Abstract
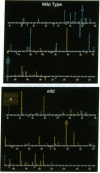
We have analyzed the spectra of UV-induced mutations in the lacI gene of a wild-type and an mfd strain of Escherichia coli. mfd strains have been recently proposed to be deficient in a factor coupling DNA repair and transcription. Analysis of UV-induced mutations occurring at adjacent pyrimidines showed that mutations in the wild-type strain arose largely from the nontranscribed strand but arose predominantly from the transcribed strand in the mfd strain. The overall strand switch was 14-fold. One mutation, G.C-->A.T in the lacI initiation codon, showed a > 300-fold shift. No effect was observed for mutations at non-pyrimidine-pyrimidine sequences. These results provide in vivo evidence for a key role of the mfd gene in controlling the strandedness of mutagenesis and support the proposed role of the mfd gene product in directing DNA excision repair to the transcribed strand of a damaged gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bockrath R. C., Palmer J. E. Differential repair of premutational UV-lesions at tRNA genes in E. coli. Mol Gen Genet. 1977 Nov 14;156(2):133–140. doi: 10.1007/BF00283485. [DOI] [PubMed] [Google Scholar]
- Bockrath R., Barlow A., Engstrom J. Mutation frequency decline in Escherichia coli B/r after mutagenesis with ethyl methanesulfonate. Mutat Res. 1987 May;183(3):241–247. doi: 10.1016/0167-8817(87)90006-x. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Hanawalt P. C. Enhanced repair of pyrimidine dimers in coding and non-coding genomic sequences in CHO cells expressing a prokaryotic DNA repair gene. Carcinogenesis. 1987 Sep;8(9):1333–1336. doi: 10.1093/carcin/8.9.1333. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Haseltine W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature. 1982 Jul 8;298(5870):189–192. doi: 10.1038/298189a0. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Seetharam S., Kraemer K. H., Seidman M. M., Bredberg A. Photoproduct frequency is not the major determinant of UV base substitution hot spots or cold spots in human cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3782–3786. doi: 10.1073/pnas.84.11.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges B. A., Dennis R. E., Munson R. J. Mutation in Escherichia coli B/r WP2 try by reversion or suppression of a chain-terminating codon. Mutat Res. 1967 Jul-Aug;4(4):502–504. doi: 10.1016/0027-5107(67)90012-7. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. RecA + -dependent mutagenesis occurring before DNA replication in UV- and -irradiated Escherichia coli. Mutat Res. 1971 Sep;13(1):1–8. doi: 10.1016/0027-5107(71)90120-5. [DOI] [PubMed] [Google Scholar]
- Cohn S. M., Lieberman M. W. The distribution of DNA excision-repair sites in human diploid fibroblasts following ultraviolet irradiation. J Biol Chem. 1984 Oct 25;259(20):12463–12469. [PubMed] [Google Scholar]
- Doudney C. O., Haas F. L. MODIFICATION OF ULTRAVIOLET-INDUCED MUTATION FREQUENCY AND SURVIVAL IN BACTERIA BY POST-IRRADIATION TR000EATMENT. Proc Natl Acad Sci U S A. 1958 May;44(5):390–401. doi: 10.1073/pnas.44.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey N., Witkin E. M., Brash D. E. Ultraviolet photoproducts at the ochre suppressor mutation site in the glnU gene of Escherichia coli: relevance to "mutation frequency decline". Mol Gen Genet. 1989 Nov;219(3):359–364. doi: 10.1007/BF00259607. [DOI] [PubMed] [Google Scholar]
- George D. L., Witkin E. M. Slow excision repair in an mfd mutant of Escherichia coli B/r. Mol Gen Genet. 1974;133(4):283–291. doi: 10.1007/BF00332704. [DOI] [PubMed] [Google Scholar]
- George D. L., Witkin E. M. Ultraviolet light-induced responses of an mfd mutant of Escherichia coli B/r having a slow rate of dimer excision. Mutat Res. 1975 Jun;28(3):347–354. doi: 10.1016/0027-5107(75)90229-8. [DOI] [PubMed] [Google Scholar]
- Halliday J. A., Zielenska M., Awadallah S. S., Glickman B. W. Colony hybridisation in Escherichia coli: a rapid procedure for determining the distribution of specific classes of mutations among a number of preselected sites. Environ Mol Mutagen. 1990;16(3):143–148. doi: 10.1002/em.2850160303. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A. Ultraviolet light repair and mutagenesis revisited. Cell. 1983 May;33(1):13–17. doi: 10.1016/0092-8674(83)90329-x. [DOI] [PubMed] [Google Scholar]
- Kantor G. J., Setlow R. B. Rate and extent of DNA repair in nondividing human diploid fibroblasts. Cancer Res. 1981 Mar;41(3):819–825. [PubMed] [Google Scholar]
- Koehler D. R., Awadallah S. S., Glickman B. W. Sites of preferential induction of cyclobutane pyrimidine dimers in the nontranscribed strand of lacI correspond with sites of UV-induced mutation in Escherichia coli. J Biol Chem. 1991 Jun 25;266(18):11766–11773. [PubMed] [Google Scholar]
- LeClerc J. E., Christensen J. R., Tata P. V., Christensen R. B., Lawrence C. W. Ultraviolet light induces different spectra of lacI sequence changes in vegetative and conjugating cells of Escherichia coli. J Mol Biol. 1988 Oct 5;203(3):619–633. doi: 10.1016/0022-2836(88)90197-0. [DOI] [PubMed] [Google Scholar]
- Madhani H. D., Bohr V. A., Hanawalt P. C. Differential DNA repair in transcriptionally active and inactive proto-oncogenes: c-abl and c-mos. Cell. 1986 May 9;45(3):417–423. doi: 10.1016/0092-8674(86)90327-2. [DOI] [PubMed] [Google Scholar]
- Mellon I., Bohr V. A., Smith C. A., Hanawalt P. C. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Hanawalt P. C. Induction of the Escherichia coli lactose operon selectively increases repair of its transcribed DNA strand. Nature. 1989 Nov 2;342(6245):95–98. doi: 10.1038/342095a0. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Nishioka H., Doudney C. O. Different modes of loss of photoreversibility of mutation and lethal damage in ultraviolet-light resistant and sensitive bacteria. Mutat Res. 1969 Sep-Oct;8(2):215–228. doi: 10.1016/0027-5107(69)90001-3. [DOI] [PubMed] [Google Scholar]
- Nishioka H., Doudney C. O. Different modes of loss of photoreversibility of ultraviolet light-induced true and suppressor mutations to tryptophan independence in an auxotrophic strain of Escherichia coli. Mutat Res. 1970 Apr;9(4):349–358. doi: 10.1016/0027-5107(70)90017-5. [DOI] [PubMed] [Google Scholar]
- Sauerbier W., Hercules K. Gene and transcription unit mapping by radiation effects. Annu Rev Genet. 1978;12:329–363. doi: 10.1146/annurev.ge.12.120178.001553. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Danforth B. N., Glickman B. W. Mechanisms of spontaneous mutagenesis: an analysis of the spectrum of spontaneous mutation in the Escherichia coli lacI gene. J Mol Biol. 1986 May 20;189(2):273–284. doi: 10.1016/0022-2836(86)90509-7. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Danforth B. N., Glickman B. W. Rapid repeated cloning of mutant lac repressor genes. Gene. 1985;39(2-3):181–189. doi: 10.1016/0378-1119(85)90312-9. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Dunn R. L., Glickman B. W. Mechanisms of ultraviolet-induced mutation. Mutational spectra in the Escherichia coli lacI gene for a wild-type and an excision-repair-deficient strain. J Mol Biol. 1987 Nov 20;198(2):187–202. doi: 10.1016/0022-2836(87)90305-6. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Dunn R. L. Spectra of spontaneous mutations in Escherichia coli strains defective in mismatch correction: the nature of in vivo DNA replication errors. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6220–6224. doi: 10.1073/pnas.84.17.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaper R. M., Dunn R. L. Spontaneous mutation in the Escherichia coli lacI gene. Genetics. 1991 Oct;129(2):317–326. doi: 10.1093/genetics/129.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaper R. M. Mechanisms of mutagenesis in the Escherichia coli mutator mutD5: role of DNA mismatch repair. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8126–8130. doi: 10.1073/pnas.85.21.8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. Gene- and strand-specific repair in vitro: partial purification of a transcription-repair coupling factor. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8232–8236. doi: 10.1073/pnas.88.18.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. Transcription preferentially inhibits nucleotide excision repair of the template DNA strand in vitro. J Biol Chem. 1990 Dec 5;265(34):21330–21336. [PubMed] [Google Scholar]
- Selby C. P., Witkin E. M., Sancar A. Escherichia coli mfd mutant deficient in "mutation frequency decline" lacks strand-specific repair: in vitro complementation with purified coupling factor. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11574–11578. doi: 10.1073/pnas.88.24.11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerdon M. J., Thoma F. Site-specific DNA repair at the nucleosome level in a yeast minichromosome. Cell. 1990 May 18;61(4):675–684. doi: 10.1016/0092-8674(90)90479-x. [DOI] [PubMed] [Google Scholar]
- Terleth C., van Sluis C. A., van de Putte P. Differential repair of UV damage in Saccharomyces cerevisiae. Nucleic Acids Res. 1989 Jun 26;17(12):4433–4439. doi: 10.1093/nar/17.12.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Okumoto D. S., Sancar A., Bohr V. A. Preferential DNA repair of (6-4) photoproducts in the dihydrofolate reductase gene of Chinese hamster ovary cells. J Biol Chem. 1989 Oct 25;264(30):18005–18010. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vrieling H., Van Rooijen M. L., Groen N. A., Zdzienicka M. Z., Simons J. W., Lohman P. H., van Zeeland A. A. DNA strand specificity for UV-induced mutations in mammalian cells. Mol Cell Biol. 1989 Mar;9(3):1277–1283. doi: 10.1128/mcb.9.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins R. J., Hart R. W. Preferential DNA repair in human cells. Nature. 1974 Jan 4;247(5435):35–36. doi: 10.1038/247035a0. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Radiation-induced mutations and their repair. Science. 1966 Jun 3;152(3727):1345–1353. doi: 10.1126/science.152.3727.1345. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet-induced mutation and DNA repair. Annu Rev Microbiol. 1969;23:487–514. doi: 10.1146/annurev.mi.23.100169.002415. [DOI] [PubMed] [Google Scholar]
- Witkin E. M., Wermundsen I. E. Targeted and untargeted mutagenesis by various inducers of SOS functions in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):881–886. doi: 10.1101/sqb.1979.043.01.095. [DOI] [PubMed] [Google Scholar]