Abstract
Hyperosmotic stress induces cytoskeleton reorganization and a net increase in cellular F-actin, but the underlying mechanisms are incompletely understood. Whereas de novo F-actin polymerization likely contributes to the actin response, the role of F-actin severing is unknown. To address this problem, we investigated whether hyperosmolarity regulates cofilin, a key actin-severing protein, the activity of which is inhibited by phosphorylation. Since the small GTPases Rho and Rac are sensitive to cell volume changes and can regulate cofilin phosphorylation, we also asked whether they might link osmostress to cofilin. Here we show that hyperosmolarity induced rapid, sustained, and reversible phosphorylation of cofilin in kidney tubular (LLC-PK1 and Madin-Darby canine kidney) cells. Hyperosmolarity-provoked cofilin phosphorylation was mediated by the Rho/Rho kinase (ROCK)/LIM kinase (LIMK) but not the Rac/PAK/LIMK pathway, because 1) dominant negative (DN) Rho and DN-ROCK but not DN-Rac and DN-PAK inhibited cofilin phosphorylation; 2) constitutively active (CA) Rho and CA-ROCK but not CA-Rac and CA-PAK induced cofilin phosphorylation; 3) hyperosmolarity induced LIMK-2 phosphorylation, and 4) inhibition of ROCK by Y-27632 suppressed the hypertonicity-triggered LIMK-2 and cofilin phosphorylation. We then examined whether cofilin and its phosphorylation play a role in the hypertonicity-triggered F-actin changes. Downregulation of cofilin by small interfering RNA increased the resting F-actin level and eliminated any further rise upon hypertonic treatment. Inhibition of cofilin phosphorylation by Y-27632 prevented the hyperosmolarity-provoked F-actin increase. Taken together, cofilin is necessary for maintaining the osmotic responsiveness of the cytoskeleton in tubular cells, and the Rho/ROCK/LIMK-mediated cofilin phosphorylation is a key mechanism in the hyperosmotic stress-induced F-actin increase.
Keywords: cytoskeleton, hypertonicty, cell volume, small GTPases
Maintenance of cellular integrity under aniso-osmotic conditions is a vital homeostatic requirement. Accordingly, several mechanisms have evolved to cope with osmotic shock and the corresponding volume perturbation, which either help restore near-normal volume or remodel the cell structure, enabling it to withstand the physical challenge (7, 20, 36). In case of hyperosmotic stress, these compensatory/adaptive responses can be classified into three main categories: activation of solute transport systems (44); transcriptional changes impacting on the synthesis of osmolyte transporters and osmolyte-synthesizing enzymes (7) and cytoskeleton reorganization, which is necessary to maintain structural integrity, and may also contribute to the realization of the other two responses (1, 11, 20, 54, 73).
Regarding cytoskeleton remodeling, hyperosmolarity has been shown to induce a net increase in F-actin content in a variety of cells (28, 29, 37, 50, 55), but neither the underlying mechanism nor the responsible signaling has been sufficiently defined. Actin polymerization depends predominantly on the number of free barbed ends, which act as nuclei to receive actin monomers. Three major mechanisms are known to account for free barbed end generation (14): de novo nucleation, mostly through the Arp2/3 complex; F-actin severing, which cuts an existing filament into two (or more) parts; and uncapping of blocked (capped) barbed ends. Previous studies from our lab have shown that hyperosmotic stress facilitates de novo F-actin assembly preferentially at the cell periphery with the involvement of cortactin-enhanced and Arp2/3-mediated nucleation (21). Although osmotic stress has been reported to impact on signaling pathways that could alter severing and uncapping (42, 47, 52, 53, 55, 57) as well, it is unknown whether such mechanisms also participate in osmotic stress-induced F-actin remodeling.
Filament severing is a “Janus-faced” process, since it may lead to a net increase or decrease in polymerized actin. Severing generates new barbed ends, which facilitates net buildup if the rate of monomer addition to these new ends overrides monomer dissociation. On the other hand, fast and extensive severing can lead to actin depolymerization and rapid loss of actin filaments. Cofilin has emerged as a central F-actin-severing protein, the activity of which is tightly regulated by phosphorylation (3, 27, 38, 46, 68). In neutrophils (46) and tumor cells (9), cofilin-mediated severing is an important component of dynamic lamellipodial activity, operating in synergy with the Arp2/3 complex (68, 69). In Dictyostelium, hyperosmolarity was shown to induce translocation of cofilin to the cortical skeleton, and cofilin overexpression increased the thickness of cortical actin bundles (1). Theoretically, increased cofilin activity or redistribution might play a role in the rapid cortical F-actin assembly in hyperosmotically shocked cells. However, an opposite scenario is also possible; namely, that downregulation of cofilin’s severing activity might contribute to the maintenance of the Arp2/3 complex-generated cortical meshwork. Importantly, cofilin phosphorylation results in decreased severing activity (71), which might help to preserve the F-actin network. However, in mammalian cells the regulation of cofilin by osmotic stress and the potential functional consequences of such an event are unknown.
The primary enzyme that phosphorylates cofilin is LIM kinase (LIMK) (6, 58). Rho kinase (ROCK I) phosphorylates LIMK1 at Thr508 (45) and LIMK2 at Thr505 (61) and enhances the ability of LIMKs to phosphorylate cofilin (39). In addition to the Rho/ROCK pathway, Cdc42 and Rac effector p21-activated kinases (PAKs) can also phosphorylate LIMK1 leading to cofilin phosphorylation (16, 23, 39). Relevant to this, our previous studies have shown that hyperosmotic stress induces a rapid and substantial increase in the level of active Rho in kidney tubular cells (19). Likewise, hyperosmotic exposure caused a fast activation of Rac and Cdc42 in neutrophils (37), Chinese hamster ovary (CHO) (21), and human embryonic kidney (HEK)-293 (66) cells.
Cognizant of this scenario we sought to determine whether hyperosmotic stress increases cofilin phosphorylation, and if so, whether this effect could be mediated by the Rho/ROCK/ LIMK and/or the Rac/PAK/LIMK pathway. We also asked whether cofilin plays a role in the osmotically induced changes in F-actin level and whether altered cofilin activity leads to net F-actin destruction or stabilization during osmotic stress. Our results suggest that hyperosmolarity promotes Rho/ROK/ LIMK-mediated cofilin phosphorylation, which in turn is an important contributor to the shrinkage-triggered rise in F-actin in kidney epithelial cells.
MATERIALS AND METHODS
Chemicals and antibodies
The enhanced chemiluminescence kit was from Amersham Pharmacia Biotech. The protease inhibitor mixture containing 0.8 mg/ml benzamidine-HCl, 0.5 mg/ml aprotinin, 0.5 mg/ml leupeptin, 0.5 mg/ml pepstatin A, and 50 mM phenylmethyl-sulfonyl fluoride (PMSF) was obtained from PharMingen and was dissolved in pure ethanol. The ROCK inhibitor Y-27632, the p38 mitogen-activated protein kinase (designated as p38) inhibitor SB-203580 and the PAK inhibitory peptide PAK18, were from Calbiochem. Rhodamine-labeled phalloidin was purchased from Cytoskeleton. The following antibodies used were polyclonal anti-phospho-cofilin (Ser3), anti-cofilin, anti-phospho-PAK1 (Ser144)/PAK2 (Ser141), and anti-phospho-LIMK1 (Thr508)/LIMK2 (Thr505) and were from Cell Signaling; monoclonal anti-Rac was from Upstate Biotechnology, LIMK1 was from BD Transduction Laboratories; monoclonal anti-Myc (9E10) and polyclonal anti-LIMK2 were from Santa Cruz Biotechnology; antibodies against β-actin, myosin light chain, and tubulin were from Sigma, whereas anti-phospho-myosin light chain was from Abcam. FITC-labeled anti-mouse and Cy3-labeled anti-rabbit and anti-mouse IgG were from Jackson Laboratories; peroxidase-conjugated anti-mouse and anti-rabbit IgG were from Amersham Pharmacia Biotech. Rac/Cdc42 Assay Reagent (PAK1 PBD, agarose) was from Upstate Biotechnology. Small interfering RNAs (siRNAs) targeted against cofilin1 (siCofilin: GGT GTT CAA TGA CAT GAAA) and nonrelevant control (siNR: AUU CUA UCA CUA GCG UGA CUU) were purchased from Dharmacon.
Cell culture
In most experiments LLC-PK1 cells, a renal tubular epithelial cell line with several proximal tubule characteristics, were used. Some experiments were conducted using Madin-Darby canine kidney (MDCK) cells (a distal tubular line), the fibroblast-like CHO cells or Ehrlich Letter Ascites (ELA) tumor cells. We previously generated an LLC-PK1 cell line that stably expresses a retroviral vector encoding for a nonphosphorylatable, dominant negative version of myosin regulatory light chain (MLC), in which T18 and S19 were replaced with alanine (AA-MLC) (18). The epithelial cell lines were grown in Dulbecco’s modified Eagle medium (DMEM), whereas the CHO (21) and ELA cells (53) were cultured in α-minimal essential medium and RPMI-1640 medium, respectively. The growth media contained 25 mM NaHCO3 and were supplemented with 10% fetal calf serum and 1% antibiotic suspension (penicillin and streptomycin, Sigma). Cells were grown in a humidified air-CO2 (19:1 ratio) atmosphere at 37°C.
Media
Bicarbonate-free RPMI 1640 was buffered with 25 mM HEPES to pH 7.4 (HPMI, osmolarity 290 ± 5 mosM). The isotonic sodium medium (290 ± 5 mosM) consisted of (in mM) 130 NaCl, 3 KCl, 1 MgCl2, 1 CaCl2, 5 glucose, and 20 HEPES (pH 7.4). If not otherwise stated, hypertonic solution was obtained by supplementing the isotonic solution with 300 mM sucrose. In experiments in which other levels of hyperosmolarity were used, the desired value was set by adding the corresponding concentration of sucrose to the isotonic medium.
Constructs and cell transfection
The plasmids encoding the Myc epitope-tagged dominant-negative (DN) forms of RhoA [RhoA(TN19)myc], Rac1 [Rac1(T17N)myc], and Cdc42 [Cdc42(T17N)myc], as well as the constitutively active (CA) RhoA [RhoA(Q63L)myc], Rac1 [Rac1(Q61L)myc, and Cdc42(Q61L)myc] were kindly provided by Dr. G. Bokoch (Scripps Research Institute, La Jolla, CA) (5) and were used as described in our earlier studies (19, 21). Expression vectors containing cDNAs encoding the constitutively active p160 Rho-associated kinase I ROK-CATmyc, (designated as CA-ROCK) and the dominant-negative p160 Rho-associated kinase I ROK-RB/PHmyc (DN-ROCK) were described previously (41, 48). All these mutants are NH2 terminally Myc tagged. The Myc-tagged constitutively active PAK1 (CA-PAK; H83,86L/ T422E) and dominant negative PAK1 (DN-PAK1; H83,86L/K299R) were kindly provided by Dr. A. S. Mak and have been used in our earlier studies (59). Transient transfection with the corresponding plasmids was performed using FuGene reagent (Roche Molecular Biochemicals) according to the manufacturer’s instructions. Routinely, cells were transfected with 1 μg of plasmid DNA per well (for 6-well plates) or 4 –5 μg of DNA per 10-cm dish. The ratio of plasmid DNA to FuGene reagent was 1 μg to 2.5 μl, respectively. The classical effects of each CA and DN construct on the cytoskeleton were verified using rhodamine phalloidin staining (not shown).
Preparation of cell extracts
Before the experimentation, confluent cell cultures were kept in serum-free HPMI for 3 h. These quiescent cells were then preincubated in isotonic medium for 10 min and subsequently subjected to various treatments as indicated. Cells were treated with isotonic or hypertonic medium for different times as stated. The medium was then aspirated, and the cells were vigorously scraped into an ice-cold lysis buffer containing 100 mM NaCl, 30 mM HEPES, 20 mM NaF, 1 mM EGTA, 1% Triton X-100, pH 7.5, which was supplemented with 1 mM Na3VO4, 1 mM PMSF, and 20 μl/ml protease inhibitor cocktail.
Western blot analysis
Cell lysates diluted in Laemmli buffer were boiled for 5 min, subjected to SDS-PAGE on 10% or 15% gels, and transferred to nitrocellulose by using a semidry apparatus (Bio-Rad). Blots were blocked in Tris-buffered saline containing 5% bovine serum albumin and incubated with the corresponding primary antibody. Binding of the antibody was visualized by using the relevant (anti-mouse, -rabbit, or -goat) peroxidase-conjugated secondary antibodies (1:3,000 dilution) with the enhanced chemiluminescence method.
Rac activity assay
The abundance of active (i.e., GTP bound) small GTPase proteins was followed by affinity pull-down assays as described (5, 37). Confluent cell cultures were serum deprived in HPMI medium for 3 h followed by a 10-min incubation in isotonic medium. Subsequently, the medium was aspirated and replaced with either isotonic or hypertonic medium for the indicated times. Cells were then scraped in ice-cold magnesium lysis buffer [containing 10% glycerol, 25 mM HEPES (pH 7.5), 150 mM NaCl, 1% Igepal CA-630, 10 mM MgCl2, 1 mM EDTA, and 1 mM Na3VO4, supplemented with 1 mM PMSF and 20 μl/ml protease inhibitor cocktail], and the lysates were precleared by brief centrifugation (1 min at 12,500 rpm). To capture active Rac, the supernatants were immediately mixed with 10 μl of a 50% suspension of glutathione beads covered with a fusion protein (≈10 μg) composed of glutathione S-transferase (GST) and the p21-binding domain of the p21-activated kinase (Upstate Biotechnology). After a 30-min rotation at 4°C, the beads were washed four times with magnesium lysis buffer, suspended in Laemmli sample buffer, and boiled for 5 min. Precipitated proteins were subjected to electrophoresis on 15% SDS-polyacrylamide gels, which was followed by Western blot analysis using anti-Rac antibody. To obtain controls for active and inactive GTPases, lysates from untreated cells were supplemented with 0.1 mM GTPγS or 1 mM GDP, respectively, and incubated for 15 min. Nucleotide binding was locked by adding 60 mM MgCl2 to the lysates, and the samples were analyzed.
Immunofluorescence microscopy
Confluent cultures grown on round, 25-mm coverslips were serum deprived for 3 h in HPMI, preincubated for 10 min in isotonic medium, and treated as specified in the figure legends. Subsequently, the cells were fixed for 30 min with 4% paraformaldehyde. The coverslips were extensively washed with PBS, incubated with 100 mM glycine in PBS for 10 min, permeabilized with 0.1% Triton X-100 in PBS for 20 min, and then blocked with 3% BSA or 1% donkey serum in PBS for 1 h. Samples to be stained with the anti-phospho-cofilin were fixed with ice-cold methanol for 6 min and blocked as described. Subsequently, the samples were incubated with primary antibodies for 1 h, washed with PBS, and incubated with fluorescently labeled secondary antibodies for 1 h. The coverslips were washed and mounted on glass slides using mounting solution (DAKO). Samples were viewed using an Olympus IX81 microscope (Melville, NY) (×60 objective), coupled to an Evolution QEi Monochrome camera (Media Cybernetics, Silver Spring, MD), and driven by the ImageQuant acquisition software.
Transfection of siRNA
Kidney tubular epithelial cells (LLC-PK1) were transfected with 20 nM nonrelevant or cofilin siRNA using Lipofectamine RNAiMAX reagent from Invitrogen as per the manufacturer’s instruction. Cells were incubated for 48 h following transfection, without any medium replacement.
Quantification of F-actin
Measurement of cellular F-actin content was based on a rhodamine phalloidin binding and extraction assay, essentially as described in Pedersen et al. (50), with slight modifications. Cells were grown to confluence in six-well plates, serum-deprived for 3 h, and preincubated with isotonic medium for 10 min, which was then aspirated and replaced with either the same isotonic or hypertonic medium (+300 mM sucrose) for 5 min. At the end of this period, the cells were fixed in the corresponding medium supplemented with 2% paraformaldehyde (final concentration) for 10 min at room temperature and placed on ice for another 30 min. The cells were then gently washed three times with PBS, permeablized for 10 min using a saponin buffer (10 mM MOPS, 5 mM EGTA, 20 mM K2HPO4, 2 mM MgSO4, 0.1% saponin, pH 6.9), and then incubated for 1 h in 250 μl of the same solution supplemented with 0.33 μM rhodamine phalloidin. The medium was aspirated, and the cells were washed three times in the MOPS solution without saponin. To extract the bound phalloidin, 2.5 ml of pure methanol were added to each well, and the samples were incubated under gentle shaking for 30 min. The entire volume of methanol from each sample was then transferred into a cuvette, and the fluorescence of each sample was measured by a Photon Technologies (PTI) fluorimeter using 540 and 576 nm for excitation and emission, respectively. The autofluorescence of methanol extracts (determined in the absence of added rhodamine phalloidin) was negligible. Both visual inspection and protein determination (performed after methanol extraction) verified that there was no loss of cells or protein during isotonic or hypertonic treatment. Relative canges in F-actin content are expressed compared with the fluorescence of the isotonic sample (100% = 1).
Protein assays
Protein concentrations were determined by bicinchoninic acid assay (BCA Assay, Pierce) using BSA as a standard.
Densitometry
Quantification of the bands was performed using a Bio-Rad GS-690 Imaging Densitometer and the Molecular Analyst program as described previously (31).
Data are presented as representative immunoblots or photomicrographs of at least three similar experiments or as the means ± SE of the number of experiments indicated (n). Statistical significance was calculated using ANOVA or Student’s t-test.
RESULTS
Hypertonicity induces cofilin phosphorylation in tubular cells
To assess the effect of hyperosmotic stress on cofilin phosphorylation, LLC-PK1 cells were exposed to either isotonic (290 mosM) or hypertonic (590 mosM) media for various times (1 to 30 min) and then lysed and subjected to Western blotting using phospho-cofilin and cofilin antibodies. Hypertonic treatment substantially increased cofilin phosphorylation, an effect that was readily apparent after 1 min, peaked after 2–10 min at about threefold over the isotonic level, and persisted for 30 min, i.e., during the entire duration of these experiments (Fig. 1A). Restoration of isotonicity after hypertonic exposure resulted in a rapid decrease in cofilin phosphorylation, which returned to the prestimulus level within a few minutes (Fig. 1B). The magnitude of cofilin phosphorylation depended on the applied osmotic concentration (Fig. 1C): a discernable increase was attained at mild osmotic stress (325–400 mosM), the maximum was reached at approximately equaling a doubling of the isotonic level (600 mosM), whereas the response tended to diminish at very high osmolarities (900 mosM). MDCK cells, another tubular cell line, also exhibited cofilin phosphorylation upon hyperosmotic stimulation; in these cells the response was even more pronounced (>5-fold) after a 10-min stimulation but appeared to be more transient in nature (Fig. 1D). In contrast, in the fibroblast-like CHO cells, cofilin was highly phosphorylated even under resting (isotonic) conditions, and hyperosmolarity failed to induce any significant change in the level of phospho-cofilin (Fig. 1E). Taken together, these results show that in kidney tubular cells hyperosmotic stress induces rapid, sustained, and reversible cofilin phosphorylation, which shows a bell-shape dependence on osmolarity.
Fig. 1.
Hyperosmolarity induces rapid and reversible cofilin phosphorylation in kidney tubular cells. A: confluent layers of LLC-PK1 proximal tubule cells grown in 6-well plates were preincubated in isotonic (Iso) medium for 10 min and then exposed to iso- or hyperosmolarity (Hyper = isotonic medium supplemented with 300 mM sucrose) for the indicated times. Cells were then lysed, and aliquots of cell lysates containing equal amounts of protein were subjected to Western blot analysis using an anti-phospho-cofilin (pcof) antibody. To check for total cofilin expression and the equality of protein loading, the blots were reprobed using antibodies against cofilin (cof) and actin. Blot is not shown for the isotonic full time course. Routinely a 5-min isotonic treatment was used as control (labeled as Iso), since maximal responses (plateau) to hyperosmolarity were usually attained at this time point. Bottom: densitometric analysis of the hyperosmolarity-induced cof phosphorylation. Data are expressed as fold change, normalized to the pcof-to-cof ratio of the isotonic sample as 1. Means ± SE for n = 12 separate experiments are shown. B: reversibility of the osmotically provoked cof phosphorylation. Cells were treated iso- or hypertonically for 5 min as in A and then isotonicity was reestablished for the indicated times (min). Subsequently cells were lysed and processed as in A. C: dependence of the hypertonicity-induced cof phosphorylation on the osmotic concentration of the medium. Osmolarity was set to the indicated values using various concentrations of sucrose added to the isotonic medium. After 10 min preincubation in the isotonic medium, cells were challenged with the corresponding level of osmolarity for 5 min and then processed as in A. Changes in pcof levels are expressed compared with isotonic control. D: Madin-Darby canine kidney (MDCK) distal tubular cells exhibit similar cof phosphorylation upon hyperosmotic treatment. Similar conditions were used as in A. E: hyperosmolarity does not increase cof phosphorylation in Chinese hamster ovary (CHO) cells.
Osmotic stress triggers a biphasic response in Rac activity and induces PAK phosphorylation in tubular cells
Depending on the stimulus and cell type, cofilin phosphorylation may be downstream to Rho/ROCK-mediated (45, 61, 67) or Rac/ p21-activated kinase (PAK)-mediated (23, 39) activation of LIMK (and see Ref. 72). Our earlier studies have shown that hyperosmotic stress induces robust and sustained Rho activation in LLC-PK1 cells (19). Whereas hyperosmolarity was also found to stimulate Rac (21, 37, 66) and PAK (10, 13, 56) in certain cell types, the osmotic responsiveness of this small GTPase has not been characterized in LLC-PK1 cells. To assess the potential involvement of the Rac pathway, we first determined the effect of hypertonic stimulation on the level of active Rac and PAK. Exposure of the cells to hypertonicity caused a small but significant increase (1.21 ± 0.04-fold; n = 4) in the precipitated (active) Rac after 30 s, which was followed by a gradual and substantial decrease below the resting level (Fig. 2A). This response was consistent with the early and transient Rac activation observed in HEK-293 cells upon osmotic stimulation (66). Since peripheral translocation of Rac is a strong indicator of Rac activation (64), we followed the Rac distribution upon osmotic stress. In resting cells, Rac was present in the cytosol in a finely punctate manner. Hypertonicity caused early and marked increase in peripheral labeling that manifested as enhanced Rac staining in a narrow line along the cell border, accompanied by Rac accumulation in perinuclear clusters (Fig. 2B). At later times peripheral labeling was still present albeit was less pronounced. Together these data may indicate early Rac activation, followed by deactivation, but they may also be consistent with the possibility that activated Rac binds preferentially to an endogenous effector, and thereby remains “invisible” for the PAK-binding domain of the GST-fusion protein, utilized in the pull-down assay. To substantiate that the apparently modest Rac activation may, nevertheless, have functional consequences, we examined the phosphorylation of PAK, a well-known downstream effect of Rac activation. Hyperosmolarity provoked transient PAK phosphorylation, which peaked at 5 min and decayed thereafter (Fig. 2C). Taken together, these data suggest that LLC-PK1 proximal tubular cells respond to hypertonic stress with a modest and transient activation of Rac and PAK, which is followed by an apparent Rac inactivation.
Fig. 2.
The effect of hyperosmolarity on Rac and PAK in LLC-PK1 cells. A: confluent layers of LLC-PK1 cells in 10-cm dishes were challenged with a hypertonic solution for the indicated time. Cells were then lysed and processed for the Rac activity assay as described under MATERIALS AND METHODS. Before the pull-down assay, an aliquot was taken from each lysate to measure total Rac. Note that hyperosmolarity induced a rapid, small, but significant increase in the amount of captured (GTP-bound) Rac, followed by a substantial decrease (n = 4, *P < 0.05 and *** P < 0.001). B: effect of hyperosmolarity on the intracellular localization of Rac. LLC-PK1 cells, grown to confluence on glass coverslips were treated iso- or hypertonically as indicated. Cells were then fixed and stained for Rac using a primary and a Cy-3-labeled secondary antibody. Note that under isotonic condition Rac distribution is primarily cytosolic, exhibiting some vesicular pattern; after exposure to hypertonicity, the most prominent Rac labeling occurs at the cell periphery, whereas the overall cytosolic staining is reduced. We often observed enhanced and polarized Rac labeling in the perinuclear area. C: hyperosmolarity induces transient PAK phosphorylation. Cells were treated as indicated, and cellular lysates were processed for Western blot analysis using a phospho-specific (Ser 142/144) PAK1/2 antibody. Results of densitometric analyses obtained from 3 independent experiments are shown.
Rho/ROCK but not rac/PAK pathway is the critical regulator of the osmotically induced cofilin phosphorylation
To delineate whether the Rac and/or Rho pathway(s) could be upstream of cofilin phosphorylation in tubular cells, we employed two strategies in the following order: 1) we examined whether constitutively active members of the Rac and Rho pathways are able to promote cofilin phosphorylation; and 2) we investigated whether genetic or pharmacological interference with Rac or Rho signaling alters the hyperosmotically induced cofilin phosphorylation.
Monolayers of LLC-PK1 cells were transfected with Myc-tagged constitutively active (CA) Rac or Rho constructs and 24 – 48 h later were doubly stained for phospho-cofilin and the Myc epitope to indentify the successfully transfected cells. As shown on Fig. 3A, cells expressing CA-Rho but not CA-Rac (Myc-positive, green) displayed enhanced phospho-cofilin labeling compared with their untransfected neighbors. In fact increased cofilin phosphorylation was readily detectable by Western blot analysis in whole cells lysates obtained from CA-Rho-transfected cultures: there was a 1.44-fold increase in the total phospho-cofilin signal, despite the low transfection efficiency (<10%), indicating that very substantial (severalfold) increase occurred in the phospho-cofilin content of CA-Rho-expressing cells (Fig. 3B). No increase in phospho-cofilin content was observed upon transfection of CA-Rac (Fig. 3C, bottom). In accordance with these findings, Myc-tagged CA-ROCK (a downstream effector of Rho) but not CA-PAK (downstream effector or Rac and Cdc42), induced enhanced phospho-cofilin labeling in tubular cells (Fig. 3C). These observations were corroborated by Western blot analysis: transfection of cultures with CA-ROCK caused a significant increase in the total phospho-cofilin content of the lysates, whereas CA-PAK had no effect, despite the fact that the overall PAK expression exceeded that of ROCK, as verified by the Myc-signal (Fig. 3D). Collectively, these findings suggest that in LLC-PK1 cells the Rho/ROCK pathway is a strong inducer of cofilin phosphorylation, whereas the Rac/PAK pathway does not appear to play a major role in the regulation of this process.
Fig. 3.
Constitutively active (CA) Rho and ROCK but not Rac and PAK induce cof phosphorylation in tubular cells. A and B: LLC-PK1 cells grown on coverslips were transfected with one of the indicated constructs (1 μg DNA) encoding for the Myc epitope-tagged constitutively active form of Rho, Rac, ROCK, and PAK. Forty-eight hours later, the cells were briefly washed, fixed, and doubly stained for phosphorylated cof (red) and the Myc epitope (green) to identify the successfully transfected cells. Identical cells on the corresponding images are marked with identical symbols (arrows or asterisks). C: cells grown in 10-cm dishes were transfected with empty vector (Mock), CA-Rho, or CA-Rac and lysed 48 h later. Proteins of the whole cell lysates were separated by SDS-PAGE and subjected to Western blotting using anti-phospho-cof. The blots were reprobed with anti-Rho and anti-Myc antibodies to detect all and endogenous Rho proteins, respectively. Despite the fact that the transfection efficiency was only a few percent (< 10%), a highly significant (P < 0.001) 1.44-fold increase was detected in the phospho-cof content of the CA-Rho-transfected monolayers. This increase corresponds to a 5- to 10-fold increase in the CA-Rho expressing cells. D: cells were transfected with empty vector or Myc-tagged CA-ROCK or CA-PAK1 and then processed as in C. Tubulin was used as loading control. Note that CA-ROCK caused a highly significant increase in the phospho-cof content of the entire cell population (with a few % transfection) originating from a large increase present in the ROCK-expressing cells. CA-PAK failed to increase cof phosphorylation despite the fact that PAK1 expression was stronger than ROCK expression, as verified by the Myc signal.
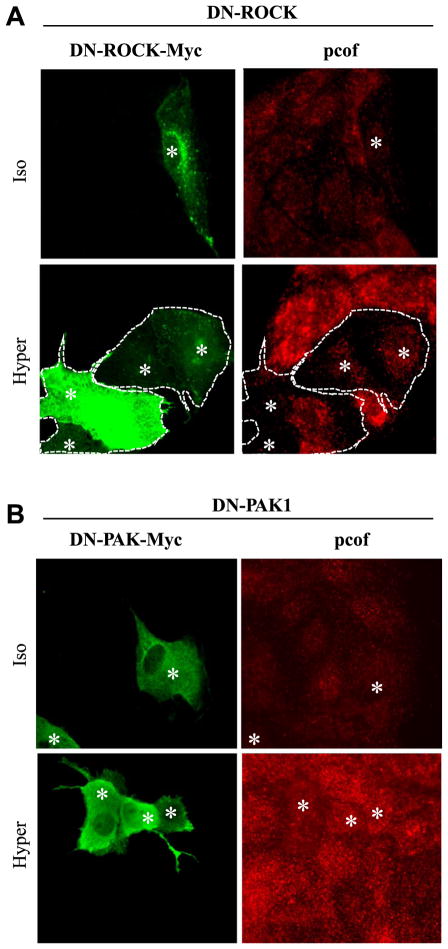
In the following experiments we sought to determine whether the osmotically induced cofilin phosphorylation was mediated by the Rho/ROCK/LIMK pathway. Hyperosmolarity induced a general increase in phospho-cofilin staining in non-transfected cells, which was present in the entire cell interior without showing accumulation in any particular compartment. Expression of dominant negative (DN) Rho fully prevented the rise in phospho-cofilin staining in the cytosol (Fig. 4A). We occasionally observed that elevated phospho-cofilin labeling remained present in the nucleus (see DISCUSSION), but the majority of the response was eliminated. In contrast, DN-Rac did not interfere with the osmotically evoked cofilin phosphorylation (Fig. 4B). In accordance with these findings, DN-ROCK but not DN-PAK abolished the hypertonicity-induced elevation in phospho-cofilin staining (Fig. 5, A and B).
Fig. 4.
Dominant negative (DN) Rho but not DN-Rac prevents the hypertonicity-induced phosphorylation of cof. Cells were transfected with Myc-tagged DN-Rho (A) or DN-Rac (B) constructs, as described under Fig. 3, treated iso- or hypertonically (as indicated), and stained for phospho-cofilin (pcof, red) and the Myc epitope (green). The presence of cells was visualized by the nuclear stain 4,6-diamidino-2-phenylindole (DAPI) (blue). To help identification of the transfected cells on the red image, the area of the corresponding cell(s) was traced with a dashed line. Note that the expressions of DN-Rho almost completely suppressed the hyperosmolarity-triggered increase in pcof staining, whereas the pcof content of DN-Rac-expressing cells is indistinguishable from their nontransfected neighbors. In DN-Rho expressors, we occasionally observed some pcof labeling in nuclear/perinuclear areas.
Fig. 5.
DN-ROCK but not DN-PAK prevents the hypertonicity-induced phosphorylation of cof. Cells were transfected with Myc-tagged DN-ROCK (A) or DN-PAK1 (B) constructs, treated iso- or hypertonically, and stained for pcof (red) and the Myc epitope (green). *Identical cells on the red and green images. In A the contours of the transfected cell clusters are also shown. Note that DN-ROCK strongly reduces the hypertonically provoked rise in pcof labeling, whereas DN-PAK had no effect.
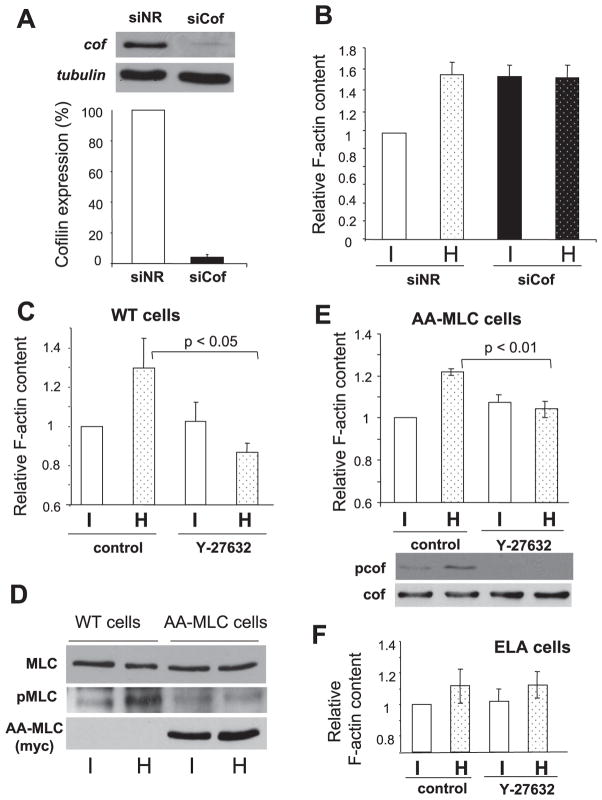
To substantiate these findings with biochemical methods on entire cell populations, we used pharmacological approaches. Pretreatment of cells with the ROCK inhibitor Y-27632 drastically reduced the basal cofilin phosphorylation under isotonic conditions and abolished its hypertonicity-triggered increase, as detected by Western blot analysis (Fig. 6A). In contrast, PAK18, a selective cell-permeable PAK inhibitor failed to prevent cofilin phosphorylation (Fig. 6C). Importantly, hyperosmolarity induced sizable and rapid LIMK2 phosphorylation that reached a plateau at approximately sixfold increase at 5 min (Fig. 6B). Moreover, the hyperosmolarity-provoked LIMK2 phosphorylation was entirely prevented by Y-27632 (Fig. 6B).
Fig. 6.
Hyperosmolarity induces ROCK-mediated LIM kinase and cof phosphorylation. A and B: cells grown in 6-well plates were preincubated for 10 min with vehicle or 10 μM Y-27632 in isotonic medium and then challenged with hyperosmolarity (300 mM sucrose) in the presence or absence of Y-27632 for the indicated times. Subsequently cells were lysed and the lysates were subjected to Western blot anaylsis using pcof and cof antibodies. Cumulated data for n = 6. B: similar experiments were performed as in A, and the lysates were probed with phospho-LIMK and LIMK2 antibodies (n = 4). Blotting with anti-LIMK1 did not provide clear, specific labeling (not shown). C: cells were preincubated with vehicle or 10 μM PAK18, a selective PAK inhibitor, for 20 min under isotonoic condition and then challenged with hyperosmolarity for 5 min in the presence or absence of the drug. Cof and pcof content were determined by Western blots. GADPH was used as a loading control. D, top: cells were pretreated in isotonic medium with or without Y-27632, treated iso-or hypertonically for 5 min (as indicated) in the absence or presence of 10 μM Y-27632, and then processed for Western blotting using an anti-phospho-p38 antibody. The blots were reprobed with anti-p38. Bottom: cells were preincubated with 10 μM SB-203580, treated in the absence or presence of the drug, and processed as stated above. Cell lysates were probed for pcof and reprobed for cof. E: similar experiments were performed as in C, and the lysates were probed with anti-p38 and anti-phospho-p38 antibodies.
A recent report indicated that in endothelial cells vascular endothelial growth factor stimulated LIMK1 by inducing its phosphorylation on Ser-323, a distinct site from the ROCK target Thr-508. This reaction was mediated by MK2, a downstream target of p38. Since hyperosmolarity is a well-known activator of p38, we asked whether p38 could be involved, either upstream or downstream of ROCK, in the hypertonicity-induced cofilin phosphorylation. Figure 6D shows that inhibition of ROCK, which prevented the hyperosmolarity-induced cofilin phosphorylation, had no effect on the hyperosmolarity-induced p38 phosphorylation. Conversely, inhibition of p38 by SB-203580 did not eliminate cofilin phosphorylation; in fact, occasionally we observed elevated basal cofilin phosphorylation under isotonic conditions after preincubation with SB-203580. Interestingly, PAK18 prevented the osmotically provoked p38 phosphorylation (Fig. 6E). Together these findings verify the efficiency of the PAK inhibitor, indicate that PAK is an important mediator of the hyperosmolarity-induced p38 phosphorylation, and argue against the possibility that p38 activation would play a major role in the osmotically induced cofilin phosphorylation in kidney tubular cells. Considered in aggregate, our results suggest that the Rho/ROCK/LIMK pathway is the central mediator of the hypertonicity-induced cofilin phosphorylation.
Functional significance of cofilin phosphorylation: involvement in F-actin increase during osmotic stress
A variety of cell types was reported to respond with cytoskeletal remodeling and particularly with a net increase in their F-actin content to hyperosmotic stress (28, 29, 37, 50, 55). The mechanism underlying this phenomenon remained largely undefined. In the following experiments we sought to address whether cofilin in general and cofilin phosphorylation in particular might play a role in the osmotically triggered F-actin response. To address this question, we compared the effect of hyperosmotic challenge on F-actin changes in control and cofilin-depleted cells. Cells were treated for 48 h either with a nonrelated siRNA or an siRNA designed to silence cofilin (siCof). The siCof construct caused a near-complete (>90%) reduction in cofilin expression, as verified by Western blot analysis (Fig. 7A). Hyperosmotic exposure caused a large increase in the total F-actin content (measured as the extractable Rhodamine-phalloidin) in control siRNA-treated cells, indicating the osmotic sensitivity of the cytoskeleton in kidney tubular cells (Fig. 7B, columns 1 and 2). Interestingly, downregulation of cofilin also led to a remarkable rise in F-actin. The magnitude of this increase was comparable to the osmotically provoked effect. Moreover, hypertonic stimulation of cofilin-depleted cells failed to elicit any further change in their F-actin content (Fig. 7B, columns 3 and 4). These results unambiguously imply that the presence of cofilin is a prerequisite for the osmotic responsiveness of kidney tubular cells, in terms of remodeling of their F-actin skeleton. However, these data do not indicate whether cofilin, or more importantly, its osmotically induced phosphorylation plays a causal role in the osmotic stress-induced F-actin change. To address this question, we tested the effect of Y-27632, which abolished the hypertonically triggered cofilin phosphorylation (Fig. 6A). Importantly, control (untreated) cells responded with a substantial increase in their F-actin content1, whereas Y-27632 completely prevented the hypertonicity-induced rise (Fig. 7C). These observations clearly indicate that the hypertonicity-induced elevation in polymerized actin is a ROCK-dependent process in LLC-PK1 cells.
Fig. 7.
Role of cof and its phosphorylation in the regulation of basal F-actin levels and osmotically induced changes in F-actin content. A: cells were transfected with 20 nM nonrelated small interfering RNA (siNR) or siRNA against cof (siCof) for 48 h as described under MATERIALS METHODS. The efficiency of siCof was evaluated by Western blots, using tubulin as a loading control (n = 9). B: cells grown in 6-well plates were transfected with siNR or siCof, and 48 h later they were serum-deprived, preincubated in isotonic medium for 10 min, and exposed to iso- (I) or hypertonicity (H, 300 mM sucrose) for an additional 5 min. Subsequently, cells were fixed, permeabilized, incubated with 0.33 μM rhodmine-labeled phalloidin for 1 h, and extensively washed. Bound phalloidin (as a measure of total F-actin) was extracted with methanol and quantified fluorimetrically as described under MATERIALS AND METHODS (n = 9). Both visual inspection and parallel protein determinations verified that the confluent layers remained intact during the extraction procedure. C: wild-type (WT) LLC-PK1 cells were incubated with vehicle or 10 μM Y-27632 for 10 min and then challenged with iso- or hypertonic medium (300 mM sucrose) for 5 min. F-actin content was measured as above (n = 5). D: LLC-PK1 cells, stably expressing AA-MLC, a nonphosphorylatable, dominant negative form of the myosin light chain, were treated as in C (n = 6). Bottom: hyperosmolarity induced cof phosphorylation in AA-MLC cells as well is shown. E: expression of AA-MLC inhibits the osmotically triggered phosphorylation of MLC. Wild-type and AA-MLC cells were treated iso- or hypertonically for 5 min. Lysates obtained from these cells were probed with antibodies against Myc (to detect Myc-tagged AA-MLC), phospho-MLC and MLC, as indicated. F: effect of hyperosmolarity and Y-27632 on F-actin content was investigated in ELA cells, under iso- and hypertonic conditions (n = 4).
Whereas the above findings are consistent with a causal role of cofilin phosphorylation in the actin response, an important caveat is that cofilin phosphorylation is not the only ROCK-dependent, cytoskeleton-modulating process. The other ROCK-mediated downstream effect is MLC phosphorylation, which, as we have shown earlier (18, 19), indeed occurs during hyperosmotic stress in LLC-PK1 cells. It was therefore conceivable that MLC phosphorylation (or the consequent MLC activation) might be the critical event. Myosin activity can be blocked pharmacologically with blebbistatin; however, this drug induces drastic alterations in basal F-actin arrangement (18) and fails to prevent MLC phosphorylation per se. Therefore, we addressed the question with a more specific tool that directly targets MLC phosphorylation and does not induce gross alteration in the F-actin structure (18). We have generated an LLC-PK1 cell line that stably expresses AA-MLC, dominant negative light chain mutant in which Thr18 and Ser19, the critical target residues for phosphorylation, were replaced with phenylalanine. In accordance with our earlier findings (18), expression of AA-MLC effectively inhibited the hypertonicity-provoked MLC phosphorylation (Fig. 7D). We considered a potential problem before testing the effect of AA-MLC on the F-actin response: it was conceivable that AA-MLC might inhibit ROCK (as a competitive inhibitor) since wild-type MLC is a direct substrate of ROCK. However, this possibility was excluded since hyperosmolarity elicited comparable cofilin phosphorylation also in AA-MLC cells, and Y-27632 eliminated the basal and stress-induced cofilin phosphorylation (see blots on Fig. 7E). We then tested the effect of hypertonicity: AA-MLC cells responded to osmotic stress with a marked increase in their F-actin content, similar to the parent cells, and Y-27632 prevented the hypertonicity-induced F-actin increase in these cells as well (Fig. 7E). Y-27632 in itself appeared to cause a slight elevation in the basal F-actin, which did not change upon hyperosmotic stress. Taken together, these observations are consistent with the notion that ROCK-mediated cofilin phosphorylation is a key contributor to the F-actin response triggered by hypertonic shock.
The finding that ROCK activity was critical for the cell shrinkage-induced F-actin rise was somewhat surprising, because this process was found to be insensitive to Y-27632 in Ehrlich ascites (EA) cells (49). Since these are suspended (nonattached) cells, it was not clear whether the difference was due to distinct cytoskeletal responses to osmotic stress in attached versus suspended cells, or it reflected cell type-specific differences in the mechanism whereby the F-actin change is brought about. To address this question, we investigated the ROCK-sensitivity of F-actin changes in ELA cells, an attached counterpart of EA cells. Hyperosmolarity provoked an increase in F-actin in ELA cells, albeit their response was smaller than that observed in LLC-PK1 cells. Importantly, Y-27632 did not have any effect on the basal F-actin level or its hypertonicity-induced elevation (Fig. 7F). These findings suggest that a similar, general response (stress-induced rise in F-actin) can be realized by different mechanisms in a cell type-specific manner (see DISCUSSION).
Effects of strong, long-term hyperosmotic stress on cofilin
Nephrotoxicant-induced, apoptosis-promoting stress has been recently shown to reduce cellular cofilin levels in LLC-PK1 cells (17). We were therefore interested whether, in addition to the regulation of cofilin phosphorylation, stronger (and longer lasting) osmotic stress might alter cofilin expression. Exposure of the cells to a medium of 900 mosM for 3 h (a condition that was accompanied by caspase-3 activation; data not shown) induced a strong decrease in their total cofilin content (Fig. 8). Moreover, this sustained and robust hyperosmotic shock was not accompanied by increased cofilin phosphorylation. In fact there was an overall decrease in the phosphorylation of the remaining cofilin compared with the isotonic levels (Fig. 8). This finding was in accordance with our observation that even at early times (5 min) strong hyperosmolarity elicited much less cofilin phosphorylation than moderate osmolarity (Fig. 1C). This suggests that the phosphorylating pathways are less efficient under these extreme conditions and/or cofilin phosphatases are also activated. In any case, these results show that hypertonicity exerts multiple and profound effects on cofilin: short-term, milder osmotic stress induces cofilin phosphorylation, whereas long-term strong osmotic stress induces cofilin degradation and may favor dephosphorylation. These changes, in turn may have major impact on the regulation of the actin skeleton during stress (see DISCUSSION).
Fig. 8.
Effect of long-term, strong hyperosmolarity on cofilin expression and phosphorylation. LLC-PK1 cells were exposed to iso-osmolarity or challenged with strong hyperosmolarity (isotonic Na+ medium supplemented with 600 mM sucrose) for 3 h. The levels of total and pcof were detected by Western blotting and are expressed as percentage compared with the isotonic control (n = 5).
DISCUSSION
The reorganization of the cytoskeleton upon osmotic stress is a ubiquitous response that has been documented in a variety of eukaryotic organisms, including yeast (11), Dictyostelium (1, 73), and mammalian cells (see Ref. 20). Despite the fact that this general phenomenon is thought to be critical for mechanical protection of the challenged cells (30, 54, 73), the underlying mechanisms remained poorly defined. Our current studies indicate that, in kidney tubular cells, hyperosmotic stress induces cofilin phosphorylation via the Rho/ROCK/ LIMK pathway, and this process is a central contributor to the ensuing increase in F-actin. Several lines of evidence support this conclusion: hyperosmotic stress provokes rapid and sustained Rho activation (19) as well as substantial LIMK phosphorylation (current work) with slightly delayed kinetics; genetic or pharmacological inhibition of Rho or ROCK nearly eliminates the shrinkage-induced cofilin phosphorylation and LIMK activation; and importantly, ROCK inhibition prevents the hypertonicity-triggered increase in F-actin. Moreover, downregulation of cofilin elevates the basal F-actin level and cancels further changes on osmotic stimulation. Together these findings imply that basal cofilin activity is essential to render the cell osmotically responsive, whereas the shrinkage-induced inhibition of cofilin activity is a key component of the response itself.
Our results not only provide evidence for the involvement of the Rho/ROCK pathway in the osmotically provoked cofilin phosphorylation but also rule out the participation of the Rac/PAK pathway. The major arguments supporting this view are that the kinetics of the transient Rac and PAK activation do not correspond to the cofilin response and, more importantly, that DN-Rac, DN-PAK, and a PAK inhibitor do not prevent the shrinkage-induced cofilin phosphorylation. In addition, CARac and CA-PAK fail to promote cofilin phosphorylation in tubular cells. The latter findings were somewhat surprising given the fact that Rac and PAK have been described as potent inducers of cofilin phosphorylation in neuronal cells (39). However, recent reports indicate that the overall role of the Rac/PAK pathway in cofilin phosphorylation is very different in epithelial cells. Specifically, both Rac (32, 60) and PAK1 (15) have been shown to promote cofilin dephosphorylation, presumably by activating the cofilin phosphatase, slingshot, and this effect seems the predominant in certain cell types. In agreement with the potential contribution of such mechanism, in some experiments we observed that CA-Rac seemed to reduce cofilin phosphorylation in hypertonically treated cells. Provided that the substantial decrease in precipitated GTP-Rac (after the initial increase) indeed reflects reduced Rac activity during osmotic shock, this effect might contribute to the maintenance of cofilin phosphorylation by suppressing cofilin phosphatase activity. Finally, it is worth mentioning that over-expression of CA-Cdc42 caused a slight increase in cofilin phosphorylation in some cells (data not shown). This finding may be due to the fact that Cdc42 can activate LIMK through myotonic dystrophy kinase-related Cdc42-binding kinase-α (62). The osmosensitivity of this pathway remains to be determined.
Whereas in tubular cells the majority of the osmotically provoked cofilin phosphorylation is mediated by the Rho/ ROCK pathway, alternative mechanisms may also contribute and may even be predominant in other cell types. A recent report shows that in endothelial cells vascular endothelial growth factor stimulates LIMK1 by inducing its phosphorylation on Ser-323, a distinct site from the ROCK target Thr-508. This reaction is mediated by MK2, a downstream target of p38. In endothelial cells, strong osmotic stress increased the activity of LIMK1 and cofilin phosphorylation in a p38-dependent manner (34). Whereas our own data clearly show that the ROCK-dependent cofilin phosphorylation is not mediated by p38, and thus represents a distinct mechanism, the involvement of a minor, p38-dependent component cannot be excluded. Such mechanism may explain our observation that DN-Rho did not fully extinguish cofilin phosphorylation in areas corresponding to the nucleus and raises the notion that nuclear cofilin phosphorylation might be separately regulated. Since MK2, p38, and LIMK can all reside in the nucleus (4, 26, 43, 70) and certain stresses can induce nuclear cofilin translocation (51), it will be interesting to determine whether the Rho/ ROCK/LIMK2 pathway is predominantly responsible for the cytosolic phosphorylation of cofilin, whereas the p38/MK2/ LIMK1 pathway for its nuclear phosphorylation.
A central finding of the present work is that the shrinkage-induced increase in F-actin critically depends on the Rho/ROCK pathway and cofilin in tubular cells. We reported earlier that in these cells the Rho/ROCK pathway mediates the hypertonicity-triggered MLC phosphorylation as well (19), and it has been shown to underlie the hypertonicity-provoked assembly of a novel cytoskeletal structure, myosin-containing striated polygonal actin networks (termed as SPAN) in endothelial cells (40). Thus this route can regulate net F-actin levels, myosin phosphorylation, and the assembly of acto-myosin, implying that it is a central mediator of the overall cytoskeletal response to osmotic stress. However, net F-actin generation is a complex process that, in addition to changes in severing, involves de novo F-actin assembly (nucleation) and altered capping as well (14). The relative contribution of these mechanisms may grossly differ in various cell types. Accordingly, the present study shows that the Rho/ROCK (cofilin) pathway is central in two kidney tubular cell lines, but it plays no major role in ELA cells, and presumably in CHO cells, which fail to respond with increased cofilin phosphorylation. It is worth noting that although ELA cells exhibit Rho activation upon osmotic stress, this response is transient (53). One of the alternative F-actin generating mechanisms may be the shrinkage-induced production of phosphatidylinositol 4,5-bisphosphate (PIP2) (42), which facilitates the dissociation of capping proteins (e.g., gelsolin) from filament ends (63) and also activates the Arp2/3 complex, thereby inducing nucleation (65). The latter process is mediated via Wiskott-Aldrich syndrome protein (WASP) family proteins, which in addition to phosphoinositides, require costimulation with Cdc42 or Rac. These small GTPases are also activated by hypertonicity in certain cells (see Ref. 20), and, at least in neutrophils (37, 55), were shown to be involved in the net F-actin elevation. Moreover, shrinkage (presumably through PIP2) activates members of the ezrin-radixin-moesin family, important organizers of the cortical cytoskeleton (53). Interestingly, downregulation of ezrin significantly reduced the hypertonicity-provoked Rho activation, suggesting that ezrin may be one of the upstream elements linking cell shrinkage to Rho (53). It is worth noting that Rho might enhance F-actin assembly in a ROCK-independent manner as well, through mDia (24). The involvement of this pathway awaits elucidation. Finally, activation of p38 may facilitate F-actin assembly either by inhibiting the filament capping-activity of heat shock protein-27 (52) or by modifying severing via the alternative phosphorylation of LIMK1 (34), as mentioned above. Clearly, these mechanisms do not act in isolation but cooperate (with different weight) in the cell-type-specific realization of the actin response. Our studies suggest that in tubular cells de novo F-actin assembly should be accompanied by decreased severing to ensure a net increase in the F-actin content. It is worth noting that in addition to phosphorylation, cofilin activity may also be modulated by other osmotically regulated factors, such as intracellular pH and PIP2 (22). Future studies are warranted to determine the impact of these parameters in various cell types.
Finally, we address the potential functional role of cofilin and its Rho/ROCK-mediated phosphorylation during osmotic stress. In Dictyostelium, the reorganization of the cortical cytoskeleton (increased F-actin content and bundling together with enhanced myosin-based contractility) were shown to be osmoprotective (35, 54, 73). Coflin was implicated in the process, as its overexpression was accompanied with increased length and thickness of cortical actin bundles during stress (1). To explain this somewhat paradoxical observation, Aizawa et al. (1) proposed that increased severing might be followed by augmented filament annealing. Potential changes in cofilin phosphorylation during osmotic shock have not been investigated in the slime mold. Our studies suggest that in mammalian cells cofilin phosphorylation and consequently decreased F-actin severing, along with enhanced MLC phosphorylation may lead to the same functional outcome: increased cortical rigidity that helps the cells withstand acute mechanical stress. This “frozen” cytoskeletal arrangement may also be responsible for the major (and therapeutically exploited) inhibitory effects of hypertonicity on processes that depend on actin dynamics, including endo- and exocytosis and cell motility (55). Regarding the kidney, hyperosmolarity-induced increase in and reorganization of F-actin has also been documented in intact medullary thick ascending limb of Henle (8). In addition to osmoprotection, such F-actin changes are thought to be involved in volume control (8) and solute transport through the regulation of the activity and trafficking of various ion transporters and water channels (2, 33). Assessment of the potential role of volume-sensitive cofilin phosphorylation in these physiological processes is an attractive area for future research.
In addition to its cytoskeletal functions, cofilin has been implicated in the regulation of apoptosis as well; upon stress cofilin was shown to translocate to the mitochondria, where it facilitates the apoptotic machinery (12). Interestingly, however, only active (nonphosphorylated) cofilin seems to participate in this process. It is therefore conceivable that rapid phosphorylation of cofilin during stress would decrease the translocation-competent pool, thereby delaying apoptosis and providing time for volume-regulatory and other adaptive mechanisms. However, the picture is more complex: Whereas short-term inhibition of cofilin may be useful both for acute mechanoprotection and delayed onset of apoptosis, the overall absence of cofilin is likely deleterious. This view is supported by our finding that deletion of cofilin enhances caspase activation, induced by strong and long-lasting hyperosmolarity (work in preparation). This scenario is likely due to the fact that cofilin downregulation substantially increases the total F-actin in the cells (Fig. 7A), and excessive actin polymerization was found to be an inducer of cellular aging and apoptosis (25). Furthermore, we and others observed that during extreme stress conditions, cofilin is degraded and dephosphorylated, events that may mark the dysregulation of cofilin and the irreversible phase of stress-induced death.
Taken together, the presence and the phosphorylation of cofilin are important for the osmotic responsiveness of the cytoskeleton, the osmotically provoked adaptive cytoskeleton rearrangement, and presumably, for the regulation of survival/ apoptosis in moderate versus extreme stress.
Acknowledgments
We thank Dr. A. Masszi for valuable comments.
GRANTS
This work was supported by research grants from the National Sciences and Engineering Research Council of Canada (A. Kapus), the Kidney Foundation of Canada (to A. Kapus and K. Szászi), the Danish National Research Council and by the Carlsberg Foundation (to S. F. Pedersen). K. Szászi is a recipient of a KRESCENT New Investigator Award (a joint award of the Kidney Foundation of Canada, Canadian Nephrology Society and Canadian Institute of Health Research).
Footnotes
References
- 1.Aizawa H, Katadae M, Maruya M, Sameshima M, Murakami-Murofushi K, Yahara I. Hyperosmotic stress-induced reorganization of actin bundles in Dictyostelium cells over-expressing cofilin. Genes Cells. 1999;4:311–324. doi: 10.1046/j.1365-2443.1999.00262.x. [DOI] [PubMed] [Google Scholar]
- 2.Alexander RT, Furuya W, Szaszi K, Orlowski J, Grinstein S. Rho GTPases dictate the mobility of the Na/H exchanger NHE3 in epithelia: role in apical retention and targeting. Proc Natl Acad Sci USA. 2005;102:12253–12258. doi: 10.1073/pnas.0409197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Levy R, Hooper S, Wilson R, Paterson HF, Marshall CJ. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr Biol. 1998;8:1049–1057. doi: 10.1016/s0960-9822(98)70442-7. [DOI] [PubMed] [Google Scholar]
- 5.Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 6.Bernard O. Lim kinases, regulators of actin dynamics. Int J Biochem Cell Biol. 2007;39:1071–1076. doi: 10.1016/j.biocel.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev. 2007;87:1441–1474. doi: 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- 8.Bustamante M, Roger F, Bochaton-Piallat ML, Gabbiani G, Martin PY, Feraille E. Regulatory volume increase is associated with p38 kinase-dependent actin cytoskeleton remodeling in rat kidney MTAL. Am J Physiol Renal Physiol. 2003;285:F336–F347. doi: 10.1152/ajprenal.00003.2003. [DOI] [PubMed] [Google Scholar]
- 9.Chan AY, Bailly M, Zebda N, Segall JE, Condeelis JS. Role of cofilin in epidermal growth factor-stimulated actin polymerization and lamellipod protrusion. J Cell Biol. 2000;148:531–542. doi: 10.1083/jcb.148.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan PM, Lim L, Manser E. PAK is regulated by PI3K, PIX, CDC42, and PP2Cα and mediates focal adhesion turnover in the hyperosmotic stress-induced p38 pathway. J Biol Chem. 2008;283:24949–24961. doi: 10.1074/jbc.M801728200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhury S, Smith KW, Gustin MC. Osmotic stress and the yeast cytoskeleton: phenotype-specific suppression of an actin mutation. J Cell Biol. 1992;118:561–571. doi: 10.1083/jcb.118.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua BT, Volbracht C, Tan KO, Li R, Yu VC, Li P. Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nat Cell Biol. 2003;5:1083–1089. doi: 10.1038/ncb1070. [DOI] [PubMed] [Google Scholar]
- 13.Clerk A, Sugden PH. Activation of p21-activated protein kinase alpha (alpha PAK) by hyperosmotic shock in neonatal ventricular myocytes. FEBS Lett. 1997;403:23–25. doi: 10.1016/s0014-5793(97)00020-3. [DOI] [PubMed] [Google Scholar]
- 14.Condeelis J. How is actin polymerization nucleated in vivo? Trends Cell Biol. 2001;11:288–293. doi: 10.1016/s0962-8924(01)02008-6. [DOI] [PubMed] [Google Scholar]
- 15.Coniglio SJ, Zavarella S, Symons MH. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol Cell Biol. 2008;28:4162–4172. doi: 10.1128/MCB.01532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dan C, Kelly A, Bernard O, Minden A. Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J Biol Chem. 2001;276:32115–32121. doi: 10.1074/jbc.M100871200. [DOI] [PubMed] [Google Scholar]
- 17.de Graauw M, Tijdens I, Cramer R, Corless S, Timms JF, van de Water B. Heat shock protein 27 is the major differentially phosphorylated protein involved in renal epithelial cellular stress response and controls focal adhesion organization and apoptosis. J Biol Chem. 2005;280:29885–29898. doi: 10.1074/jbc.M412708200. [DOI] [PubMed] [Google Scholar]
- 18.DiCiano-Oliveira C, Lodyga M, Fan L, Szaszi K, Hosoya H, Rotstein OD, Kapus A. Is myosin light-chain phosphorylation a regulatory signal for the osmotic activation of the Na+-K+-2Cl− cotransporter? Am J Physiol Cell Physiol. 2005;289:C68–C81. doi: 10.1152/ajpcell.00631.2004. [DOI] [PubMed] [Google Scholar]
- 19.DiCiano-Oliveira C, Sirokmany G, Szaszi K, Arthur WT, Masszi A, Peterson M, Rotstein OD, Kapus A. Hyperosmotic stress activates Rho: differential involvement in Rho kinase-dependent MLC phosphorylation and NKCC activation. Am J Physiol Cell Physiol. 2003;285:C555–C566. doi: 10.1152/ajpcell.00086.2003. [DOI] [PubMed] [Google Scholar]
- 20.DiCiano-Oliveira C, Thirone AC, Szaszi K, Kapus A. Osmotic stress and the cytoskeleton: the R(h)ole of Rho GTPases. Acta Physiol (Oxf) 2006;187:257–272. doi: 10.1111/j.1748-1716.2006.01535.x. [DOI] [PubMed] [Google Scholar]
- 21.Di Ciano C, Nie Z, Szaszi K, Lewis A, Uruno T, Zhan X, Rotstein OD, Mak A, Kapus A. Osmotic stress-induced remodeling of the cortical cytoskeleton. Am J Physiol Cell Physiol. 2002;283:C850–C865. doi: 10.1152/ajpcell.00018.2002. [DOI] [PubMed] [Google Scholar]
- 22.dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–473. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- 23.Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 24.Geneste O, Copeland JW, Treisman R. LIM kinase and Diaphanous cooperate to regulate serum response factor and actin dynamics. J Cell Biol. 2002;157:831–838. doi: 10.1083/jcb.200203126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gourlay CW, Ayscough KR. The actin cytoskeleton: a key regulator of apoptosis and ageing? Nat Rev Mol Cell Biol. 2005;6:583–589. doi: 10.1038/nrm1682. [DOI] [PubMed] [Google Scholar]
- 26.Goyal P, Pandey D, Siess W. Phosphorylation-dependent regulation of unique nuclear and nucleolar localization signals of LIM kinase 2 in endothelial cells. J Biol Chem. 2006;281:25223–25230. doi: 10.1074/jbc.M603399200. [DOI] [PubMed] [Google Scholar]
- 27.Gungabissoon RA, Bamburg JR. Regulation of growth cone actin dynamics by ADF/cofilin. J Histochem Cytochem. 2003;51:411–420. doi: 10.1177/002215540305100402. [DOI] [PubMed] [Google Scholar]
- 28.Hallows KR, Law FY, Packman CH, Knauf PA. Changes in cytoskeletal actin content, F-actin distribution, and surface morphology during HL-60 cell volume regulation. J Cell Physiol. 1996;167:60–71. doi: 10.1002/(SICI)1097-4652(199604)167:1<60::AID-JCP7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Hallows KR, Packman CH, Knauf PA. Acute cell volume changes in anisotonic media affect F-actin content of HL-60 cells. Am J Physiol Cell Physiol. 1991;261:C1154–C1161. doi: 10.1152/ajpcell.1991.261.6.C1154. [DOI] [PubMed] [Google Scholar]
- 30.Insall RH. Cyclic GMP and the big squeeze. Osmoregulation Curr Biol. 1996;6:516–518. doi: 10.1016/s0960-9822(02)00530-4. [DOI] [PubMed] [Google Scholar]
- 31.Kapus A, Szaszi K, Sun J, Rizoli S, Rotstein OD. Cell shrinkage regulates Src kinases and induces tyrosine phosphorylation of cortactin, independent of the osmotic regulation of Na+/H+ exchangers. J Biol Chem. 1999;274:8093–8102. doi: 10.1074/jbc.274.12.8093. [DOI] [PubMed] [Google Scholar]
- 32.Kligys K, Claiborne JN, DeBiase PJ, Hopkinson SB, Wu Y, Mizuno K, Jones JC. The slingshot family of phosphatases mediates Rac1 regulation of cofilin phosphorylation, laminin-332 organization, and motility behavior of keratinocytes. J Biol Chem. 2007;282:32520–32528. doi: 10.1074/jbc.M707041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klussmann E, Tamma G, Lorenz D, Wiesner B, Maric K, Hofmann F, Aktories K, Valenti G, Rosenthal W. An inhibitory role of Rho in the vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem. 2001;276:20451–20457. doi: 10.1074/jbc.M010270200. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi M, Nishita M, Mishima T, Ohashi K, Mizuno K. MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J. 2006;25:713–726. doi: 10.1038/sj.emboj.7600973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuwayama H, Ecke M, Gerisch G, Van Haastert PJ. Protection against osmotic stress by cGMP-mediated myosin phosphorylation. Science. 1996;271:207–209. doi: 10.1126/science.271.5246.207. [DOI] [PubMed] [Google Scholar]
- 36.Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 37.Lewis A, Di Ciano C, Rotstein OD, Kapus A. Osmotic stress activates Rac and Cdc42 in neutrophils: role in hypertonicity-induced actin polymerization. Am J Physiol Cell Physiol. 2002;282:C271–C279. doi: 10.1152/ajpcell.00427.2001. [DOI] [PubMed] [Google Scholar]
- 38.Maciver SK, Hussey PJ. The ADF/cofilin family: actin-remodeling proteins. Genome Biol. 2002;3:3007. doi: 10.1186/gb-2002-3-5-reviews3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 40.Malek AM, Xu C, Kim ES, Alper SL. Hypertonicity triggers RhoA-dependent assembly of myosin-containing striated polygonal actin networks in endothelial cells. Am J Physiol Cell Physiol. 2007;292:C1645–C1659. doi: 10.1152/ajpcell.00533.2006. [DOI] [PubMed] [Google Scholar]
- 41.Masszi A, Di Ciano C, Sirokmany G, Arthur WT, Rotstein OD, Wang J, McCulloch CA, Rosivall L, Mucsi I, Kapus A. Central role for Rho in TGF-β1-induced α-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol Renal Physiol. 2003;284:F911–F924. doi: 10.1152/ajprenal.00183.2002. [DOI] [PubMed] [Google Scholar]
- 42.Nasuhoglu C, Feng S, Mao Y, Shammat I, Yamamato M, Earnest S, Lemmon M, Hilgemann DW. Modulation of cardiac PIP2 by cardioactive hormones and other physiologically relevant interventions. Am J Physiol Cell Physiol. 2002;283:C223–C234. doi: 10.1152/ajpcell.00486.2001. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen MB, Christensen ST, Hoffmann EK. Effects of osmotic stress on the activity of MAPKs and PDGFR-beta-mediated signal transduction in NIH-3T3 fibroblasts. Am J Physiol Cell Physiol. 2008;294:C1046–C1055. doi: 10.1152/ajpcell.00134.2007. [DOI] [PubMed] [Google Scholar]
- 44.O’Neill WC. Physiological significance of volume-regulatory transporters. Am J Physiol Cell Physiol. 1999;276:C995–C1011. doi: 10.1152/ajpcell.1999.276.5.C995. [DOI] [PubMed] [Google Scholar]
- 45.Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- 46.Okada K, Takano-Ohmuro H, Obinata T, Abe H. Dephosphorylation of cofilin in polymorphonuclear leukocytes derived from peripheral blood. Exp Cell Res. 1996;227:116–122. doi: 10.1006/excr.1996.0256. [DOI] [PubMed] [Google Scholar]
- 47.Okada T, Otani H, Wu Y, Kyoi S, Enoki C, Fujiwara H, Sumida T, Hattori R, Imamura H. Role of F-actin organization in p38 MAP kinase-mediated apoptosis and necrosis in neonatal rat cardiomyocytes subjected to simulated ischemia and reoxygenation. Am J Physiol Heart Circ Physiol. 2005;289:H2310–H2318. doi: 10.1152/ajpheart.00462.2005. [DOI] [PubMed] [Google Scholar]
- 48.Oshiro N, Fukata Y, Kaibuchi K. Phosphorylation of moesin by rho-associated kinase (Rho-kinase) plays a crucial role in the formation of microvilli-like structures. J Biol Chem. 1998;273:34663–34666. doi: 10.1074/jbc.273.52.34663. [DOI] [PubMed] [Google Scholar]
- 49.Pedersen SF, Hoffmann EK. Possible interrelationship between changes in F-actin and myosin II, protein phosphorylation, and cell volume regulation in Ehrlich ascites tumor cells. Exp Cell Res. 2002;277:57–73. doi: 10.1006/excr.2002.5529. [DOI] [PubMed] [Google Scholar]
- 50.Pedersen SF, Mills JW, Hoffmann EK. Role of the F-actin cytoskeleton in the RVD and RVI processes in Ehrlich ascites tumor cells. Exp Cell Res. 1999;252:63–74. doi: 10.1006/excr.1999.4615. [DOI] [PubMed] [Google Scholar]
- 51.Pendleton A, Pope B, Weeds A, Koffer A. Latrunculin B or ATP depletion induces cofilin-dependent translocation of actin into nuclei of mast cells. J Biol Chem. 2003;278:14394–14400. doi: 10.1074/jbc.M206393200. [DOI] [PubMed] [Google Scholar]
- 52.Pichon S, Bryckaert M, Berrou E. Control of actin dynamics by p38 MAP kinase-Hsp27 distribution in the lamellipodium of smooth muscle cells. J Cell Sci. 2004;117:2569–2577. doi: 10.1242/jcs.01110. [DOI] [PubMed] [Google Scholar]
- 53.Rasmussen M, Alexander RT, Darborg BV, Mobjerg N, Hoffmann EK, Kapus A, Pedersen SF. Osmotic cell shrinkage activates ezrin/ radixin/moesin (ERM) proteins: activation mechanisms and physiological implications. Am J Physiol Cell Physiol. 2008;294:C197–C212. doi: 10.1152/ajpcell.00268.2007. [DOI] [PubMed] [Google Scholar]
- 54.Rivero F, Koppel B, Peracino B, Bozzaro S, Siegert F, Weijer CJ, Schleicher M, Albrecht R, Noegel AA. The role of the cortical cytoskeleton: F-actin crosslinking proteins protect against osmotic stress, ensure cell size, cell shape and motility, and contribute to phagocytosis and development. J Cell Sci. 1996;109:2679–2691. doi: 10.1242/jcs.109.11.2679. [DOI] [PubMed] [Google Scholar]
- 55.Rizoli SB, Rotstein OD, Parodo J, Phillips MJ, Kapus A. Hypertonic inhibition of exocytosis in neutrophils: central role for osmotic actin skeleton remodeling. Am J Physiol Cell Physiol. 2000;279:C619–C633. doi: 10.1152/ajpcell.2000.279.3.C619. [DOI] [PubMed] [Google Scholar]
- 56.Roig J, Huang Z, Lytle C, Traugh JA. p21-activated protein kinase gamma-PAK is translocated and activated in response to hyperosmolarity. Implication of Cdc42 and phosphoinositide 3-kinase in a two-step mechanism for gamma-PAK activation. J Biol Chem. 2000;275:16933–16940. doi: 10.1074/jbc.M001627200. [DOI] [PubMed] [Google Scholar]
- 57.Schafer DA, Jennings PB, Cooper JA. Dynamics of capping protein and actin assembly in vitro: uncapping barbed ends by polyphosphoinositides. J Cell Biol. 1996;135:169–179. doi: 10.1083/jcb.135.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scott RW, Olson MF. LIM kinases: function, regulation and association with human disease. J Mol Med. 2007;85:555–568. doi: 10.1007/s00109-007-0165-6. [DOI] [PubMed] [Google Scholar]
- 59.Sebe A, Masszi A, Zulys M, Yeung T, Speight P, Rotstein OD, Nakano H, Mucsi I, Szaszi K, Kapus A. Rac, PAK and p38 regulate cell contact-dependent nuclear translocation of myocardin-related transcription factor. FEBS Lett. 2008;582:291–298. doi: 10.1016/j.febslet.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sehgal BU, DeBiase PJ, Matzno S, Chew TL, Claiborne JN, Hopkinson SB, Russell A, Marinkovich MP, Jones JC. Integrin beta4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J Biol Chem. 2006;281:35487–35498. doi: 10.1074/jbc.M606317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J Biol Chem. 2001;276:670–676. doi: 10.1074/jbc.M007074200. [DOI] [PubMed] [Google Scholar]
- 62.Sumi T, Matsumoto K, Shibuya A, Nakamura T. Activation of LIM kinases by myotonic dystrophy kinase-related Cdc42-binding kinase alpha. J Biol Chem. 2001;276:23092–23096. doi: 10.1074/jbc.C100196200. [DOI] [PubMed] [Google Scholar]
- 63.Sun HQ, Yamamoto M, Mejillano M, Yin HL. Gelsolin, a multifunctional actin regulatory protein. J Biol Chem. 1999;274:33179–33182. doi: 10.1074/jbc.274.47.33179. [DOI] [PubMed] [Google Scholar]
- 64.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 65.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 66.Uhlik MT, Abell AN, Johnson NL, Sun W, Cuevas BD, Lobel-Rice KE, Horne EA, Dell’Acqua ML, Johnson GL. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat Cell Biol. 2003;5:1104–1110. doi: 10.1038/ncb1071. [DOI] [PubMed] [Google Scholar]
- 67.Vardouli L, Moustakas A, Stournaras C. LIM-kinase 2 and cofilin phosphorylation mediate actin cytoskeleton reorganization induced by transforming growth factor-beta. J Biol Chem. 2005;280:11448–11457. doi: 10.1074/jbc.M402651200. [DOI] [PubMed] [Google Scholar]
- 68.Wang W, Eddy R, Condeelis J. The cofilin pathway in breast cancer invasion and metastasis. Nat Rev Cancer. 2007;7:429–440. doi: 10.1038/nrc2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yokoo T, Toyoshima H, Miura M, Wang Y, Iida KT, Suzuki H, Sone H, Shimano H, Gotoda T, Nishimori S, Tanaka K, Yamada N. p57Kip2 regulates actin dynamics by binding and translocating LIM-kinase 1 to the nucleus. J Biol Chem. 2003;278:52919–52923. doi: 10.1074/jbc.M309334200. [DOI] [PubMed] [Google Scholar]
- 71.Zebda N, Bernard O, Bailly M, Welti S, Lawrence DS, Condeelis JS. Phosphorylation of ADF/cofilin abolishes EGF-induced actin nucleation at the leading edge and subsequent lamellipod extension. J Cell Biol. 2000;151:1119–1128. doi: 10.1083/jcb.151.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao ZS, Manser E. PAK and other Rho-associated kinases–effectors with surprisingly diverse mechanisms of regulation. Biochem J. 2005;386:201–214. doi: 10.1042/BJ20041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zischka H, Oehme F, Pintsch T, Ott A, Keller H, Kellermann J, Schuster SC. Rearrangement of cortex proteins constitutes an osmoprotective mechanism in Dictyostelium. EMBO J. 1999;18:4241–4249. doi: 10.1093/emboj/18.15.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]