Abstract
After vaccination, memory CD8+ T cells migrate to different organs to mediate immune surveillance. In most nonlymphoid organs, following an infection, CD8+ T cells differentiate to become long-lived effector-memory cells, thereby providing long-term protection against a secondary infection. In this study, we demonstrated that Ag-specific CD8+ T cells that migrate to the mouse brain following a systemic Listeria infection do not display markers reminiscent of long-term memory cells. In contrast to spleen and other nonlymphoid organs, none of the CD8+ T cells in the brain reverted to a memory phenotype, and all of the cells were gradually eliminated. These nonmemory phenotype CD8+ T cells were found primarily within the choroid plexus, as well as in the cerebrospinal fluid-filled spaces. Entry of these CD8+ T cells into the brain was governed primarily by CD49d/VCAM-1, with the majority of entry occurring in the first week postinfection. When CD8+ T cells were injected directly into the brain parenchyma, cells that remained in the brain retained a highly activated (CD69hi) phenotype and were gradually lost, whereas those that migrated out to the spleen were CD69low and persisted long-term. These results revealed a mechanism of time-bound immune surveillance to the brain by CD8+ T cells that do not reside in the parenchyma.
Listeria monocytogenes is an intracellular pathogen that causes disease in immunocompromised hosts. The most severe outcomes in persons infected by L. monocytogenes occur when the bacteria invade the CNS, which can result in death (1). During primary infection, L. monocytogenes is controlled mainly by innate immunity (2, 3). In the brain, a primary infection with L. monocytogenes is controlled mainly by CD8+ T cells and NK cells, which develop after several days (1). Protection against a peripheral secondary infection is mediated mainly by CD8+ T cells through a mechanism that is IFN-γ independent (4) but perforin (5) and TNF (6) dependent.
CD8+ T cells are stimulated by APCs, which display peptides from endogenously derived Ags (intracellular bacteria, viruses, or tumors) on MHC class I molecules (7). After differentiation, CD8+ T cells secrete cytokines and mediate specific cytotoxicity (by perforin- and Fas-dependent pathways) toward infected cells and tumors (8–10). A vast majority (>95%) of Ag-specific T cells activated at the onset of the immune response are eliminated, and only a small portion of those T cells survive (<5%) for extended periods (11–13). These long-lived memory T cells possess the unique ability to respond rapidly and specifically to Ags (14, 15).
After activation of CD8+ T cells, the expression of various cell surface molecules is differentially modulated (15–17); however, CD44 is one of the few proteins that is persistently elevated on memory T cells, irrespective of their activation status (14, 18). Memory T cells have been segregated broadly into two phenotypic and functional subsets: effector memory cells (CD44hiCD62Llow CCR7−) and central memory cells (CD44hiCD62LhiCCR7+) (19–23). Central memory cells persist within the lymphoid organs, whereas effector memory cells home to nonlymphoid organs (24). Although both subsets can express cytokines (25), central memory cells can proliferate more efficiently and, consequently, provide better long-term protection (26).
T cells, after activation, traffic to various nonlymphoid organs to provide rapid protection against reinfection (24, 27–29). Although the blood–brain barrier (BBB) exists to prevent the exposure of the brain to various soluble mediators from systemic sites, multiple mechanisms of T cell trafficking to the brain have been proposed (30, 31). In this study, we evaluated the trafficking of Ag-specific CD8+ T cells to the brain during systemic infection of mice with the intracellular pathogen L. monocytogenes. Our results indicated that CD8+ T cells that reside in the brain display cell surface markers typical of early effectors and are found mainly outside of the parenchyma. Following a secondary brain infection, these cells move through cerebrospinal fluid (CSF)-filled spaces and into the parenchymal region affected by the infection. In the absence of a brain infection, these cells never revert to a memory phenotype and are gradually eliminated, thereby providing time-bound immune surveillance to the brain. Activated CD8+ T cells transferred directly into the brain parenchyma undergo gradual and complete erosion within the brain, but they can survive and convert to memory cells in the periphery following migration out of the brain. Thus, in the absence of Ag in the brain, CD8+ T cells may not convert to memory cells within this organ.
Materials and Methods
Bacterial strains
OVA-expressing L. monocytogenes (LM-OVA), as described previously (32), was grown in brain–heart infusion (BHI) media (DIFCO Laboratories, Detroit MI) to OD600nm = 0.4–0.8. At midlog phase, bacteria were harvested and frozen in 20% glycerol and stored at −80°C. CFU were determined by performing serial dilutions on plates.
Mice and immunizations
Female C57BL/6J mice were obtained at 6–8 wk of age from The Jackson Laboratories (Bar Harbor, ME). CD45.1+ OT-1 and CD45.2+ OT-1 mice were bred in-house. Mice were maintained in the animal facility at the National Research Council of Canada-Institute for Biological Sciences, in accordance with the guidelines of the Canadian Council on Animal Care. For immunizations with LM-OVA, frozen stocks were thawed and diluted in 0.9% NaCl, and mice were inoculated i.v. via the lateral tail vein with 1 × 104 LM-OVA suspended in 200 μl 0.9% NaCl.
For intracranial (i.c.) injections, mice were deeply anesthetized with isoflurane in O2. The scalp was cut to expose the skull, and a 30-gauge burr hole was made through the skull above the left anterior forebrain. Intracranial injections of 1 × 102–104 LM-OVA in 5 μl saline were performed using a Hamilton syringe fitted with a 31-gauge blunt-end cannula. The cannula was left in situ for an additional 1 min to prevent reflux along the injection tract. A plug of sterile surgical bone wax was used to cover the hole in the skull, and mice were given bupivacaine (long-acting local anesthetic) before the skin was sutured and the suture ties were glued.
Assessment of bacterial burden
Spleens and brains from infected mice were homogenized in RPMI 1640 medium. CFU were determined by plating 100-μl aliquots of serial 10-fold dilutions in 0.9% saline on BHI agar plates.
Isolation of cells from spleen, brain, lungs, and liver
A transcardial perfusion was performed in mice before isolation of organs. Mice were deeply anesthetized with isoflurane in O2. The right atrial chamber of the heart was lacerated with scissors. A 25-gauge needle attached to a heparinized cannula attached to a PBS-filled syringe was inserted into the left ventricular chamber. Mice were perfused with 50–60 ml PBS, and various organs were removed. Spleen cell suspensions were made by pressing the spleens against the frosted ends of glass slides. Cell suspensions from nonlymphoid organs were prepared by chopping the organ into small pieces, incubating with collagenase (0.5 mg for 10 min at 37°C), and incubating for an additional 5 min at 37°C with 1 mM EDTA in RPMI 1640. The tissue was then pushed through a 100-μm cell strainer (Falcon 2360 cell strainer), using a plunger from a 10-ml syringe. The strainer was washed with RPMI 1640, and the cells were spun down at 1700 rpm for 10 min at 5°C. Cells were resuspended in 1 ml RPMI 1640 plus 1 ml 60% Percoll (GE Healthcare) and then layered onto 70% Percoll. After centrifugation at 2000 rpm for 30 min at 5°C, the lymphocytes were collected from the interface. The cells were then washed in 20 ml PBS, resuspended in 1% BSA in PBS, and stained for flow cytometry, as described previously.
In vitro stimulation of OT-1 cells
OT-1 spleen cells (45.1+ or 45.2+) were incubated (100 × 106 cells/flask) with LM-OVA (1 × 102). After 16–18 h, cells were washed and cultured for two more days with media containing gentamicin (50 μg/ml). CD8+ T cells were purified using magnetic-selection beads (Miltenyi Biotec) and injected i.c. in 5 μl HBSS into the anterior forebrain. In some cases, after activation (as described above), activated CD8+ T cells were kept in media containing IL-7 (1 ng/ml) for additional time periods, with cultures split on a daily basis to avoid overcrowding. Cells were processed for injection as described above.
Assessment of the fate and phenotype of Ag-specific CD8+ T cells
For evaluation of the fate and phenotype of OVA257–264-specific CD8+ T cells in vivo, aliquots of cells were incubated in 200 μl PBS plus 1% BSA with anti-CD16/32 at 4°C. After 10 min, cells were stained with anti-CD8 Ab and PE–H-2Kb OVA257–264 tetramer (Beckman Coulter, Fullerton, CA) at room temperature for 30 min. Cells were washed and stained with various Abs (anti-CD62L, anti–IL-2Rα, anti–IL-7Rα, anti-CD69, anti-CD11a, anti-CD44) for 30 min on ice. All Abs were obtained from BD Biosciences (Mississauga, ON, Canada). Cells were washed with PBS, fixed in 0.5% formaldehyde, and acquired on a BD FACSCanto flow cytometer. In some experiments, CD45.1+ OT-1 cells were adoptively transferred into CD45.2+ recipients, and the transferred cells were tracked after staining cells with anti-CD45.1 Abs.
Functional Abs
Blocking Abs against CD49d (9C10 and R1-2; BD Biosciences) and VCAM-1 (clone 429; eBioscience) were injected i.p. (100 μg/mouse). A rat isotype control, IgG2a κ (eBioscience), was used in control injections compared with the VCAM-1–blocking Ab, and normal rat IgG was used as a control for CD49d blocking. The number of injections and their timing are detailed in the appropriate figure legends. The CD8 cell-depletion Ab (clone 2.43) was produced in our laboratory by a standard Protein G-purification protocol from hybridoma supernatant. It was dialyzed against PBS, and its functional concentration was determined by an ELISA assay.
Imaging
A standard immunofluorescence-staining protocol was used for labeling 10-μm frozen sections cut from unfixed, PBS-perfused brains. The sections were fixed with 4% paraformaldehyde for 15–20 min, washed three times for 10 min in PBS, and then blocked and permeabilized with PBS containing 0.4% Triton X-100 and 10% donkey serum. Following the blocking/permeabilization of the sections, they were sequentially incubated with the different primary and secondary Abs, with three 10-min washes with PBS in between Ab incubations. Abs were diluted in a solution of PBS containing 0.04% Triton X-100 and 5% FBS and incubated either overnight at 4°C or for 1 h at room temperature. Primary Abs included goat anti-glial fibrillary acidic protein (C-19; Santa Cruz) used at 1:100, rat anti-CD8a (clone 53–6.7; BD Biosciences) used at 1:200, mouse anti-CD45.1 (clone A20; BD Biosciences) used at 1:200, rabbit anti-smooth muscle actin (Abcam) used at 1:200, and rabbit anti-pan cyto-keratin (Santa Cruz, H-240) used at 1:50. Secondary Abs were donkey anti-goat or donkey anti-rabbit Alexa Fluor 488 (Invitrogen), highly cross-adsorbed donkey anti-rat Cy3 (Jackson ImmunoResearch), donkey anti-mouse Alexa Fluor 546 (Invitrogen), and highly cross-adsorbed donkey anti-rabbit Cy5 (Jackson ImmunoResearch). Hoechst dye was used to label cell nuclei by diluting a 1 mg/ml solution 1:5000 in PBS and incubating it for 15 min on the sections, prior to a final wash and mounting in Dako fluorescence mounting medium. Images were acquired on a Zeiss Axiovert 200M microscope with an AxioCamHR3 camera, using appropriate filter cubes. Extended-focus images were generated by acquiring Z-series (at the optimal step size to satisfy the Nyquist frequency) using either a 20× (NA = 0.5) or 40× (NA = 0.75) Neofluar objective. Images were subsequently processed in Axiovision 4.7 (Zeiss), and tiff images were assembled with Adobe Photoshop and Adobe Illustrator (Adobe Systems) for the figures. The picture of the brain during cryosectioning was taken using an iPhone3 GS (Apple).
Results
Migration of effector phenotype CD8+ T cells to the brain following systemic LM-OVA infection
It was shown that L. monocytogenes (10403S), when given i.v. to mice, results in an acute infection that lasts until day 5–7 (32). We determined whether this strain of L. monocytogenes would also reach the brain of infected mice with the injection of 104 bacteria. Dilutions of the entire brain homogenate were plated on agar plates to detect bacterial colonies. As shown in Fig. 1A, LM-OVA was detectable in the spleens, but not the brain, of infected mice. By day 10, LM-OVA was undetectable in the spleens of infected mice. Not a single colony of LM-OVA was detectable in the brains of infected mice at any of the time intervals.
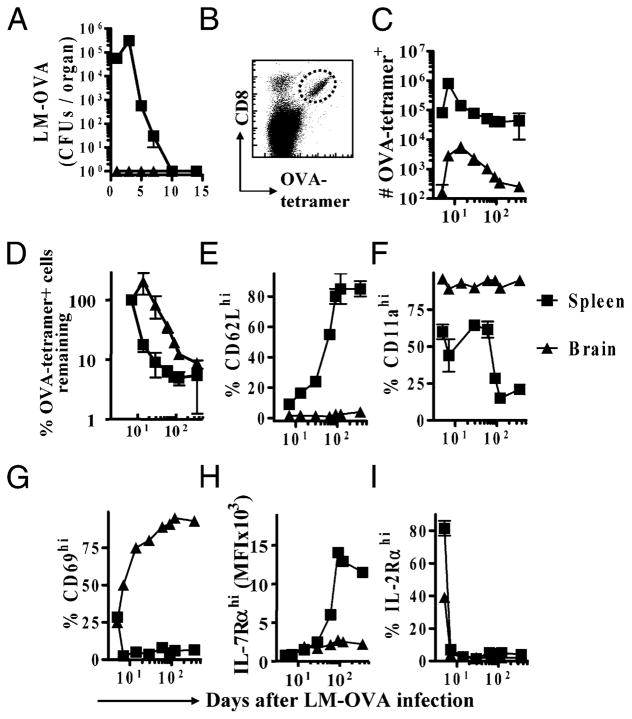
FIGURE 1.
Ag-specific CD8+ T cells in the brain display a persistently activated phenotype in response to a peripheral infection. C57BL/6J mice were infected i.v. with LM-OVA (104). At various time intervals, spleens and brains were removed from mice after cardiac perfusion. A, Cell suspensions were prepared, and serial dilutions were plated on BHI agar plates to determine the bacterial burden (CFU/organ). B–I, C57BL/6J mice were injected i.v. with 1 × 103 OT-1 TCR transgenic CD8+ T cells and challenged within 5–7 d with LM-OVA (1 × 104, i.v.). At various time intervals postinfection, spleens and brains were removed from mice after cardiac perfusion. B, Cells were stained with OVA-tetramer and anti-CD8 Abs and analyzed by flow cytometry. C, The numbers of OVA-tetramer+ CD8+ T cells in the brain and spleen were evaluated. D, To compare the rates of attrition, the percentage of cells remaining past day 5 postinfection were calculated, with numbers at the day 7 time point set as 100%. Cells were also stained with Abs against CD62L (E), CD11a (F), CD69 (G), IL-7Rα (H), and IL-2Rα (I), and the expression of these molecules on OVA-tetramer+ CD8+ T cells was evaluated in the brain and spleen. Each experiment involved the analysis of at least four mice/group and was repeated at least twice.
We then determined the relative migration of CD8+ T cells to the brain in LM-OVA–infected mice that had been adoptively transferred with low numbers of OVA-responsive OT-1 cells prior to infection. Mice were perfused first with 50–100 ml of buffer, and the spleen and brain were removed. This was done to remove traces of blood from brain vasculature. Lymphocytes in the brain were detected by flow cytometry using forward- versus side-scatter plots to separate them from glial and neuronal cells, and the remaining cells were plotted for CD8 and OVA257–264-tetramer staining (Fig. 1B). There was a rapid increase in the numbers of OVA-specific CD8+ T cells in the spleen that peaked at day 7 of LM-OVA infection and subsequently underwent attrition (Fig. 1C). This was followed by a stable pool of memory CD8+ T cells. The numbers of OVA-specific CD8+ T cells in the brain did not peak at day 7, and the numbers increased steadily until day 15, which was indicative of a progressive migration of cells to the brain (Fig. 1C). In the long term, the relative numbers of OVA-specific CD8+ T cells in the brain declined in a similar fashion to those in the spleen (Fig. 1D). This resulted in a loss of OVA-specific CD8+ T cells in the brain down to levels close to the detection limit, whereas a stable pool of memory cells remained in the spleen (Fig. 1D). Interestingly, OVA-specific CD8+ T cells in the brain were persistently CD62LlowCD11ahi (Fig. 1E, 1F). Furthermore, OVA-specific CD8+ T cells in the brain expressed high levels of the early activation marker, CD69, even at day 180 postinfection (Fig. 1G). CD8+ T cells in the brain also expressed persistently reduced levels of the memory cell marker, IL-7Rα, whereas the cells in the spleen differentiated progressively into memory (IL-7Rαhi) cells (Fig. 1H). IL-2Rα expression was quickly lost on the OVA-specific CD8+ T cells in both brain and spleen (Fig. 1I). These results indicated that CD8+ T cells that migrate into the brain following activation in the periphery display a cell surface expression profile typical of early effectors, not memory cells, even at later time periods postinfection.
Because the experiments described above involved adoptive transfer of small numbers of OT-1 cells, we addressed whether the results would be similar for endogenous OVA-specific CD8+ T cells. Following LM-OVA infection in the absence of OT-1 cells, endogenous OVA-responsive CD8+ T cells migrated to various organs. OVA257–264-tetramer+ CD8+ T cells were found in the liver, lungs, brain, and spleen at 30 d postinfection (Fig. 2). Again, the OVA-specific CD8+ T cells in the brain were unique in exhibiting the lowest expression of CD62L and IL-7Rα. OVA-specific CD8+ T cells displayed similar IFN-γ expression in the spleen, lungs, and brain (Fig. 3B, 3C), despite the differences in CD62L and IL-7Rα expression, Proliferation of OVA-specific CD8+ T cells in the spleen and brain was also similar as measured by BrdU uptake (Fig. 3A), with cells displaying maximal proliferation around day 7 and return to homeostatic levels beyond day 15 postinfection. Thus, the cell surface profile of CD8+ T cells that home to the brain is distinctly unique in comparison with CD8+ T cells in other lymphoid or nonlymphoid organs.
FIGURE 2.
The endogenous CD8+ T cell response in various organs following LM-OVA infection. Spleens, lungs, livers, and brains were collected 30 d following i.v. infection of C57BL/6J mice with LM-OVA (1 × 104). No OT-1 cells were used in this case. A, The numbers of OVA-specific CD8+ T cells in each organ were quantified by FACS analysis. B, The percentage of OVA-specific CD8+ T cells expressing high levels of CD62L and IL-7Rα was assessed by FACS analysis at various time points over a 35-d period following LM-OVA infection (1 × 104, i.v.). This was assessed from spleens, lungs, livers, brains, and peripheral blood (PBL). Each experiment involved analysis of at least four mice/group and was repeated at least twice.
FIGURE 3.
Normal function of CD8+ T cells in the brain. C57BL/6J mice were injected with OT-1 and infected with LM-OVA, as described in Fig. 1. Three days prior to the harvest of spleens and brain, BrdU (0.8 mg/ml) was incorporated into the drinking water of mice, which was changed daily. A, Cells were stained with anti-CD8 Abs and OVA-tetramers, followed by intracellular staining for BrdU. Numbers in the graph indicate the percentage of BrdU+ CD8+ T cells among the tetramer+ cells. B, Numbers of IFN-γ–secreting tetramer+ CD8+ T cells were evaluated after staining cells first with OVA-tetramers and anti-CD8 Ab, followed by the stimulation of cells for 1 h with OVA257–264 peptide. Numbers in the figure indicate the percentage of IFN-γ-secreting CD8+ T cells among the tetramer+ cells. C, The percentage of OVA-tetramer+ CD8+ T cells expressing IFN-γ in spleen, lungs, and brain collected at day 30 postinfection, as assessed by flow cytometry. Each experiment involved analysis of at least four mice/group and was repeated at least twice.
CD8+ T cells respond to fight a secondary infection of LM-OVA in the brain
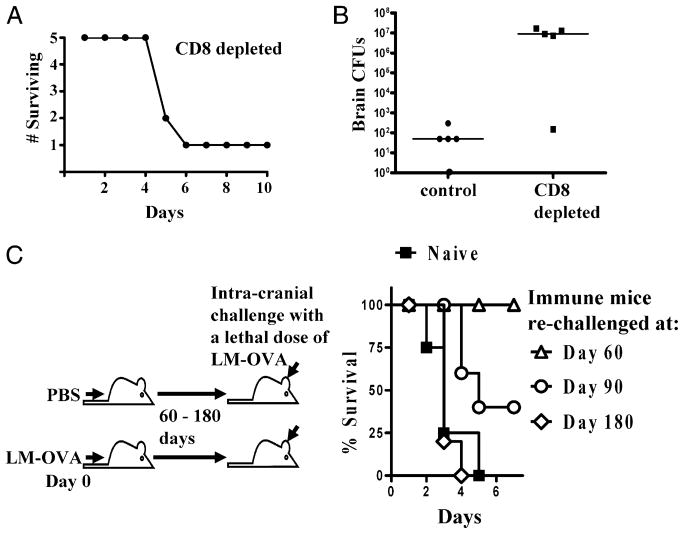
CD8+ T cells are known to be a major player in the response to a primary brain infection of L. monocytogenes (1). To assess the importance of the CD8+ T cell response in the brain in fighting a potential secondary infection, we examined the response of mice to a brain rechallenge of LM-OVA following CD8+ T cell depletion. We first examined the survival of LM-OVA–immunized animals depleted of CD8+ T cells using an anti-CD8 depletion Ab (clone 2.43) and then given an i.c. rechallenge of LM-OVA (1 × 102). A second injection of the anti-CD8 depletion Ab was given following the i.c. infection. Depletion of OVA-specific CD8+ T cells with this Ab was complete by 24 h postinjection in brain and spleen (Fig. 4). Of the five mice that were rechallenged in the brain, four lost weight and died over the course of 6 d, and one gained weight and showed no sign of illness by the end of 10 d (Fig. 5A). We then immunized two groups of five mice with LM-OVA, as described in Fig. 1 (with 5 × 103 OT-1 splenocytes adoptively transferred prior to infection). Two weeks later, one group was depleted of CD8+ T cells using the anti-CD8 depletion Ab, and the second group was given a PBS injection. The mice were then rechallenged with LM-OVA (1 × 102) i.c. and given a second injection of anti-CD8 depletion Ab or PBS. All of the PBS-injected animals controlled the infection, showing low bacterial burdens in the brain at 3 d post rechallenge (Fig. 5B), and all had gained weight and appeared healthy prior to being sacrificed. In contrast, four of the five CD8-depleted mice had a high bacterial burden in the brain and had lost approximately one fifth (20.6%) of their weight, on average. A fifth CD8-depleted mouse, in line with the prior survival experiment, had a low bacterial burden and had gained weight.
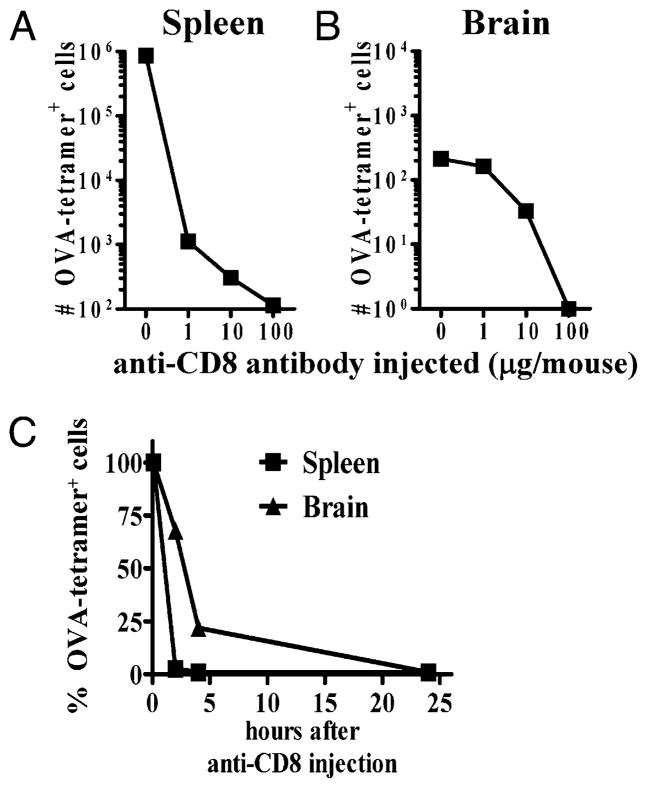
FIGURE 4.
Systemic administration of anti-CD8 Ab deletes CD8+ T cells in the brain. C57BL/6J mice were injected i.v. with OT-1 and LM-OVA (1 × 104), as described in Fig. 1. At day 30 after primary infection, mice were injected i.p. with different amounts of anti-CD8 depletion Ab (clone 2.43) and evaluated for the reduction in the numbers of OVA-specific CD8+ T cells in the spleen (A) and brain (B) at 24 h. C, Also at day 30, mice were injected i.p. (100 μg, once) with depleting anti-CD8 Ab, and tissues were collected over a 24-h time period. At various times post-Ab injection, spleens and brains were removed, and the relative numbers of OVA-tetramer+ CD8+ T cells were evaluated by flow cytometry. The numbers of OVA-tetramer+ CD8+ T cells in the control Ab-treated mice were factored to 100%, and the relative numbers in anti-CD8 Ab-treated mice were evaluated relative to control Ab-treated mice. Each experiment involved analysis of at least four mice/group and was repeated at least twice.
FIGURE 5.
Time-bound protection against LM-OVA rechallenge in the brain. C57BL/6J mice were injected i.v. with LM-OVA (1 × 104) in each experiment. A, To examine the ability of mice to clear a secondary LM-OVA infection in the brain, we first examined the survival of a group of mice that had CD8+ T cells depleted using the anti-CD8 clone 2.43 Ab (see Materials and Methods and Fig. 4 for additional information). These mice were rechallenged with an i.c. infection (1 × 102 LM-OVA in 5 μl) 2 wk following the initial i.v. infection. All mice lived for 4 d following the rechallenge, with one mouse surviving well beyond this. This mouse was sacrificed at day 10 with no apparent illness or weight loss. B, Two more groups of five mice had 1 × 103 OT-1 splenocytes adoptively transferred i.v. and then were infected i.v. with 1 × 104 LM-OVA. Two weeks later, these mice received either i.p. injections of CD8 cell-depletion Ab or a PBS control. We then administered an i.c. rechallenge of 1 × 102 LM-OVA and collected brains 3 d thereafter. The bacterial burden (CFU) was determined with serial dilutions plated on BHI agar plates. Complete CD8+ cell depletion was confirmed by a FACS analysis of spleens using anti-CD8-labeling Ab in the experiments in A and B (data not shown). C, At days 60, 90, or 180 post-i.v. infection, mice were challenged i.c. with LM-OVA in 5 μl, and the survival of mice was monitored. A >20% reduction in the weight of mice was scored as moribund, and mice were sacrificed at that point. This experiment involved the analysis of five mice/group and was repeated twice.
We also examined the survival of LM-OVA–immunized mice in long-term experiments to assess the duration of protection provided by the immunization. Mice with 1 × 103 OT-1 CD8+ T cells adoptively transferred i.v. were subsequently infected i.v. with LM-OVA (104) to generate a memory CD8+ T cell response. To evaluate protection in the brain, mice were given an i.c. rechallenge (at days 60, 90, and 180) of LM-OVA. All of the naive mice succumbed to infection within the first week (Fig. 5C). Mice that were rechallenged i.c. with LM-OVA at day 60 after the primary infection survived. When vaccinated mice were rechallenged on day 90, ~50% survived; none of the vaccinated mice survived when rechallenge was given at day 180. Protection in the liver, lungs, and spleen was not abrogated at late time intervals in response to an i.v. or intranasal LM-OVA challenge (data not shown).
CD49d and VCAM-1 govern the initial homing of CD8+ T cells to the brain
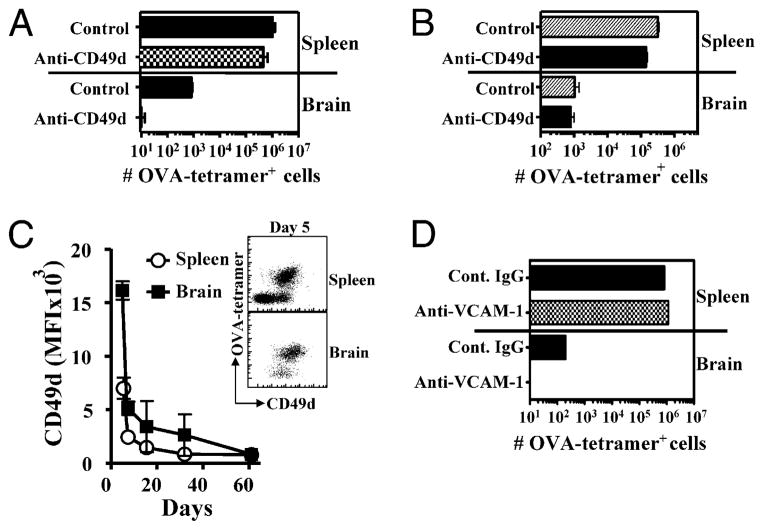
The α4 integrin (CD49d) adhesion protein is well known to mediate the entry of T cells into the CNS in animal models of multiple sclerosis (33–35). We evaluated the role of CD49d in mediating the homing of OVA-specific CD8+ T cells to the brain following i.v. LM-OVA infection. Interestingly, anti-CD49d treatment of mice during initial priming resulted in a drastic reduction in the homing of OVA-specific CD8+ T cells to the brain (Fig. 6A). Migration of CD8+ T cells was not influenced by the absence of P-selectin glycoprotein ligand 1, P-selectin, ICAM, LFA1, CCR2, CCR5, or CCR6 (S. MacLean and S. Sad, unpublished observations). We then determined whether anti-CD49d Ab treatment had any influence on the CD8+ T cells that had immigrated to the brain prior to Ab treatment. At a time point (day 60) when memory CD8+ T cells were already formed in the periphery and CD8+ T cells were detectable in the brain, we began anti-CD49d Ab treatment. As is evident in Fig. 6B, the numbers of pre-existing CD8+ T cells in the brain were not modulated by anti-CD49d Ab treatment. These results indicated that CD49d function is not required for the entry or retention of CD8+ T cells in the brain at 60 d postinfection.
FIGURE 6.
CD49d/VCAM-1 mediated homing of CD8+ T cells to the brain postinfection. A and B, C57BL/6J mice were injected with OT-1 and LM-OVA (1 × 104), as described in Fig. 1. Separate groups of mice received Abs against CD49d i.p.: 100 μg each on days 2–6 (A) or on days 62–66 after LM-OVA infection (B). Control mice received normal rat IgG. Twenty-four hours after the last Ab injection, brains were removed, and the relative numbers of OVA-tetramer+ CD8+ T cells were evaluated after staining cells with anti-CD8 Abs and OVA-tetramers. C, C57BL/6J mice were injected with OT-1 and LM-OVA (1 × 104), as described in Fig. 1, and the expression of CD49d on OVA-tetramer+ CD8+ T cells in the spleen and brain was evaluated at various time intervals. D, C57BL/6 mice were treated as in A, with anti–VCAM-1–blocking Ab; isotype-matched control IgG was used for the i.p. injections. Brains were removed 24 h after the last Ab injection, and the relative numbers of OVA-tetramer+ CD8+ T cells were evaluated after staining cells with anti-CD8 Abs and OVA-tetramers. Experiments in A–C involved analysis of at least four mice/group and were repeated at least twice; the experiment in D involved the analysis of four mice and was performed once.
We evaluated the expression of CD49d on OVA-specific CD8+ T cells as they differentiated and migrated to the brain. The expression of CD49d was highest at day 5 postinfection (Fig. 6C), and cells that had migrated to the brain expressed the highest levels of CD49d. In both spleen and brain, the numbers of CD49dhi CD8+ T cells decreased progressively to undetectable levels. Taken together, these results suggested that the expression of CD49d is high in Ag-specific CD8+ T cells early on, and this is important for their trafficking to the brain. CD49d interacts with VCAM-1 in the brain on both inflamed endothelial cells (33, 34) and on epithelial cells of the choroid plexus (CP) (35) in the animal model of multiple sclerosis, experimental autoimmune en-cephalomyelitis (EAE). Therefore, we assessed whether blocking with an anti–VCAM-1 Ab following LM-OVA infection during the priming phase would have an effect similar to CD49d blocking. Indeed, this resulted in the blocking of OVA-specific CD8+ T cell entry into the brain, comparable to the CD49d blocking (Fig. 6D). Stopping the blockade of CD49d–VCAM-1 after the first 25 d of treatment resulted in a rebound in the numbers of OVA-specific CD8+ T cells in the brain, indicating that CD8+ T cells may continue to cycle into the brain for ≥1 mo (Supplemental Fig. 1A). After the initial migration of OVA-CD8+ T cells during the primary response, blockade of CD49d during LM-OVA rechallenge in the brain seemed to have little influence on the migration of CD8+ T cells (Supplemental Fig. 1B, 1C).
Localization of Ag-specific CD8+ T cells in the brain
We used an anti-CD8 Ab to deplete CD8+ T cells in the systemic compartments. Because Abs do not cross the BBB, a depletion of brain-derived CD8+ T cells would indicate that these cells may be in spaces accessible to blood. The main exception to Ab permeability in brain is in the CP, where IgG can freely pass through fenestrated blood vessels (36). Anti-CD8 Ab was injected at day 30 after LM-OVA infection, a time point when there is little systemic inflammation present, because L. monocytogenes is cleared rapidly by innate immune cells within the first week of infection (3). Higher doses of anti-CD8 Ab were required to delete cells in the brain compared with the spleen. When 1 μg of Ab was injected, there was a 1000-fold reduction in the numbers of OVA-specific CD8+ T cells in the spleen, but no effect was noted on CD8+ T cells in the brain (Fig. 4A, 4B). When the dose of anti-CD8 Ab was increased substantially, deletion of OVA-specific CD8+ T cells in the brain was noted. At 2 h after Ab treatment, there was complete deletion of CD8+ T cells in the spleen (~99%), whereas only ~30% of cells were deleted in the brain. At 24 h, complete deletion of OVA-specific CD8+ T cells was observed in the brain (Fig. 4C).
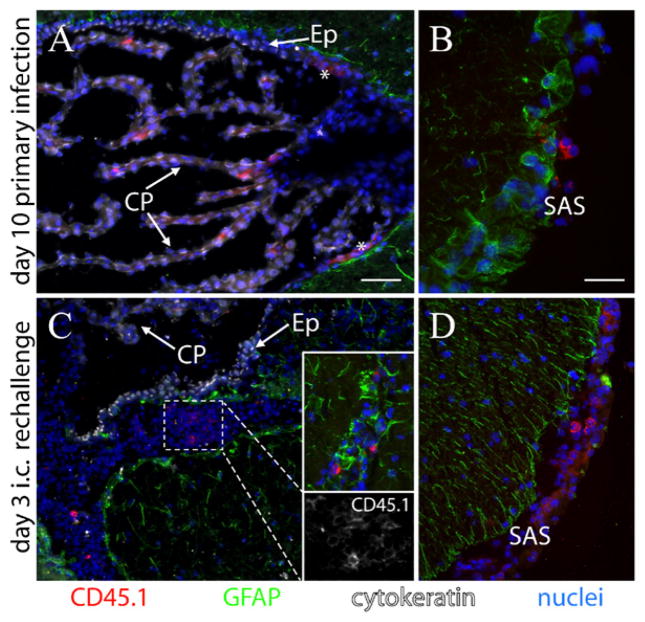
We used frozen tissue sections to examine where in the brain OVA-specific T cells were located. In individual mouse brains examined at days 10 and 14 post-i.v. infection, CD45.1+ OT-1 CD8+ T cells that had been injected into CD45.1−CD45.2+ hosts were observed primarily within the CP (Fig. 7A). These cells were also observed in the subarachnoid space (SAS) underlying the meninges surrounding the brain (Fig. 7B). Of the 53 cells that were positive for CD45.1 or CD8a observed in these two brains, 43 were in the CP, 10 were in the SAS, and none were in the parenchyma. In the CP, these T cells typically displayed a very polarized appearance (with the nucleus restricted to one side of the cell), in contrast to those found in the SAS, which exhibited CD45.1 staining more evenly distributed around the cell. Similar results were obtained when cells were stained with an anti-CD8a Ab (Supplemental Fig. 2). In the absence of an LM-OVA infection, this lymphocyte staining in the brain was not observed in a mouse injected i.v. with naive OT-1 CD8+ T cells (Supplemental Fig. 2).
FIGURE 7.
CD8+ T cell migration into the brain following systemic LM-OVA infection and i.c. rechallenge. A and B, Photomicrographs taken 10 d post-i.v. injection of LM-OVA (1 × 103) into a CD45.2+ host previously injected with CD45.1+ OT-1 CD8+ T cells, as described in Fig. 1. In the brain, CD45.1+ cells were found only in the CP (A) and the SAS (B). Asterisks in A denote diffuse staining, consistently observed near the ependymal (Ep)–CP transition zone of the third ventricle with CD45.1 Ab (shown) and CD8 Ab (not shown). C, At day 30 after vaccination, we rechallenged a group of vaccinated mice with LM-OVA i.c. (1 × 103). Three days later, masses of cells had moved into a space on the parenchymal side of the ependymal lining of the third ventricle. Only a subset of these was CD45.1+ cells (bottom inset; CD45.1 staining alone is shown from a cluster of these cells). Individual CD45.1+ cells were also observed in the parenchyma in the region of the brain near the injection site (top inset). The other prominent location of CD45.1+ cells at this time point, and later, was within the SAS (D). Scale bars, 50 μm (A, C, D) and 25 μm (B). Individual mice were evaluated at days 10 and 14 post-i.v. infection and days 3, 5, 7, and 10 post-i.c. rechallenge. All staining was repeated at least twice.
We then determined where these CD8+ T cells (CD45.1+) migrated to, in response to an i.c. rechallenge of LM-OVA. Previously vaccinated (104 LM-OVA, i.v.) mice were rechallenged on day 30 in the anterior forebrain with LM-OVA (103). Limited CD45.1 or CD8a staining was observed in the CP in individual brains at 3, 5, 7, and 10 d postrechallenge. Rather, these cells were found in large clusters in CSF-filled spaces, including the ventricles (Fig. 7C) and SAS (Fig. 7D). Hundreds of CD8a+ and CD45.1+ cells were observed in these two locations in each brain. Many of these cells were also observed in the parenchyma around the injection site of LM-OVA (Fig. 7C, top inset), although not in large clusters. In mice that received a control saline brain injection instead of LM-OVA rechallenge, the CD45.1+ cells remained in the CP and were not observed to move out into regions where these cells had migrated to following LM-OVA rechallenge (Supplemental Fig. 3).
Activated OVA-specific CD8+ T cells can migrate from the parenchyma to the lymphoid compartment
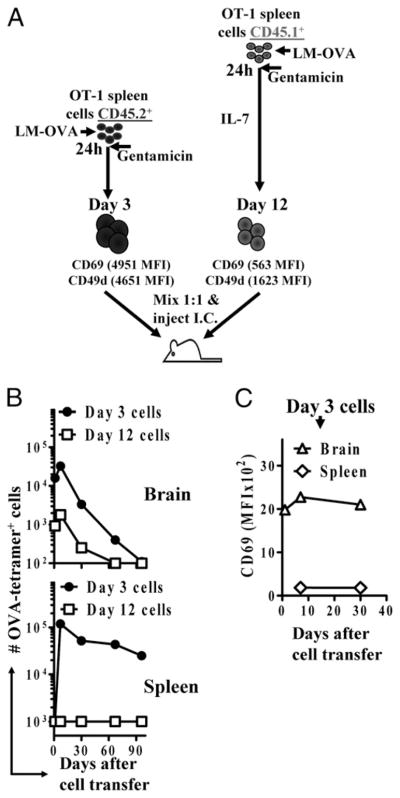
We then went on to investigate the fate of OVA-specific T cells injected directly into the brain parenchyma. We wished to determine whether cells that were stimulated with LM-OVA would remain in the parenchyma or migrate out of the brain upon transfer. We injected recipient mice simultaneously with a mixture of cells that were stimulated in vitro with LM-OVA 3 d (CD45.2+) or 12 d (CD45.1+) prior to transfer. In both cases, CD8+ T cells were purified, mixed 1:1, and injected (~1 × 106 cells) into CD45.2+ C57BL/6J hosts (Fig. 8A). Although a million cells were transferred, far fewer cells were recovered at 24 h after transfer, with the recently stimulated cells displaying better initial take. Beyond day 1, cells underwent expansion followed by a protracted and total contraction in the brain (Fig. 8B). At day 1 after transfer, cells were not detectable in the spleen. Interestingly, recently stimulated cells were detectable at day 7 in the spleens, indicating that activated CD8+ T cells have the potential to migrate out of the parenchyma back into the peripheral circulation. Cells that migrated to the spleen underwent little contraction subsequently, whereas their counterparts that remained in the brain underwent protracted and massive contraction (Fig. 8B). More interestingly, in the day-3 cells, although the CD8+ T cell population in the brain remained CD69hi, the injected OT-1 cells found in the spleen were CD69low (Fig. 8C). These results indicated that the same population undergoes differential contraction in the spleen and brain, which correlates with the phenotype of the cells in each organ. Cells that migrated from the brain to the spleen persisted and expressed undetectable levels of CD69, whereas cells that remained in the brain underwent contraction and expressed high levels of CD69.
FIGURE 8.
Activated CD8+ T cells that migrate out of the brain undergo a reduced contraction in the periphery. OT-1 spleen cells were stimulated in vitro for 3 d (CD45.2+) or 12 d (CD45.1+), as described in Materials and Methods. A, CD8+ T cells were quantified and mixed 1:1 and injected i.c. (~106/mouse) into the anterior forebrain of naive C57BL/6J recipients. At various time intervals, the numbers of CD45.1+ and CD45.2+ OVA-specific CD8+ T cells (B) and the expression of CD69 (C) were evaluated in the spleen and brain of recipient mice by flow cytometry. Each experiment involved the analysis of at least four mice/group and was repeated at least twice.
To find where the OT-1 cells injected into the parenchyma can migrate to, we injected in vitro-activated OT-1 CD8+ T cells, as described in Fig. 8 (3 d postactivation), to track the fate of these cells by immunohistochemistry. Approximately one million cells were injected into the anterior forebrain of naive mice (Fig. 9A). At 1–3 d postinjection, clusters of cells were observed in only two locations: the region adjacent to the bottom of the injection tract and CSF-filled spaces. The CSF-filled spaces included the SAS and the lateral ventricle on the side ipsilateral to the injection (Fig. 9B–D). They were not observed on the side contralateral to the injection. Within the lateral ventricle where they were present, cells were located both inside and outside of the CP (Fig. 9C, 9D). These cells had a polarized morphology similar to those observed in the CP in response to an i.v. LM-OVA infection (Fig. 7). Cells remaining inside the CP were still easily observable by 3 d postinjection (Fig. 9D).
FIGURE 9.
Ag-specific CD8+ T cells injected into the parenchyma can migrate into the CP. OVA-specific CD8+ T cells were generated, as described in Fig. 8, and used at day 3 following activation. A, Horizontal profile of an embedded, sectioned mouse brain injected with activated OT-1 CD8+ T cells. The injections were placed in the upper left region of the brain (red circle). B, A cluster of CD45.1+ cells (shown) and CD8+ cells (not shown) were easily observable near the bottom of the injection tracts. Outside of the injection tracts, cells were mainly found in CSF-filled spaces. In the lateral ventricle on the side ipsilateral to the injection, CD45.1+ (shown) and CD8+ (not shown) cells could be found entering into and within the CP at 1 d (C) and 3 d (D) postinjection. Scale bars, 50 μm (B) and 25 μm (C, D). Two mice each were evaluated at 1 and 3 d post-injection, and staining was repeated at least twice. lv, lateral ventricle; 3rd v, third ventricle.
Discussion
Subsequent to the initial pathogen encounter, <10% of primed CD8+ T cells persist as memory cells (11, 14). Subsets of these memory T cells home to lymphoid (central memory cells) and nonlymphoid (effector memory cells) organs to mediate protection against a subsequent encounter with the same pathogen (21, 27). From a host’s perspective, this model of T cell differentiation makes sense because pathogen encounters may occur often in nonlymphoid areas, and memory T cells in these areas (effector-memory cells) may provide rapid protection against the pathogen. However, nonlymphoid organs vary in physiology and function (37, 38). Therefore, it is pertinent to ask whether similar T cell-trafficking and -homing mechanisms apply to all nonlymphoid organs. The brain is a nonlymphoid organ that is uniquely separated from systemic traffic by the BBB and the blood–CSF barrier (BCSFB). Thus, T cells that immigrate to the brain will have to cross an extra hurdle. Therefore, important questions are how and under what circumstances do T cells home to the brain and how long do T cells persist there to mediate protection.
The selective migration of a subset of activated T cells (effector-memory) to nonlymphoid organs has been shown in various models (20, 21). These T cells express reduced levels of the lymph node-homing receptor CD62L and chemokine receptor CCR7 (21) and mediate effector functions rapidly (24). Our results indicated graded expression of CD62L in differentiating CD8+ T cells (Fig. 2), and this most likely influences the subsequent migration of the cells (37). An important aspect of CD8+ T cells in the brain was their persistently elevated expression of CD69, a marker of early activation. Indeed, T cells in the brain have been shown to express high levels of CD69 following viral infections (39, 40). CD69+ CD8+ T cells are generated immediately after activation by LM-OVA, and such cells are undetectable in the spleen and other lymphoid organs by day 7 postactivation (41). Our results suggested the possibility that rather than being deleted rapidly, CD69hi CD8+ T cells continue to circulate through the brain.
Our results indicated that the initial homing of CD8+ T cells to the brain is primarily dependent on CD49d and VCAM-1. In the brain, VCAM-1 is expressed in the CP epithelium and parenchymal blood vessels (35, 42). Because blocking CD49d function had no effect on the brain CD8+ T cell population by 60 d following infection, the cells either eventually became noncirculating residents in the brain or entered in low numbers through a CD49d-independent mechanism at this later time. Our preliminary evidence indicated that CD8+ T cells continue to cycle into the brain for ≥1 month following systemic infection (Supplemental Fig. 1A). However, we have not been able to determine whether some of this entry is CD49d/VCAM-1 independent. Further work with selective VCAM-1 ablation in a transgenic model would likely be necessary to determine this precisely.
The requirement for CD49d as a key mediator of homing of Ag-specific CD8+ T cells to the brain is consistent with its role in EAE (33). CD49d forms the α-chain of α4β1 integrin (VLA-4), which is expressed on most peripheral lymphocytes, thymocytes, and monocytes (43). α4β1 integrins interact with VCAM-1 to mediate cell–cell interactions (43–45). Abs against α4β1 integrins were shown to prevent EAE in mice (33), and an inhibitory α4 integrin Ab is one of the most potent drugs used to stem the progression of multiple sclerosis in humans (31). It would be of interest to determine whether the role of α4 integrin mediating T cell entry into the brain changes over time in multiple sclerosis, because this may have implications for drug efficacy. It is interesting that the initial mechanism of T cell entry into the brain may be generalized between an autoimmune condition and a response to bacterial infection.
Our histological analysis showed the Ag-specific CD8+ cells entering the brain following an i.v. infection resided in the CP and SAS (Fig. 7). The majority of CD4+ T cells in human CSF have been considered to migrate through the CP (30, 46). The CP is the main structure of the BCSFB; blood vessels within the CP have a limited barrier function (36, 42). Lymphocytes within the stroma of the CP have exited the blood, but they remain behind a barrier formed by the CP epithelium. It was proposed that lymphocytes migrate across this epithelial barrier and through the CSF as a normal path of migration through the human brain under normal physiological conditions (30). Interestingly, T cells are found in disproportionately high numbers in human CSF compared with blood, indicating their selective migration into the CSF. Our data support the idea that T cells migrate through the CP and into the CSF (with cells being found in the CSF of the SAS in this study) following a systemic bacterial infection. Following a reinfection in the brain of immunized mice, the Ag-specific CD8+ T cells were found to have expanded in the ventricles and SAS and entered into the brain parenchyma near the site of infection (Fig. 7).
A recent study demonstrated that CD8+ T cells can form long-term resident memory cells in the brain (47). However, this was in the context of a viral infection in the brain. In this case, CD103 was found to be a marker of CD8+ T cells that were retained in the brain, with its expression appearing after these cells were in the brain for ~20 d. As the investigators of that study pointed out, and in agreement with our study, long-term resident CD103+ cells did not form in the absence of Ag in the brain. Indeed, we only found CD103+ CD8+ T cells in the brain following a rechallenge of LM-OVA injected into the brain (Supplemental Fig. 4). CD103 is not expressed on brain CD8+ T cells in the case of a systemic LM-OVA infection in which Ag is not present in the brain (47) (K. Young, S. MacLean, S. Sad, unpublished observations).
Our study indicated that although activated T cells home to CP, they are progressively eliminated over the period of a few months in the mouse brain. Because these cells do not express memory markers, it is understandable that they do not persist. We propose that effector T cells, not memory cells, play a key role in mediating immune surveillance in the brain following a peripheral infection. This subset of T cells likely circulates through the BCSFB, a normal route of T cell entry into the noninflamed CNS. Only following infection within the brain do these cells undergo a secondary activation and migrate into the parenchyma. The lack of expression of memory markers on CD8+ T cells in the brain may ensure that the cells that traffic to the brain do not persist there for the host’s lifetime, thereby avoiding undesirable inflammation of the brain every time a host encounters an infection in the peripheral compartment.
Supplementary Material
Acknowledgments
We thank Bodgan Zurakowski for performing i.c. injections.
This work was supported by funds from the National Research Council of Canada and a grant from the Canadian Institutes of Health Research.
Abbreviations used in this article
- BBB
blood–brain barrier
- BCSFB
blood–cerebrospinal fluid barrier
- BHI
brain–heart infusion
- CP
choroid plexus
- CSF
cerebrospinal fluid
- EAE
experimental autoimmune encephalomyelitis
- i.c
intracranial(ly)
- LM-OVA
OVA-expressing Listeria monocytogenes
- SAS
subarachnoid space
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Hayashi T, Nagai S, Fujii H, Baba Y, Ikeda E, Kawase T, Koyasu S. Critical roles of NK and CD8+ T cells in central nervous system listeriosis. J Immunol. 2009;182:6360–6368. doi: 10.4049/jimmunol.0803798. [DOI] [PubMed] [Google Scholar]
- 2.Edelson BT, Unanue ER. Immunity to Listeria infection. Curr Opin Immunol. 2000;12:425–431. doi: 10.1016/s0952-7915(00)00112-6. [DOI] [PubMed] [Google Scholar]
- 3.Unanue ER. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr Opin Immunol. 1997;9:35–43. doi: 10.1016/s0952-7915(97)80156-2. [DOI] [PubMed] [Google Scholar]
- 4.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 5.Kägi D, Ledermann B, Bürki K, Hengartner H, Zinkernagel RM. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur J Immunol. 1994;24:3068–3072. doi: 10.1002/eji.1830241223. [DOI] [PubMed] [Google Scholar]
- 6.White DW, Harty JT. Perforin-deficient CD8+ T cells provide immunity to Listeria monocytogenes by a mechanism that is independent of CD95 and IFN-gamma but requires TNF-alpha. J Immunol. 1998;160:898–905. [PubMed] [Google Scholar]
- 7.Bevan MJ. Antigen presentation to cytotoxic T lymphocytes in vivo. J Exp Med. 1995;182:639–641. doi: 10.1084/jem.182.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 9.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 10.Henkart PA. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity. 1994;1:343–346. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 12.Sprent J, Tough DF. T cell death and memory. Science. 2001;293:245–248. doi: 10.1126/science.1062416. [DOI] [PubMed] [Google Scholar]
- 13.Wong P, Pamer EG. CD8 T cell responses to infectious pathogens. Annu Rev Immunol. 2003;21:29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- 14.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 15.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 16.Dutton RW, Swain SL, Bradley LM. The generation and maintenance of memory T and B cells. Immunol Today. 1999;20:291–293. doi: 10.1016/s0167-5699(98)01415-7. [DOI] [PubMed] [Google Scholar]
- 17.Walunas TL, Bruce DS, Dustin L, Loh DY, Bluestone JA. Ly-6C is a marker of memory CD8+ T cells. J Immunol. 1995;155:1873–1883. [PubMed] [Google Scholar]
- 18.Budd RC, Cerottini JC, Horvath C, Bron C, Pedrazzini T, Howe RC, MacDonald HR. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- 19.Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J Immunol. 1998;161:5338–5346. [PubMed] [Google Scholar]
- 20.Usherwood EJ, Hogan RJ, Crowther G, Surman SL, Hogg TL, Altman JD, Woodland DL. Functionally heterogeneous CD8(+) T-cell memory is induced by Sendai virus infection of mice. J Virol. 1999;73:7278–7286. doi: 10.1128/jvi.73.9.7278-7286.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 22.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 23.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 24.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 25.Unsoeld H, Krautwald S, Voehringer D, Kunzendorf U, Pircher H. Cutting edge: CCR7+ and CCR7− memory T cells do not differ in immediate effector cell function. J Immunol. 2002;169:638–641. doi: 10.4049/jimmunol.169.2.638. [DOI] [PubMed] [Google Scholar]
- 26.Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 27.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 28.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, Haqqani AS, Kreymborg K, Krug S, Moumdjian R, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9:137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- 30.Ransohoff RM, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 31.Kerfoot SM, Norman MU, Lapointe BM, Bonder CS, Zbytnuik L, Kubes P. Reevaluation of P-selectin and alpha 4 integrin as targets for the treatment of experimental autoimmune encephalomyelitis. J Immunol. 2006;176:6225–6234. doi: 10.4049/jimmunol.176.10.6225. [DOI] [PubMed] [Google Scholar]
- 32.Dudani R, Chapdelaine Y, Faassen Hv H, Smith DK, Shen H, Krishnan L, Sad S. Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. J Immunol. 2002;168:5737–5745. doi: 10.4049/jimmunol.168.11.5737. [DOI] [PubMed] [Google Scholar]
- 33.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 34.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steffen BJ, Breier G, Butcher EC, Schulz M, Engelhardt B. ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am J Pathol. 1996;148:1819–1838. [PMC free article] [PubMed] [Google Scholar]
- 36.Seitz RJ, Heininger K, Schwendemann G, Toyka KV, Wechsler W. The mouse blood-brain barrier and blood-nerve barrier for IgG: a tracer study by use of the avidin-biotin system. Acta Neuropathol. 1985;68:15–21. doi: 10.1007/BF00688950. [DOI] [PubMed] [Google Scholar]
- 37.Román E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med. 2002;196:957–968. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freitas AA, Rocha B. Population biology of lymphocytes: the flight for survival. Annu Rev Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- 39.Bergmann CC, Altman JD, Hinton D, Stohlman SA. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J Immunol. 1999;163:3379–3387. [PubMed] [Google Scholar]
- 40.van der Most RG, Murali-Krishna K, Ahmed R. Prolonged presence of effector-memory CD8 T cells in the central nervous system after dengue virus encephalitis. Int Immunol. 2003;15:119–125. doi: 10.1093/intimm/dxg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luu RA, Gurnani K, Dudani R, Kammara R, van Faassen H, Sirard JC, Krishnan L, Sad S. Delayed expansion and contraction of CD8+ T cell response during infection with virulent Salmonella typhimurium. J Immunol. 2006;177:1516–1525. doi: 10.4049/jimmunol.177.3.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolburg H, Paulus W. Choroid plexus: biology and pathology. Acta Neuropathol. 2010;119:75–88. doi: 10.1007/s00401-009-0627-8. [DOI] [PubMed] [Google Scholar]
- 43.Holzmann B, McIntyre BW, Weissman IL. Identification of a murine Peyer’s patch—specific lymphocyte homing receptor as an integrin molecule with an alpha chain homologous to human VLA-4 alpha. Cell. 1989;56:37–46. doi: 10.1016/0092-8674(89)90981-1. [DOI] [PubMed] [Google Scholar]
- 44.Ferguson TA, Kupper TS. Antigen-independent processes in antigen-specific immunity. A role for alpha 4 integrin. J Immunol. 1993;150:1172–1182. [PubMed] [Google Scholar]
- 45.Romanic AM, Graesser D, Baron JL, Visintin I, Janeway CA, Jr, Madri JA. T cell adhesion to endothelial cells and extracellular matrix is modulated upon transendothelial cell migration. Lab Invest. 1997;76:11–23. [PubMed] [Google Scholar]
- 46.Kivisäkk P, Mahad DJ, Callahan MK, Trebst C, Tucky B, Wei T, Wu L, Baekkevold ES, Lassmann H, Staugaitis SM, et al. Human cerebro-spinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci USA. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.