was described previously [15]. Secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA).
2.2. Cell culture and treatments
LLC-PK1/AT1 cells, a kind gift of Dr. R.C. Harris (Vanderbilt University School of Medicine, Nashville, TN) were used as in our previous studies [12]. For Ca2+ deprivation, after washing with phosphate-buffered saline (Gibco), cells were incubated in serum- and CaCl2-free DMEM (low-Ca2+ medium, LCM, Gibco). Control samples were incubated with serum-free DMEM containing Ca2+ (SFM).
2.3. Plasmids and transfection
pSMA-Luc containing the 765 bp portion of the rat SMA promoter was a kind gift from Dr. R.A. Nemenoff [16]. Constitutively active myc-tagged Rac (CA-Rac, Rac1Q61L) and dominant negative (DN) Rac (Rac1T17N) were described previously [17]. Myc-tagged PAK1 mutants (CA-PAK: PAK1H83,86L/T422E and DN-PAK: PAK1H83,86L/K299R) were from Dr. A.S. Mak [18]. YFP-tagged p21 binding domain (PBD) of PAK1 was a kind gift from Dr. Sergio Grinstein (The Hospital for Sick Children, Toronto, ON, Canada). The DN-p38 construct (p38αT180A/Y182F) was kindly provided by Dr. A. Klip (The Hospital for Sick Children, Toronto, ON, Canada) [19]. For promoter studies cells on 6-well plates were co-transfected with 0.5 μg pSMA-Luc, 0.05 μg pRL-TK internal control (Promega, Madison, WI) and 2 μg of the corresponding vector using FuGENE6 (Roche Laval, QC, Canada) as described [11]. Firefly and Renilla luciferase activities were measured with the Dual Luciferase Reporter Assay System (Promega) using a Berthold Lumat LB 9507 luminometer (Bad Wildbad, Germany). Results are expressed as fold increase over pcDNA3 controls in SFM showing the scatter within each experiment (mean ± S.E., n ≥ 6). For immunofluorescence microscopy, cells on glass coverslips were transfected with 2 μg expression vector 48 h prior to the experiments.
2.4. Immunofluorescence microscopy
Immunostaining of confluent LLC-PK1 cells was performed as described [12]. Samples were viewed using an Olympus IX81 microscope (Melville, NY) coupled to an Evolution QEi Monochrome camera (Media Cybernetics, Silver Spring, MD). For detecting in vivo Rac activity cells were transfected with PBD-YFP 2 days prior to the treatment. Loci of Rac activation were monitored using a Leica DMIRE2 inverted fluorescence microscope equipped with a Hamamatsu Back- Thinned EM-CCD camera and spinning disk confocal scan head.
2.5. Rac and Cdc42 activity assays and Western blotting
Active small G-proteins were captured with PAK p21-binding-domain (PBD)-GST fusion protein using a Rac activation Kit (Upstate Biotechnology). Western blotting and densitometry were performed as described [12].
2.6. Nuclear extraction
Nuclear extracts were prepared as in [12] using the NE-PER Kit from Pierce Biotechnology (Rockford, IL). Equal amounts (10 μg) of nuclear extracts were analyzed by Western blotting.
3. Results
3.1. Rac and PAK are stimulated by contact disassembly and contribute to the injury-dependent activation of the SMA promoter and MRTF translocation
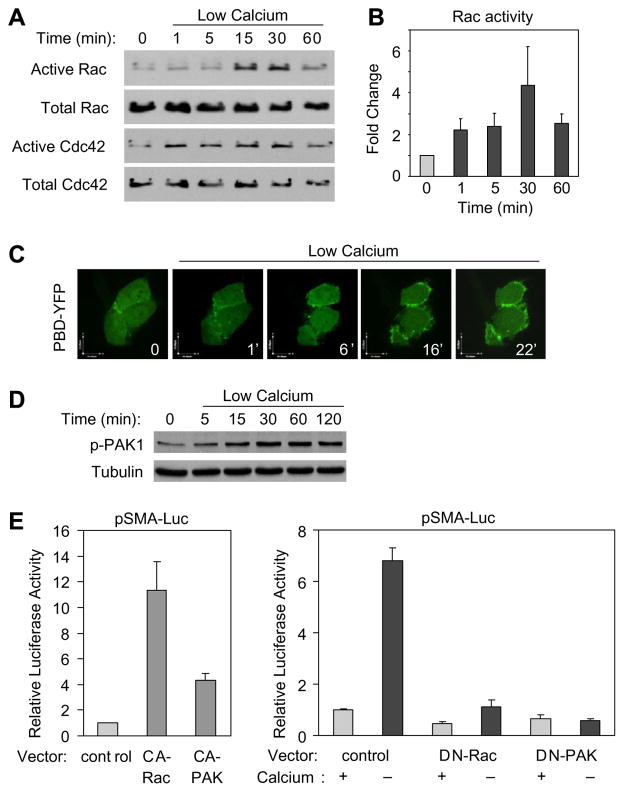
To assess the potential role of Rac and Cdc42 in the cell contact- dependent regulation of the SMA promoter, initially we tested the effect of Ca2+ removal-triggered contact disassembly on these GTPases using an affinity pull-down assay that precipitates their active (GTP-bound) form. Exchanging the serum- free medium (SFM) to a low-Ca2+ medium (LCM) induced a sizable activation of Rac and Cdc42 that continued to increase up to 30 min (Fig. 1A and B). To substantiate this finding and localize Rac activation, we performed live confocal video-imaging on LLC-PK1 cells transfected with YFP-p21-binding domain (PBD) of p21-activated kinase (PAK). Ca2+ removal caused strong peripheral accumulation of YFP-PBD within a few minutes (Fig. 1C), which was most prominent in cells with moderate YFP-PBD expression and definitive contact disassembly. To assess whether Rac/Cdc42 activation translates into downstream signaling events, we investigated the effect of contact disassembly on PAK phosphorylation. LCM facilitated rapid and sustained PAK1 phosphorylation (Fig. 1D). Next we examined the functional significance of Rac and PAK activation in the regulation of the SMA promoter, a key target for myofibroblast transition. Cells were transfected with the pSMA-Luc reporter system along with either an empty vector or constitutively active (CA) or dominant negative (DN) mutants of Rac and PAK. CA-Rac as well as CA-PAK stimulated the SMA promoter (Fig. 1E), consistent with the SRF-activating capacity of CA-Rac [20]. Importantly, both DN-Rac and DN-PAK potently inhibited the LCM-triggered promoter activation (Fig. 1E).
Fig. 1.
Rac/Cdc42 and PAK are activated by contact disassembly and contribute to the ensuing activation of the SMA promoter. (A) Confluent LLC-PK1 cells were serum-deprived and either incubated in SFM or LCM for the indicated times. Active Rac/Cdc42 was captured by PBD-GST beads. (B) Densitometric analysis of Rac activation is expressed as fold increase over control (mean ± S.E., n = 3). (C) Cells were transfected with PBD-YFP and treated with LCM. Images of the transfected cells (within a confluent monolayer) were taken at the indicated times. (D) Active PAK1 was detected by a phospho-specific antibody. (E) Cells were cotransfected with pSMA-Luc and pRL-TK plasmid along with either empty vector (Control) or with one of the test plasmids, as indicated. Cells were incubated in SFM or LCM, and 24 h later luciferase assays were performed.
Since MRTF is a cytoskeleton-regulated cofactor essential for the activation of SRF-dependent genes, including SMA [1,3], we asked whether Rac and/or PAK impacts on MRTF localization. Similar to Ca2+ removal (Fig. 2A and [12]), CA-Rac and CA-PAK induced nuclear translocation of MRTF (Fig. 2B). Importantly, DN-Rac and DN-PAK substantially reduced the LCM-induced nuclear accumulation of MRTF (Fig. 2B and C). Together these findings imply that contact injury not only activates Rac and PAK, but their activation contributes to the nuclear translocation or retention of MRTF and the ensuing SMA promoter activation.
Fig. 2.
Rac and PAK participate in the contact disassembly-induced nuclear translocation of MRTF. (A) Cells were stimulated with LCM for the indicated times and the localization of endogenous MRTF was visualized by immunostaining. (B) Cells transfected with Myc-tagged CA-Rac, CA-PAK, DN-Rac or DN-PAK, were treated with SFM or LCM for 1 h, and then doubly stained for MRTF (red) and Myc (green). Arrows indicate identical transfected cells. (C) MRTF localization (cytoplasmic, homogeneous, and nuclear) was quantified as in Ref. [12].
3.2. Rac and PAK do not act on MRTF via modifying cofilin or MLC phosphorylation
Previous studies have implicated Rho as the primary regulator of MRTF localization, via its effects on the actin skeleton. Rho acts on actin by two major pathways: it activates mDia, leading to polymerization, and induces Rho kinase (ROK)- mediated cofilin phosphorylation, which reduces F-actin severing [14]. Additionally, we have shown that ROK-dependent MLC phosphorylation plays a permissive role in MRTF translocation [12]. Since both cofilin and MLC phosphorylation may also be downstream of the Rac/PAK pathway (reviewed in [14,21]), we considered that Rac might act via contributing to these effects. Indeed, LCM increased cofilin phosphorylation (Fig. 3A and C). However, DN-Rac and DN-PAK failed to affect cofilin phosphorylation (Fig. 3B). Since we have shown that LCM rapidly activates Rho as well [12], we considered whether this is the critical stimulus for cofilin phosphorylation. Indeed, the ROK inhibitor Y-27632 abolished this response (Fig. 3C and D), and also prevented the nuclear translocation of MRTF (Fig. 3E and F). These data indicate that Rac activation is neither sufficient nor necessary for cofilin phosphorylation. Further, although CA-Rac or CA-PAK provoked MLC phosphorylation, DN-Rac or DN-PAK did not eliminate the LCM-induced response (not shown). We therefore concluded that other mechanisms must be responsible for the Rac/PAK-mediated regulation of MRTF.
Fig. 3.
Rac and PAK do not act on MRTF translocation via phosphorylation of cofilin. (A) Cells were incubated in LCM, and their lysates probed for phospho- and total cofilin. (B) Cells transfected with DN-Rac or DN-PAK were treated as in Fig. 2B, and doubly stained for phospho-cofilin and Myc. To assess the role of ROK, cells were pretreated with 20 μM Y-27632 for 30 min where indicated, and then exposed to SFM or LCM for 1 h. Cofilin phosphorylation was determined by Western blotting (C) or immunostaining (D). Nuclear translocation of MRTF under these conditions was determined by nuclear extraction and Western blotting (E) or immunostaining (F).
3.3. p38 is activated by contact injury and plays an essential role in the nuclear accumulation and activity of MRTF
Since p38 is a potential target of Rac [22], and has been implicated in SMA expression [16], we examined its involvement in the contact-dependent responses. LCM induced rapid phosphorylation of p38 as well as heat shock protein 27 (Hsp27) (Fig. 4A and B), a downstream target of p38 and regulator of the actin skeleton. The latter effect was blocked by the specific p38 inhibitor, SB203580 (Fig. 4B). More importantly, SB203580 or the expression of a DN-p38 strongly reduced the contact disruption-triggered activation of the SMA promoter (Fig. 4C). Next we tested whether this effect might be related to altered nucleocytoplasmic traffic of MRTF. Inhibition of p38 dramatically reduced the LCM-provoked nuclear accumulation of MRTF, as revealed by immunoblots performed on nuclear fractions or by immunofluorescence microscopy (Fig. 4D–F). In agreement with our previous results scratch-wounding of the epithelium also promoted nuclear accumulation of MRTF [12], and inhibition of p38 suppressed this effect as well (Fig. 4F). During these experiments we noted that LCM induced an upward shift in the molecular mass of MRTF. Nuclei isolated from stimulated cells contained predominantly the higher Mw species. SB203580 nearly abolished this shift (Fig. 4G).
Fig. 4.
Activation of p38 by contact disassembly regulates nuclear accumulation of MRTF and the SMA promoter. (A) Phospho-p38 was detected in lysates from confluent cultures treated with LCM for the indicated times. (B) Cultures were pretreated with DMSO or 10 μM SB203580 for 30 min prior to incubation with SFM or LCM for 1 h. Phospho-Hsp27 was detected by Western blotting. Membranes were reprobed for Hsp27. (C) The pSMA-Luc/pRL-TK reporter system was either transfected alone or with DN-p38, and the cells were pretreated with 10 μM SB203580 before Ca2+ removal (24 h). (D) Cells were treated as in B and the MRTF content of nuclear extracts was detected by Western blotting. Membranes were reprobed for histones. (E) Western blots in D were quantified by densitometry. Nuclear MRTF from LCM-treated cells corresponds to 100% (n = 3). (F) Nuclear translocation of MRTF upon Ca2+ removal (1 h, upper row) or scratch-wounding of the monolayer with a pipette tip (6 h, lower row) was followed in control and SB203580-pretreated samples by immunostaining. (G) LCM caused retarded migration of MRTF in total cell lysates (arrows), which was prevented by SB203580.
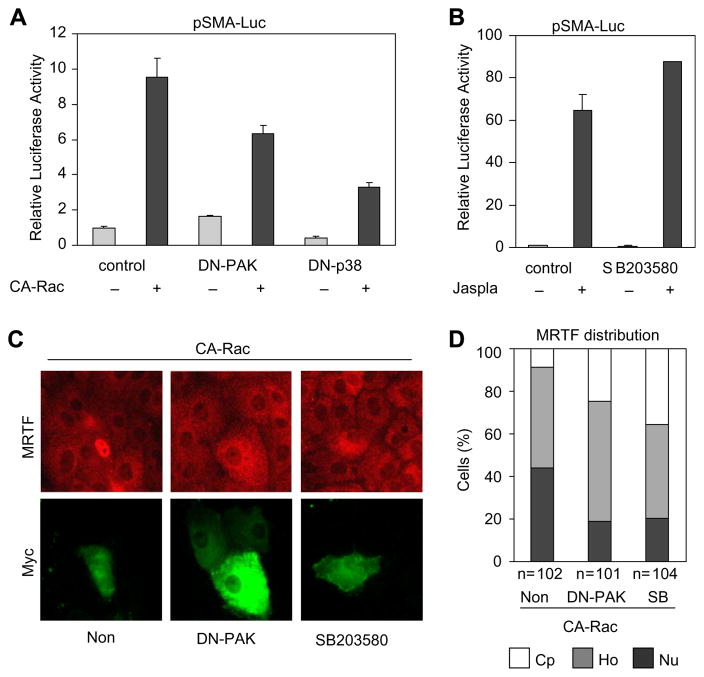
While Rac, PAK and p38 might constitute consecutive elements of the same signaling pathway, each can be activated by multiple mechanisms and can exert independent downstream effects, particularly on the cytoskeleton. To assess whether PAK and p38 are – at least partially – downstream to Rac activation in MRTF regulation, we transfected cells with CA-Rac along with DN-PAK or DN-p38. These DN constructs significantly reduced (but did not abolish) the CA-Rac- induced activation of the SMA promoter (Fig. 5A). Accordingly, inhibition of PAK or p38 mitigated the CA-Rac-induced nuclear accumulation of MRTF (Fig. 5C and D). While p38 plays an important role in the contact injury-provoked SMA promoter activation, we considered that robust actin polymerization might be sufficient for this effect, even in the absence of the facilitating impact of p38. Indeed, the dramatic activation of the SMA promoter by the F-actin- polymerizing drug jasplakinolide was not affected by p38 inhibition (Fig. 5B).
Fig. 5.
Rac activates MRTF partially through PAK and p38. (A) pSMA-Luc/pRL-TK reporters were cotransfected with 0.5 μg CA-Rac and 2 μg empty vector (control) or DN constructs as indicated. Luciferase assays were performed 48 h later. (B) SMA promoter activation upon 1 μM Jasplakinolide treatment (24 h, Jaspla) was determined in control or SB203580 pretreated (30 min) samples. (C) Cells were transfected with CA-Rac (0.5 μg, 48 h) along with empty vector (Non) or DN-PAK. Where indicated, cells were treated with SB203850 30 min prior to transfection. Cells were doubly stained for MRTF (red) and Myc (green). (D) MRTF localization (cytoplasmic: Cp, homogeneous: Ho, and nuclear: Nu) was quantified.
Taken together, p38 activation (in addition to Rho signaling) is an essential input in the injury-induced nuclear traffic of MRTF, and may be one of the mediators of the impact of Rac on this process.
4. Discussion
The present study defines novel mechanisms, involving Rac, PAK and p38, in the cell contact-dependent regulation of MRTF, and provides new insights into mechanisms through which the injury of the intercellular junctions facilities EMT. Our finding that Rac is activated upon contact disassembly may seem somewhat unexpected, inasmuch as earlier studies reported a decrease in Rac activity upon the addition of the Ca2+- chelator EGTA to epithelial cells [23,24]. However, we observed that prolonged (30 min) EGTA treatment caused blebbing in many cells, while LCM did not exert any obvious toxic effect. Moreover, novel findings give credence to a contact disassembly- induced Rac-activating mechanism. First, Rap is stimulated by contact disruption, and this small GTPase has been placed upstream of Rac [24]. Further, the GDP/GTP exchange factor GEF-H1 that has recently been identified as the activator of Rho upon contact disassembly [25], can also act as a Rac-GEF [26]. Moreover, PAK activation, was proposed to facilitate the Rac-activating potency of GEF-H1 [27]. Thus, PAK activation, either downstream or independent of Rac may represent a positive feedback mechanism. In any case, our results show that acute contact injury leads to both Rho [12] and Rac activation, and each of these is indispensable for the ensuing activation of the SMA promoter. Although the injury-induced activation of small GTPases is transient, this initial response is required for full-blown EMT that develops over several days in the presence of contact disruption and TGF-β1 [12].
One of the central findings of this study is that p38 is an important regulator of MRTF localization and activity. Contact disruption activated p38 in epithelial cells, and this process was necessary both for the shift in the Mw of MRTF, and its accumulation and action in the nucleus. Our data suggest that p38 might act, at least partially, downstream of Rac and PAK. Nonetheless, both Rac and PAK are known to induce F-actin polymerization, which can facilitate MRTF translocation without p38. We propose that p38 might regulate the G-actin affinity of MRTF (and/or enhance Hsp27-mediated actin polymerization), thereby facilitating the combinatorial effect of other F-actin-polymerizing inputs.
In a landmark paper, Miralles et al. [7] reported that serum stimulation of fibroblasts led to a shift in the Mw of MRTF. This was attributed to phosphorylation, which was partially sensitive to the inhibition of ROK and ERK. We propose a critical role for p38 in the modification and traffic of MRTF. It remains to be clarified whether p38 mediates direct or indirect phosphorylation of MRTF, and whether this occurs in the cytosol or in the nucleus. While impaired nuclear accumulation of MRTF apparently argues for a cytosolic effect, this is not necessarily so; MRTF has recently been reported to rapidly shuttle between the cytosol and the nucleus, and a reduction in its rate-limiting efflux was proposed to be the primary mechanism of regulation [28]. p38 may be necessary for increased influx or reduced efflux and/or enhanced interaction of MRTF with its intranuclear partners (e.g. SRF). It will be interesting to define, whether p38 facilities the dissociation of actin from MRTF in the cytosol (necessary for influx) and/or prevents its association with actin in the nucleus (proposed to be necessary for efflux [28]). p38, being a general stress kinase, may link various stress conditions to MRTF regulation. While p38 activation facilitates MRTF nuclear accumulation, our preliminary data suggest that it is not sufficient. Future studies should define the exact molecular mechanism whereby this major stress kinase contributes to the regulation of MRTF, a key transcription factor for myogenic differentiation and epithelial– myofibroblast transition.
Acknowledgments
This work was supported by grants from the Kidney Foundation of Canada (A.K. and K.S.), NSERC (A.K.) a Premier‘s Research Excellence Award to A.K. and a KRESCENT New Investigator Award (K.S.).
Abbreviations
- CA
constitutively active
- DN
dominant negative
- EMT
epithelial–mesenchymal transition
- LCM
low-Ca2+ medium
- MLC
myosin light chain
- MRTF
myocardin-related transcription factor
- PAK
p21-activated kinase
- PBD
p21 binding domain
- ROK
Rho kinase
- SFM
serum-free medium
- SMA
α-smooth muscle actin
- YFP
yellow fluorescent protein
References
- 1.Pipes GC, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 2006;20:1545–1556. doi: 10.1101/gad.1428006. [DOI] [PubMed] [Google Scholar]
- 2.Parmacek MS. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res. 2007;100:633–644. doi: 10.1161/01.RES.0000259563.61091.e8. [DOI] [PubMed] [Google Scholar]
- 3.Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 2007;292:C70–C81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 5.Wang DZ, et al. Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc Natl Acad Sci USA. 2002;99:14855–14860. doi: 10.1073/pnas.222561499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mack CP, Somlyo AV, Hautmann M, Somlyo AP, Owens GK. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J Biol Chem. 2001;276:341–347. doi: 10.1074/jbc.M005505200. [DOI] [PubMed] [Google Scholar]
- 7.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 8.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 10.Kalluri R, Neilson EG. Epithelial–mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masszi A, Fan L, Rosivall L, McCulloch CA, Rotstein OD, Mucsi I, Kapus A. Integrity of cell–cell contacts is a critical regulator of TGF-beta 1-induced epithelial-to-myofibroblast transition: Role for beta-catenin. Am J Pathol. 2004;165:1955–1967. doi: 10.1016/s0002-9440(10)63247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan L, et al. Cell contact-dependent regulation of epithelial–myofibroblast transition via the rho–rho kinase-phospho- myosin pathway. Mol Biol Cell. 2007;18:1083–1097. doi: 10.1091/mbc.E06-07-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braga VM, Del Maschio A, Machesky L, Dejana E. Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol Biol Cell. 1999;10:9–22. doi: 10.1091/mbc.10.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 15.Sasazuki T, et al. Identification of a novel transcriptional activator, BSAC, by a functional cloning to inhibit tumor necrosis factor-induced cell death. J Biol Chem. 2002;277:28853–28860. doi: 10.1074/jbc.M203190200. [DOI] [PubMed] [Google Scholar]
- 16.Garat C, Van Putten V, Refaat ZA, Dessev C, Han SY, Nemenoff RA. Induction of smooth muscle alpha-actin in vascular smooth muscle cells by arginine vasopressin is mediated by c-Jun amino-terminal kinases and p38 mitogenactivated protein kinase. J Biol Chem. 2000;275:22537–22543. doi: 10.1074/jbc.M003000200. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, Han J, Sells MA, Chernoff J, Knaus UG, Ulevitch RJ, Bokoch GM. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J Biol Chem. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]
- 18.Webb BA, Eves R, Crawley SW, Zhou S, Cote GP, Mak AS. PAK1 induces podosome formation in A7r5 vascular smooth muscle cells in a PAK-interacting exchange factor-dependent manner. Am J Physiol Cell Physiol. 2005;289:C898–C907. doi: 10.1152/ajpcell.00095.2005. [DOI] [PubMed] [Google Scholar]
- 19.Somwar R, et al. A dominant-negative p38 MAPK mutant and novel selective inhibitors of p38 MAPK reduce insulin-stimulated glucose uptake in 3T3-L1 adipocytes without affecting GLUT4 translocation. J Biol Chem. 2002;277:50386– 50395. doi: 10.1074/jbc.M205277200. [DOI] [PubMed] [Google Scholar]
- 20.Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 21.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 22.Minden A, Lin A, Claret FX, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell–cell adhesion sites. J Cell Sci. 2001;114:1829–1838. doi: 10.1242/jcs.114.10.1829. [DOI] [PubMed] [Google Scholar]
- 24.Balzac F, Avolio M, Degani S, Kaverina I, Torti M, Silengo L, Small JV, Retta SF. E-cadherin endocytosis regulates the activity of Rap1: a traffic light GTPase at the crossroads between cadherin and integrin function. J Cell Sci. 2005;118:4765–4783. doi: 10.1242/jcs.02584. [DOI] [PubMed] [Google Scholar]
- 25.Samarin SN, Ivanov AI, Flatau G, Parkos CA, Nusrat A. Rho/ROCK-II signaling mediates disassembly of epithelial apical junctions. Mol Biol Cell. 2007 doi: 10.1091/mbc.E07-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren Y, Li R, Zheng Y, Busch H. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J Biol Chem. 1998;273:34954–34960. doi: 10.1074/jbc.273.52.34954. [DOI] [PubMed] [Google Scholar]
- 27.Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci. 2005;118:1861–1872. doi: 10.1242/jcs.02313. [DOI] [PubMed] [Google Scholar]
- 28.Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749– 1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]