Abstract
Background:
Persistent pain after breast cancer surgery affects up to 60% of patients. Early identification of those at higher risk could help inform optimal management. We conducted a systematic review and meta-analysis of observational studies to explore factors associated with persistent pain among women who have undergone surgery for breast cancer.
Methods:
We searched the MEDLINE, Embase, CINAHL and PsycINFO databases from inception to Mar. 12, 2015, to identify cohort or case–control studies that explored the association between risk factors and persistent pain (lasting ≥ 2 mo) after breast cancer surgery. We pooled estimates of association using random-effects models, when possible, for all independent variables reported by more than 1 study. We reported relative measures of association as pooled odds ratios (ORs) and absolute measures of association as the absolute risk increase.
Results:
Thirty studies, involving a total of 19 813 patients, reported the association of 77 independent variables with persistent pain. High-quality evidence showed increased odds of persistent pain with younger age (OR for every 10-yr decrement 1.36, 95% confidence interval [CI] 1.24–1.48), radiotherapy (OR 1.35, 95% CI 1.16–1.57), axillary lymph node dissection (OR 2.41, 95% CI 1.73–3.35) and greater acute postoperative pain (OR for every 1 cm on a 10-cm visual analogue scale 1.16, 95% CI 1.03–1.30). Moderate-quality evidence suggested an association with the presence of preoperative pain (OR 1.29, 95% CI 1.01–1.64). Given the 30% risk of pain in the absence of risk factors, the absolute risk increase corresponding to these ORs ranged from 3% (acute postoperative pain) to 21% (axillary lymph node dissection). High-quality evidence showed no association with body mass index, type of breast surgery, chemotherapy or endocrine therapy.
Interpretation:
Development of persistent pain after breast cancer surgery was associated with younger age, radiotherapy, axillary lymph node dissection, greater acute postoperative pain and preoperative pain. Axillary lymph node dissection provides the only high-yield target for a modifiable risk factor to prevent the development of persistent pain after breast cancer surgery.
Despite a 10-year survival rate of 83%,1,2 between 25% and 60% of surviving patients who have undergone surgery for breast cancer experience persistent postsurgical pain,3–9 which is associated with reduced quality of life and functional impairment.10–13 Systematic reviews summarizing proposed risk factors for persistent pain after breast cancer surgery — including demographic, intraoperative and postoperative factors — have had several limitations, including outdated searches, inadequate attention to risk-of-bias assessment, lack of statistical pooling of measures of association and failure to evaluate the quality of evidence.5,10–14 We conducted a systematic review and meta-analysis of observational studies to identify risk factors for persistent pain after breast cancer surgery, addressing the limitations of previous reviews.
Methods
We completed our systematic review in accordance with the MOOSE statement15 and registered our protocol with PROSPERO (registration CRD42014013338).
Data sources and searches
Our searches, with no language restrictions, encompassed the MEDLINE, Embase, CINAHL and PsycINFO databases from inception to Mar. 12, 2015 (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151276/-/DC1), as well as review of reference lists of eligible studies and 6 previous systematic reviews.5,10–14
We included cohort or case–control studies that explored risk factors for persistent pain after breast cancer surgery using an adjusted analysis. We used criteria of the International Association for the Study of Pain (IASP) to define persistent postsurgical pain as pain that develops after surgical intervention and lasts at least 2 months, with exclusion of other potential causes for the pain.16 Studies were ineligible if they included, in all available models, significant associations with variables collected after baseline; in such instances, the status of the predictor may be a result, rather than a cause, of the pain (see Appendix 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151276/-/DC1). When more than 50% of study populations overlapped between articles, we included only the study with the largest sample size.
Study selection
Ten reviewers (L.W., S.A.K., B.R., H.Y.K., A.K., Y.C., S.C., C.P.B.deA., S.R.P., Z.I.) worked in pairs to screen, independently and in duplicate, the titles and abstracts of identified citations and, subsequently, the full texts of potentially eligible studies. The reviewers resolved disagreements by discussion or with the help of an adjudicator (L.W. or J.W.B.).
Data extraction and quality assessment
We used criteria from Users’ Guides to the Medical Literature17 to assess risk of bias, including representativeness of the study population, validity of outcome assessment, loss to follow-up and whether predictive models were optimally adjusted (Appendix 3, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151276/-/DC1). Using standardized, pilot-tested data extraction forms and a detailed instruction manual, pairs of reviewers extracted data from 10 articles independently and in duplicate. Piloting was accomplished by having each reviewer team extract data from the same 2 articles. After 100% agreement was achieved for these 10 articles, data were extracted from each remaining article by a single reviewer and verified by a second reviewer. Disagreements were resolved by discussion. If a study reported multiple regression models, we used predefined criteria to select 1 model for data extraction (Appendix 4, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151276/-/DC1).18
Data synthesis and analysis
We measured inter-rater agreement of full-text screening with the kappa statistic (κ).19 We reported the median and interquartile range (IQR) for intensity of persistent pain across eligible studies, converting all reported measures of pain intensity to a 10-cm visual analogue scale.20 When investigators reported the association of body mass index (BMI) or age as categorical data, we converted to continuous data (Appendix 5, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151276/-/DC1).21,22
We pooled all factors assessed for an association with persistent pain that were reported by more than 1 study, and present odds ratios (ORs) and associated 95% confidence intervals (CIs). If a study provided the measure of association as a relative risk, we converted the relative risk to an OR.23 We used random-effects models for all meta-analyses.24
When pooling was not possible, we explored the consistency of association between pooled results and studies reporting the same predictors that could not be pooled. We used the following 3 criteria to identify predictors that were not amenable to pooling and showed promise for future research: a statistically significant association with persistent pain of p ≤ 0.01, a large magnitude of association (OR ≥ 2.0) and a sample size of 500 or more.
To avoid overestimating the strength of association by restricting statistical pooling to predictors that appeared in adjusted regression models, we imputed an OR of 1 for predictors that were tested in bivariable analyses but because of nonsignificance were excluded from adjusted analyses or were included in multivariable analyses with the only information provided being that they were “not significant.” We imputed an associated variance for all such predictors using the hot deck approach.25
To calculate the absolute risk increase for each predictor amenable to meta-analysis, we estimated the baseline risk for persistent postsurgical pain (30% in the low-risk group, who underwent sentinel lymph node biopsy) using data from the study with the largest sample size among studies at low risk of bias.26 We performed all statistical analyses using Stata statistical software version 13.1. All comparisons were 2-tailed, with a threshold p of 0.05.
Publication bias
For meta-analyses with at least 10 studies,22,27 we assessed publication bias by visual assessment of asymmetry of the funnel plot and performed the Begg rank correlation test28 and the Egger test.29
Subgroup analyses, meta-regression and sensitivity analyses
We evaluated heterogeneity for all pooled estimates through visual inspection of forest plots,27 because statistical tests of heterogeneity can be misleading when sample sizes are large and CIs are therefore narrow.30
We generated 4 a priori hypotheses to explain variability between studies, assuming larger association with persistent pain and (1) a high pain threshold (moderate to severe pain v. no to mild pain), (2) trials having greater risk of bias (on a component-by-component basis), (3) longer duration of follow-up and (4) larger proportion of patients lost to follow-up. We did not conduct subgroup analyses if there was only 1 study in a given subgroup. We performed sensitivity analyses examining the effect of imputing data for nonsignificant postulated predictors and of converting categorical data for BMI and age to continuous data.
Quality of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to summarize the quality of evidence for all meta-analyses.27,31 Given the high baseline risk we found for persistent pain after surgery for breast cancer (30%), we estimated that a 10% increase in the absolute risk would likely be sufficient for clinicians to address modifiable risk factors, which can be directly targeted in an effort to prevent persistent pain. We estimated that an absolute difference in risk of 20% between groups at low and high risk for persistent pain would be sufficient for clinicians to selectively target nonmodifiable risk factors to identify high-risk candidates for intervention. Therefore, we rated down for imprecision if the 95% CI associated with the risk difference included 10% for modifiable risk factors, or 20% for nonmodifiable risk factors.
Results
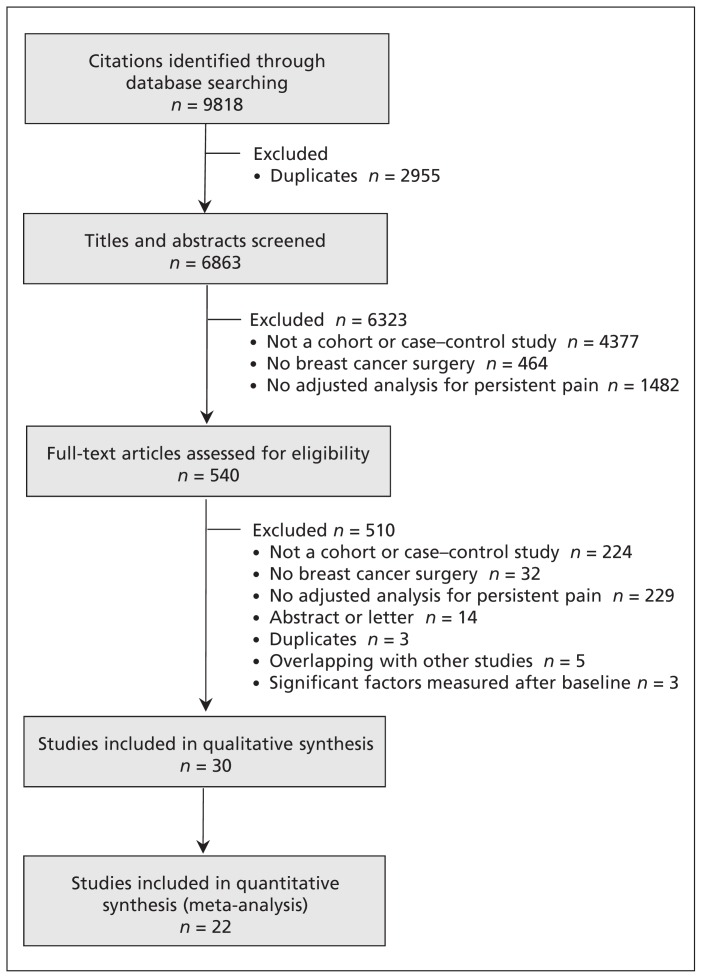
Of 6863 unique records, 492 English and 48 non-English language articles were potentially eligible; of these, 29 cohort studies26,32–59 and 1 case–control study60 proved eligible for our review (Figure 1). We excluded 5 studies with overlapping populations and 3 studies reporting significant factors that were collected after baseline (Appendix 2). There was near-perfect agreement (κ = 0.86) between reviewers at the full-text review stage. For the 3 studies in which eligibility was unclear, we obtained clarification from the authors.37,38,61 Among 8 studies for which some data needed for our analysis were not included in the published report, we obtained missing data from the authors of 2 studies.59,60
Figure 1:
Flow diagram of study selection.
Definitions of persistent pain varied across the studies (Appendix 6, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151276/-/DC1). Persistent postsurgical pain was reported at least 3 months after breast cancer surgery (range 3.28–72.50 mo) in all eligible studies. Seven studies reported that other causes of persistent pain had been excluded,32,37,38,42,44,46,52 but only 1 study explicitly used the IASP criteria for defining persistent postsurgical pain.44 The median sample size was 416 (IQR 250–772), and the median duration of follow-up was 24 months (IQR 12–42 mo) (Appendix 7, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151276/-/DC1).26,32–60 In 11 (37%) of the 30 studies, regression models included only variables that were significant in bivariable analysis (and thus more vulnerable to chance),32,34,41,42,44,46,48,49,57–59 and 22 (73%) of the 30 studies failed to present data for nonsignificant predictors in their adjusted analysis.32,34,37,39–50,53,55–60
Twenty-three studies reported the prevalence of persistent pain after breast surgery, with a median prevalence of 37.5% (IQR 30%–51%).26,32–38,40–42,44–49,51,52,54,57–59 Twenty studies reported the intensity of persistent postsurgical pain;26,33–40,43–45,47,48,50,52,53,56–58 the median value was 3.22 cm (IQR 2.75–4.12 cm) on a 10-cm visual analogue scale (where values < 4 cm correspond to mild pain, values 4–7 cm correspond to moderate pain, and values > 7 cm correspond to severe pain62).
Risk of bias
Reported protection against bias among studies exploring predictors of persistent pain was limited, with 26 (87%) of the 30 studies not meeting at least 1 of our risk-of-bias criteria (Appendix 8, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151276/-/DC1).32, 34–52, 54 – 57,59,60 All but 3 studies55,57,60 (90%) reported adequately adjusted regression models. We detected no evidence of publication bias (Table 1; funnel plots available by request to the authors).
Table 1:
GRADE evidence profile: predictors of persistent pain after breast cancer surgery
| Predictor | Quality assessment | Relative effect (95% CI) | Anticipated absolute effect | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall | Baseline risk* | Risk difference (95% CI) | ||
| Age: every 10-yr decrement | |||||||||
| 11 030 patients (22 studies), median follow-up 12 mo | No serious risk of bias† | No serious inconsistency | No serious indirectness | No serious imprecision | Undetected; symmetric funnel plot; Begg test p = 0.8; Egger test p = 0.8 | High | OR 1.36 (1.24–1.48) | 30% for age 70 yr‡ | 7% more (5% to 9% more) patients with per 10-yr decrement of age having persistent pain |
| Radiotherapy: yes v. no | |||||||||
| 9468 patients (16 studies), median follow-up 23.5 mo | No serious risk of bias† | No serious inconsistency | No serious indirectness | No serious imprecision | Undetected; symmetric funnel plot; Begg test p = 0.6; Egger test p = 0.2 | High | OR 1.35 (1.16–1.57) | 30% | 7% more (3% to 10% more) patients with radiotherapy having persistent pain |
| Axillary lymph node dissection (ALND): yes v. no or ALND v. sentinel lymph node biopsy | |||||||||
| 7699 patients (13 studies), median follow-up 12 mo | No serious risk of bias† | No serious inconsistency | No serious indirectness | No serious imprecision | Undetected; symmetric funnel plot; Begg test p > 0.9; Egger test p = 0.5 | High | OR 2.41 (1.73–3.35) | 30% | 21% more (13% to 29% more) patients with ALND having persistent pain |
| Acute postoperative pain, measured with 10-cm pain scale: better indicated by lower values | |||||||||
| 1387 patients (5 studies), median follow-up 17.5 mo | No serious risk of bias† | No serious inconsistency | No serious indirectness | No serious imprecision | Uncertain: only 5 studies | High | OR 1.16 (1.03–1.30) | 30% for 1 cm on a 10-cm scale‡ | 3% more (1% to 6% more) patients with per 1-cm increment of acute pain on 10-cm pain scale having persistent pain |
| Preoperative pain: yes v. no | |||||||||
| 2504 patients (8 studies) median follow-up 7.5 mo | No serious risk of bias† | No serious inconsistency | No serious indirectness | Serious imprecision§ | Uncertain: only 8 studies | Moderate | OR 1.29 (1.01–1.64) | 30% | 6% more (0% to 11% more) patients with preoperative pain having persistent pain |
| BMI: every 5-point increment | |||||||||
| 3178 patients (8 studies) median follow-up 12 mo | No serious risk of bias† | No serious inconsistency | No serious indirectness | No serious imprecision¶ | Uncertain: only 8 studies | High | OR 1.11 (0.99–1.24) | 30% for BMI 25 kg/m2‡ | 2% more (0% to 5% more) patients with per 5-point increment of BMI having persistent pain |
| Breast surgery: BCS v. mastectomy/modified radical mastectomy | |||||||||
| 8566 patients (17 studies), median follow-up 17.5 mo | No serious risk of bias† | No serious inconsistency | No serious indirectness | No serious imprecision¶ | Undetected; symmetric funnel plot; Begg test p = 0.2; Egger test p = 0.8 | High | OR 1.08 (0.90–1.30) | 30% | 2% more (2% less to 6% more) patients with BCS having persistent pain |
| Chemotherapy: yes v. no | |||||||||
| 8481 patients (17 studies), median follow-up 12 mo | No serious risk of bias† | No serious inconsistency | No serious indirectness | No serious imprecision¶ | Undetected; symmetric funnel plot; Begg test p = 0.6; Egger test p > 0.9 | High | OR 1.12 (0.98–1.29) | 30% | 2% more (0% less to 6% more) patients with chemotherapy having persistent pain |
| Endocrine therapy: yes v. no | |||||||||
| 8312 (11 studies), median follow-up 27 mo | No serious risk of bias† | No serious inconsistency | No serious indirectness | No serious imprecision¶ | Undetected; symmetric funnel plot; Begg test p = 0.3; Egger test p = 0.2 | High | OR 1.07 (0.94–1.22) | 30% | 1% more (1% less to 4% more) patients with endocrine therapy having persistent pain |
Note: BCS = breast-conserving surgery; BMI = body mass index; CI = confidence interval; GRADE = Grading of Recommendations Assessment, Development and Evaluation; OR = odds ratio.
Baseline risk based on the subpopulation of patients undergoing sentinel lymph node biopsy with lowest absolute risk of persistent pain in the study with the largest sample size among the studies at low risk of bias.26
Quality was not rated down on the basis of risk of bias, because the subgroup analyses and meta-regression did not show any significant difference between each risk-of-bias component and the estimates of association.
The reference groups for age, acute postoperative pain and BMI were obtained from the largest study among those with low risk of bias that explored each of these predictors (i.e., age 70 as reference,26 BMI of 2541 and acute postoperative pain of 1 cm on a 10-cm visual analogue pain scale37).
Quality was rated down on the basis of imprecision because the 95% CI associated with the risk difference included the predefined threshold of 10% for modifiable factors, which means that clinical actions based on the estimate of the lower or upper boundary may be different.
Quality was not rated down on the basis of imprecision, even though the 95% CI for the pooled effect overlapped a risk difference of 0 (no effect), because clinical actions based on the estimate of the lower or upper boundary would not change, according to the predefined threshold of ≥ 20% for nonmodifiable factors.
Predictors of persistent pain
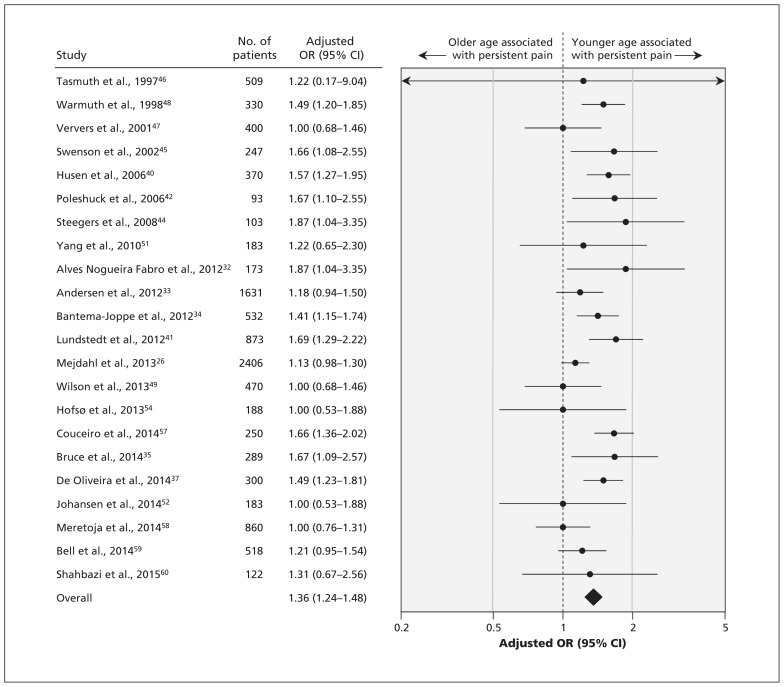
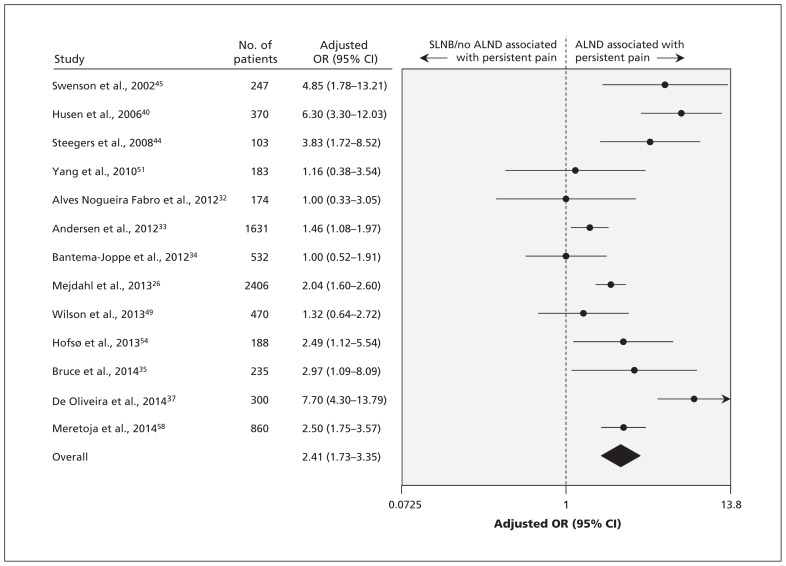
The 30 studies, involving a total of 19 813 patients, reported the association of 77 factors with the development of persistent pain. On the basis of our criteria, we conducted meta-analyses for 9 predictors of persistent pain. High-quality evidence showed a significant association between persistent pain after breast cancer surgery and 2 nonmodifiable factors (Table 1): younger age (OR for every 10-yr decrement 1.36, 95% CI 1.24–1.48 [Figure 2]; absolute risk increase 7% [95% CI 5%–9%] for every 10-yr decrement from age 70) and radiotherapy (OR 1.35, 95% CI 1.16–1.57 [forest plot available by request to the authors]; absolute risk increase 7%, 95% CI 3%–10%). We found a significant association between persistent pain and 3 modifiable factors (Table 1): axillary lymph node dissection (OR 2.41, 95% CI 1.73–3.35, high-quality evidence by GRADE [Figure 3]; absolute risk increase 21%, 95% CI 13%–29%), greater acute postoperative pain (OR for every 1-cm increment on a 10-cm visual analogue scale 1.16, 95% CI 1.03–1.30, high-quality evidence by GRADE [forest plot available by request]; absolute risk increase 3% [95% CI 1%–6%] for every 1-cm increment on a 10-cm visual analogue scale) and presence of preoperative pain (OR 1.29, 95% CI 1.01–1.64, moderate-quality evidence by GRADE [forest plot available by request]; absolute risk increase 6%, 95% CI 0%–11%).
Figure 2:
Meta-analysis of the association between persistent pain and age (per 10-year decrement). CI = confidence interval, OR = odds ratio.
Figure 3:
Meta-analysis of the association between persistent pain and axillary lymph node dissection (ALND). CI = confidence interval, OR = odds ratio, SLNB = sentinel lymph node biopsy.
High-quality evidence showed no association between persistent pain and BMI (OR for every 5-point increment 1.11, 95% CI 0.99–1.24), type of breast surgery (breast-conserving surgery v. mastectomy or modified radical mastectomy, OR 1.08, 95% CI 0.90–1.30), chemotherapy (OR 1.12, 95% CI 0.98–1.29) or endocrine therapy (OR 1.07, 95% CI 0.94–1.22) (Table 1; forest plots available by request). The results from the 7 studies37,39,42,50,53,55,56 that reported 1 or more of the 9 predictors that we subjected to meta-analysis but whose data could not be pooled were consistent with our pooled analyses (Appendix 9, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151276/-/DC1).
Appendices 10 and 11 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151276/-/DC1) present the associations with persistent pain for the 68 factors that were not amenable to meta-analysis. Two of these factors (overall comorbidity and radiotherapy dosage) met our criteria as promising for future study.
Subgroup analyses, meta-regression and sensitivity analyses
We found no evidence to support a difference in associations with predictive factors when considering different thresholds for defining persistent pain, representativeness of the study population, whether a validated measure to capture pain was used, duration of follow-up or the proportion of patients lost to follow-up (Appendix 12, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151276/-/DC1). The finding that predictive power did not differ across thresholds for defining persistent pain was strengthened by 2 cohort studies that used separate regression models for both high and low thresholds of persistent pain and reported similar associations across predictors.26,35 Whether or not we incorporated missing data for nonsignificant predictors or converted categorical data for age and BMI to continuous data (Appendix 13, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151276/-/DC1) did not appreciably influence the results.
Interpretation
We found high-quality evidence that younger age, radiotherapy, axillary lymph node dissection and greater acute postoperative pain were associated with persistent pain after breast cancer surgery, and we found moderate-quality evidence for an association with preoperative pain. The strongest of these associations was with axillary lymph node dissection, with an absolute increase in risk of persistent pain of 21%. High-quality evidence showed that BMI, type of breast surgery, chemotherapy and endocrine therapy were not associated with persistent pain (Table 1). Investigators have tested 68 additional predictors that could not be statistically pooled (Appendices 10 and 11). Preliminary evidence suggested that 2 of these predictors may warrant additional study: overall comorbidity and radiotherapy dosage.
The most recent systematic review that explored risk factors for persistent pain after breast cancer surgery identified 8 studies that met our eligibility criteria.5 That review presented a qualitative synthesis concluding that axillary lymph node dissection and radiotherapy appeared to be risk factors for persistent pain.5 We have confirmed and quantified these associations through meta-analyses, and we have established 3 additional risk factors: younger age, the presence of preoperative pain and greater acute postoperative pain. We also identified high-quality evidence that BMI, type of breast cancer surgery, chemotherapy and endocrine therapy are not important predictors.
Typically, investigators present associations with predictors of persistent breast cancer pain as relative measures (e.g., OR, relative risk). However, it is the absolute risk increase that must guide clinical decision-making. Most efforts to reduce persistent postsurgical pain have focused on pharmacologic approaches to reduce preoperative or acute postoperative pain. Recent systematic reviews have found no compelling evidence to support the prevention of persistent postsurgical pain by perioperative administration of intravenous ketamine, oral gabapentin, oral pregabalin, nonsteroidal anti-inflammatory drugs, intravenous steroids, oral N-methyl-d-aspartate blockers, oral mexiletine, intravenous fentanyl, intravenous lidocaine, oral venlafaxine or inhaled nitrous oxide.63,64 Given that the absolute increase in chronic pain associated with preoperative or greater postoperative pain is modest (Table 1), any reduction in persistent pain achieved through pharmacologic reduction of perioperative pain will likely be obscured by the random error from all other determinants of long-term pain. In other words, it would require a very large (and thus implausible) reduction of perioperative pain to result in detectable effects in randomized trials examining the impact on chronic postoperative pain.
We found one association in which the absolute increase would be sufficient to suggest targeted interventions. Women who underwent axillary lymph node dissection experienced a 21% increase in the absolute risk of chronic postoperative pain. Although axillary staging is associated with persistent pain, the risks of omitting axillary nodal sampling include increasing the number of patients who are understaged and undertreated and who experience reduced survival.65 Thus, omission of axillary staging is not an appropriate approach to modifying pain risk. However, modification of surgical procedures related to axillary dissection constitutes a promising stand-alone target for risk reduction. Preliminary evidence suggests that sentinel lymph node biopsy, rather than standard axillary treatment,66 may reduce the risk of chronic pain after breast cancer surgery. Moreover, preservation of intercostobrachial nerves during axillary lymph node dissection reduces the incidence of postmastectomy pain syndrome after surgery67,68 and reduces the risk of sensory deficits after axillary clearance.69 Accordingly, the American Society of Clinical Oncology now recommends sentinel lymph node biopsy for patients with early-stage breast cancer, followed by dissection only if the biopsy result is positive,70 because this approach is associated with less pain and equivalent rates of axillary relapse compared with axillary dissection.71
Awareness of nonmodifiable risk factors could influence management by allowing identification of women at high risk of postoperative pain who might then be targeted for interventions — for example, psychotherapy or interventions such as paravertebral blocks in addition to general anesthesia, for which preliminary evidence suggests possible benefit.72 We postulated that increases of absolute risk of 20% or more would be required to warrant targeting a high-risk population: none of the individual nonmodifiable associations that we identified met this threshold (Table 1). However, a combination of risk factors might serve to identify a population warranting special attention.
Strengths and limitations
The strengths of our review include explicit eligibility criteria and a comprehensive search with no language restrictions, which identified 23 cohort studies that were not included in previous systematic reviews.5,10–14 We assessed the risk of bias in individual studies and used the GRADE approach to appraise the quality of evidence. We converted the intensity of persistent postsurgical pain to a 10-cm visual analogue scale across studies to optimize the interpretation of our findings. We have presented statistical pooling of associations between predictive factors and the risk of persistent pain. Our approach included imputing data for missing nonsignificant predictors (to avoid overestimating associations), and we conducted subgroup and sensitivity analyses that confirmed robust associations. Finally, we have presented not only relative but also absolute risk increases, which greatly strengthens inferences about the importance of the associations and possible implications for clinical care.
Our study had some limitations. We were unable to pool data for predictors from studies that used different continuous outcome measures to assess persistent pain in linear regression models.36,39,43,50,53,55,56 However, the results from these studies were consistent with the results from studies amenable to pooling. We used the IASP criteria for the definition of persistent pain in this review; however, 14 of the included studies did not report whether their assessment of persistent postsurgical pain excluded other causes of pain;26,33–36,43,45,47,48,50,51,54,56,58 as such, they may have overestimated the prevalence of persistent pain.
Conclusion
Development of persistent pain after breast cancer surgery was associated with younger age, radiotherapy, axillary lymph node dissection, greater acute postoperative pain and preoperative pain. Axillary lymph node dissection provided the only high-yield target for a modifiable risk factor, and no single nonmodifiable risk factor changed risk sufficiently to define a target population for an intervention to prevent persistent pain. Future research should establish the association between overall comorbidity, radiotherapy dosage and persistent postsurgical pain, and determine whether axillary nerve-sparing techniques are effective for reducing chronic pain after breast surgery.
Acknowledgements
For screening the full texts of non-English articles, the authors thank Toshiaki A. Furukawa, Department of Health Promotion and Human Behavior and Department of Clinical Epidemiology, Kyoto University Graduate School of Medicine and School of Public Health, Kyoto, Japan; Sun Makosso-Kallyth, Michael G. DeGroote Institute for Pain Research and Care, McMaster University, Hamilton, Ont.; Inge H.F. Reininga, Department of Trauma Surgery, and Sandra Brouwer, Department of Health Sciences, Community and Occupational Medicine, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; Behnam Sadeghirad and Nigar Sekercioglu, Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, Ont.; Dmitry Shiktorov, Canadian Centre for Clinical Trials, Vaughan, Ont.; and Kari Tikkinen, Departments of Urology and of Public Health, Helsinki University Hospital and University of Helsinki, Helsinki, Finland. The authors thank Qi Zhou and Diane Heels-Ansdell, Department of Clinical Epidemiology and Biostatistics, McMaster University, for statistical advice. They also thank Robin Bell and Penelope Robinson, School of Public Health and Preventive Medicine, Monash University, Monash, Australia; Sayed Hossein Davoodi, Cancer Research Center, and Roghayeh Shahbazi, National Institute and Faculty of Nutrition and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran; and Lena Engqvist Boman, Department of Learning, Informatics, Management and Ethics, Karolinska Institutet, Stockholm, Sweden, for providing supplementary information or answering queries regarding their studies. No financial compensation was provided to any of these individuals.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Data sharing: The relevant data in this study are available from the authors.
Contributors: Li Wang and Jason Busse conceived the study design; Li Wang, Sean Kennedy, Beatriz Romerosa, Henry Kwon, Alka Kaushal, Yaping Chang, Samantha Craigie, Carlos de Almeida, Rachel Couban, Shawn Parascandalo and Zain Izhar acquired the data; Li Wang performed the data analysis; Li Wang, Gordon Guyatt, Susan Reid, James Khan, Michael McGillion and Jason Busse interpreted the data and findings. Gordon Guyatt and Jason Busse provided methodological support and study supervision. Li Wang and Jason Busse drafted the manuscript, and all of the authors revised the manuscript for important intellectual content. In addition, all of the authors approved the final version for publication and agreed to act as guarantors of the work. Li Wang and Jason Busse have full access to all of the study data and had final responsibility for the decision to submit for publication.
Funding: No funds were received for the preparation of this manuscript. Li Wang is supported by a Michael G. DeGroote Postdoctoral Fellowship. The funding organization had no role in the design and conduct of the study; in the collection, analysis or interpretation of the data; or in the preparation, review or approval of the manuscript.
References
- 1.Canadian cancer statistics 2014. Toronto: Canadian Cancer Society; 2014. Available: www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2014-EN.pdf?la=en (accessed 2015 July 20). [Google Scholar]
- 2.Breast cancer facts and figures 2013–2014. Atlanta: American Cancer Society; 2013. Available: www.cancer.org/acs/groups/content/@research/documents/document/acspc-042725.pdf (accessed 2015 July 20). [Google Scholar]
- 3.Jung BF, Ahrendt GM, Oaklander AL, et al. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain 2003;104:1–13. [DOI] [PubMed] [Google Scholar]
- 4.Kehlet H, Jensen Troels S, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- 5.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain 2011;12:725–46. [DOI] [PubMed] [Google Scholar]
- 6.Brummett CM. Chronic pain following breast surgery. Tech Reg Anesth Pain Manage 2011;15:124–32. [Google Scholar]
- 7.Smith HS, Wu SX. Persistent pain after breast cancer treatment. Ann Palliat Med 2012;1:182–94. [DOI] [PubMed] [Google Scholar]
- 8.Cregg R, Anwar S, Farquhar-Smith P. Persistent postsurgical pain. Curr Opin Support Palliat Care 2013;7:144–52. [DOI] [PubMed] [Google Scholar]
- 9.Haroutiunian S, Nikolajsen L, Finnerup NB, et al. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain 2013;154:95–102. [DOI] [PubMed] [Google Scholar]
- 10.Hidding JT, Beurskens CH, van der Wees PJ, et al. Treatment related impairments in arm and shoulder in patients with breast cancer: a systematic review. PLoS One 2014;9:e96748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai RJ, Dennis LK, Lynch CF, et al. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol 2009;16:1959–72. [DOI] [PubMed] [Google Scholar]
- 12.Liu CQ, Guo Y, Shi JY, et al. Late morbidity associated with a tumour-negative sentinel lymph node biopsy in primary breast cancer patients: a systematic review. Eur J Cancer 2009;45: 1560–8. [DOI] [PubMed] [Google Scholar]
- 13.Lee TS, Kilbreath SL, Refshauge KM, et al. Prognosis of the upper limb following surgery and radiation for breast cancer. Breast Cancer Res Treat 2008;110:19–37. [DOI] [PubMed] [Google Scholar]
- 14.Levangie PK, Drouin J. Magnitude of late effects of breast cancer treatments on shoulder function: a systematic review. Breast Cancer Res Treat 2009;116:1–15. [DOI] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 16.Macrae WA, Davies HTO. Chronic postsurgical pain. In: Crombie IL, Linton S, Croft P, et al., editors. Epidemiology of pain. Seattle: IASP Press; 1999:125–42. [Google Scholar]
- 17.Randolph AG, Cook D, Guyatt G. Prognosis. In: Guyatt G, Rennie D, Meade M, et al., editors. Users’ guides to the medical literature: a manual for evidence-based clinical practice. 3rd ed New York: McGraw-Hill; 2015:421–9. [Google Scholar]
- 18.Tendal B, Nuesch E, Higgins JP, et al. Multiplicity of data in trial reports and the reliability of meta-analyses: empirical study. BMJ 2011;343:d4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- 20.Thorlund K, Walter SD, Johnston BC, et al. Pooling health-related quality of life outcomes in meta-analysis — a tutorial and review of methods for enhancing interpretability. Res Synth Methods 2011;2:188–203. [DOI] [PubMed] [Google Scholar]
- 21.Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683–91. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. London (UK): The Cochrane Collaboration; 2011. Available: www.cochrane-handbook.org (accessed 2015 July 20). [Google Scholar]
- 23.Wang Z. Converting odds ratio to relative risk in cohort studies with partial data information. J Stat Softw 2013;55:1–12. [Google Scholar]
- 24.Murad MH, Montori VM, Ioannidis JPA, et al. Fixed-effects and random-effects models. In: Guyatt G, Rennie D, Meade M, et al., editors. Users’ guides to the medical literature: a manual for evidence-based clinical practice. 3rd ed New York: McGraw-Hill; 2015:507–14. [Google Scholar]
- 25.Gelman A, Hill J. Missing-data imputation. In: Gelman A, Hill J, editors. Data analysis using regression and multilevel/hierarchical models. New York: Cambridge University Press; 2006:529–44. [Google Scholar]
- 26.Mejdahl MK, Andersen KG, Gartner R, et al. Persistent pain and sensory disturbances after treatment for breast cancer: six year nationwide follow-up study. BMJ 2013;346:f1865. [DOI] [PubMed] [Google Scholar]
- 27.Iorio A, Spencer FA, Falavigna M, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015;350:h870. [DOI] [PubMed] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rücker G, Schwarzer G, Carpenter JR, et al. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol 2008;8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alves Nogueira Fabro E, Bergmann A, do Amaral E, Silva B, et al. Post-mastectomy pain syndrome: incidence and risks. Breast 2012;21:321–5. [DOI] [PubMed] [Google Scholar]
- 33.Andersen KG, Jensen MB, Kehlet H, et al. Persistent pain, sensory disturbances and functional impairment after adjuvant chemotherapy for breast cancer: cyclophosphamide, epirubicin and fluorouracil compared with docetaxel + epirubicin and cyclophosphamide. Acta Oncol 2012;51:1036–44. [DOI] [PubMed] [Google Scholar]
- 34.Bantema-Joppe EJ, Schilstra C, de Bock GH, et al. Simultaneous integrated boost irradiation after breast-conserving surgery: physician-rated toxicity and cosmetic outcome at 30 months’ followup. Int J Radiat Oncol Biol Phys 2012;83: e471–7. [DOI] [PubMed] [Google Scholar]
- 35.Bruce J, Thornton AJ, Powell R, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain 2014; 155:232–3. [DOI] [PubMed] [Google Scholar]
- 36.Caffo O, Amichetti M, Ferro A, et al. Pain and quality of life after surgery for breast cancer. Breast Cancer Res Treat 2003; 80:39–48. [DOI] [PubMed] [Google Scholar]
- 37.De Oliveira GS, Jr, Chang R, Khan SA, et al. Factors associated with the development of chronic pain after surgery for breast cancer: a prospective cohort from a tertiary center in the United States. Breast J 2014;20:9–14. [DOI] [PubMed] [Google Scholar]
- 38.De Oliveira GS, Jr, Bialek JM, Nicosia L, et al. Lack of association between breast reconstructive surgery and the development of chronic pain after mastectomy: a propensity matched retrospective cohort analysis. Breast 2014;23:329–33. [DOI] [PubMed] [Google Scholar]
- 39.Hack TF, Kwan WB, Thomas-MacLean RL, et al. Predictors of arm morbidity following breast cancer surgery. Psychooncology 2010;19:1205–12. [DOI] [PubMed] [Google Scholar]
- 40.Husen M, Paaschburg B, Flyger HL. Two-step axillary operation increases risk of arm morbidity in breast cancer patients. Breast 2006;15:620–8. [DOI] [PubMed] [Google Scholar]
- 41.Lundstedt D, Gustafsson M, Steineck G, et al. Risk factors of developing long-lasting breast pain after breast cancer radiotherapy. Int J Radiat Oncol Biol Phys 2012;83:71–8. [DOI] [PubMed] [Google Scholar]
- 42.Poleshuck EL, Katz J, Andrus CH, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain 2006;7:626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rief W, Bardwell WA, Dimsdale JE, et al. Long-term course of pain in breast cancer survivors: a 4-year longitudinal study. Breast Cancer Res Treat 2011;130:579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steegers MA, Wolters B, Evers AW, et al. Effect of axillary lymph node dissection on prevalence and intensity of chronic and phantom pain after breast cancer surgery. J Pain 2008; 9:813–22. [DOI] [PubMed] [Google Scholar]
- 45.Swenson KK, Nissen MJ, Ceronsky C, et al. Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancer. Ann Surg Oncol 2002;9:745–53. [DOI] [PubMed] [Google Scholar]
- 46.Tasmuth T, Kataja M, Blomqvist C, et al. Treatment-related factors predisposing to chronic pain in patients with breast cancer — a multivariate approach. Acta Oncol 1997;36:625–30. [DOI] [PubMed] [Google Scholar]
- 47.Ververs JMMA, Roumen RMH, Vingerhoets AJJM, et al. Risk, severity and predictors of physical and psychological morbidity after axillary lymph node dissection for breast cancer. Eur J Cancer 2001;37:991–9. [DOI] [PubMed] [Google Scholar]
- 48.Warmuth MA, Bowen G, Prosnitz LR, et al. Complications of axillary lymph node dissection for carcinoma of the breast: a report based on a patient survey. Cancer 1998;83:1362–8. [DOI] [PubMed] [Google Scholar]
- 49.Wilson GC, Quillin RC, 3rd, Hanseman DJ, et al. Incidence and predictors of neuropathic pain following breast surgery. Ann Surg Oncol 2013;20:3330–4. [DOI] [PubMed] [Google Scholar]
- 50.Winters ZE, Haviland J, Balta V, et al. Integration of patient-reported outcome measures with key clinical outcomes after immediate latissimus dorsi breast reconstruction and adjuvant treatment. Br J Surg 2013;100:240–51. [DOI] [PubMed] [Google Scholar]
- 51.Yang EJ, Park WB, Seo KS, et al. Longitudinal change of treatment-related upper limb dysfunction and its impact on late dysfunction in breast cancer survivors: a prospective cohort study. J Surg Oncol 2010;101:84–91. [DOI] [PubMed] [Google Scholar]
- 52.Johansen S, Fossa K, Nesvold IL, et al. Arm and shoulder morbidity following surgery and radiotherapy for breast cancer. Acta Oncol 2014;53:521–9. [DOI] [PubMed] [Google Scholar]
- 53.Tian Y, Schofield PE, Gough K, et al. Profile and predictors of long-term morbidity in breast cancer survivors. Ann Surg Oncol 2013;20:3453–60. [DOI] [PubMed] [Google Scholar]
- 54.Hofsø K, Rustøen T, Cooper BA, et al. Changes over time in occurrence, severity, and distress of common symptoms during and after radiation therapy for breast cancer. J Pain Symptom Manage 2013;45:980–1006. [DOI] [PubMed] [Google Scholar]
- 55.Roth RS, Lowery JC, Davis J, et al. Preoperative affective distress and somatic complaints predict persistent pain after postmastectomy breast reconstruction. Eur J Plast Surg 2007; 29:227–33. [Google Scholar]
- 56.Waters EA, Liu Y, Schootman M, et al. Worry about cancer progression and low perceived social support: implications for quality of life among early-stage breast cancer patients. Ann Behav Med 2013;45:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Couceiro TC, Valença MM, Raposo MC, et al. Prevalence of post-mastectomy pain syndrome and associated risk factors: a cross-sectional cohort study. Pain Manag Nurs 2014;15:731–7. [DOI] [PubMed] [Google Scholar]
- 58.Meretoja TJ, Leidenius MHK, Tasmuth T, et al. Pain at 12 months after surgery for breast cancer. JAMA 2014;311:90–2. [DOI] [PubMed] [Google Scholar]
- 59.Bell RJ, Robinson PJ, Nazeem F, et al. Persistent breast pain 5 years after treatment of invasive breast cancer is largely unexplained by factors associated with treatment. J Cancer Surviv 2014;8:1–8. [DOI] [PubMed] [Google Scholar]
- 60.Shahbazi R, Akbari ME, Hashemian M, et al. High body mass index and young age are not associated with post-mastectomy pain syndrome in breast cancer survivors: a case–control study. Iran J Cancer Prev 2015;8:29–35. [PMC free article] [PubMed] [Google Scholar]
- 61.Boman L, Bjorvell H, Langius A, et al. Two models of care as evaluated by a group of women operated on for breast cancer with regard to their perceived well-being. Eur J Cancer Care (Engl) 1999;8:87–96. [DOI] [PubMed] [Google Scholar]
- 62.Gerbershagen HJ, Rothaug J, Kalkman CJ, et al. Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. Br J Anaesth 2011;107:619–26. [DOI] [PubMed] [Google Scholar]
- 63.Chaparro LE, Smith SA, Moore RA, et al. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev 2013;7:CD008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth 2015;114:10–31. [DOI] [PubMed] [Google Scholar]
- 65.Bland KI, Scott-Conner CE, Menck H, et al. Axillary dissection in breast-conserving surgery for stage I and II breast cancer: a National Cancer Data Base study of patterns of omission and implications for survival. J Am Coll Surg 1999;188:586–95. [DOI] [PubMed] [Google Scholar]
- 66.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multi-center trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006;98:599–609. [DOI] [PubMed] [Google Scholar]
- 67.Kostanyan M. Intercostobrachial syndrome after nerve-sparing axillary lymph node dissection. Eur J Cancer 2014;50:S127. [Google Scholar]
- 68.Zhu JJ, Liu XF, Zhang PL, et al. Anatomical information for intercostobrachial nerve preservation in axillary lymph node dissection for breast cancer. Genet Mol Res 2014;13:9315–23. [DOI] [PubMed] [Google Scholar]
- 69.Abdullah TI, Iddon J, Barr L, et al. Prospective randomized controlled trial of preservation of the intercostobrachial nerve during axillary node clearance for breast cancer. Br J Surg 1998;85:1443–5. [DOI] [PubMed] [Google Scholar]
- 70.Lyman GH, Temin S, Edge SB, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2014;32:1365–83. [DOI] [PubMed] [Google Scholar]
- 71.Blanchard DK, Donohue JH, Reynolds C, et al. Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg 2003;138:482–7, discussion 487–8. [DOI] [PubMed] [Google Scholar]
- 72.Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a Cochrane systematic review and meta-analysis. Br J Anaesth 2013;111:711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]