Abstract
The U.S. Department of Health and Human Services addresses clear communication in the informed consent process as part of the Notice of Proposed Rulemaking for revisions to the Common Rule. However, prior research has shown that participants may not fully comprehend research studies despite completion of an informed consent process. Our main goal was to provide plain language information about donation processes to a cancer biobank to supplement an informed consent form. We developed and conducted cognitive testing with supplemental brochures that clearly communicated information about three different models for consent (notice, broad and study-specific) to future use of biospecimens. During the brochures development process, we conducted qualitative, semi-structured, individual, in-person cognitive interviews among 14 women to examine participants’ perceptions of the brochures. Each participant provided feedback regarding the understandability, graphics and layout, and cultural appropriateness of the brochures. Our findings demonstrate that these methods may be used to tailor consent form brochures, such as the ones developed here, to other populations. This study therefore adds to our understanding of how best to present content to help women from two different racial groups make informed decisions about participation in a cancer biobank.
INTRODUCTION
The Federal Policy for the Protection of Human Subjects (i.e., the Common Rule) details U.S. law regarding the ethical conduct of research involving humans[1]. Research participants’ informed consent is a key requirement[2]. Informed consent is intended to empower prospective research participants by providing the information they need to make an informed decision about research participation. Accordingly, the regulations require disclosure of specific information during the informed consent process, including study procedures, explanations of the risks and benefits of participation, and how to withdraw from a study. To fulfill the goals of informed consent, researchers need to adequately and clearly communicate this information to participants [3, 4]. This issue has taken on particular importance as the U.S. Department of Health and Human Services addresses clear communication in the informed consent process as part of the Notice of Proposed Rulemaking for revisions to the Common Rule[5]. However, prior research has shown that participants may not fully comprehend research studies despite completion of an informed consent process[6-10].
These issues are particularly salient in the context of cancer. Research with stored biospecimens can provide a greater understanding of cancer etiology and discovery of new therapeutic modalities[11-14]. Participation in biobanks by large numbers of diverse participants is critical to reaching translational research goals in cancer and reducing cancer disparities[15].
To achieve the clear communication of information about a research study necessary for informed consent, health literacy is critical to consider[16]. Health literacy has been defined as the degree to which individuals can obtain, process, and understand basic health information and services needed to make appropriate health decisions[17]. Health literacy is a critical predictor of health outcomes, health care utilization, and health knowledge, and prior studies have shown a relationship between limited health literacy and lower comprehension of informed consent forms[10]. In one study, Donovan-Kicken and colleagues[16] found that those with limited health literacy had lower self-efficacy in understanding consent and disclosure statements, which led to feeling less informed about study participation and the associated risks.
The use of plain language, or strategies focused on clear and simple communication[18], may improve comprehension of information presented during the consent process. Various recommendations have been made for the use of plain language strategies in developing informed consent forms[19, 20]. The National Institutes of Health recommends writing consent documents at an eighth grade reading level[21]. Other guidelines have emphasized the use of plain language strategies such as graphics and images to supplement text, presenting topics in a clear and descriptive way, and providing adequate white space throughout the document[20, 22].
However, findings from prior studies testing whether such plain language strategies improve comprehension of a consent form have yielded mixed results. Walters and Hamrell[23] compared a consent form written at a 10th grade reading level with a form at the 6th grade level and found no significant difference in comprehension. Tait and colleagues[24] found that combining plain language strategies, such as supplementing text with graphics, shortening message length, and increasing white space and font size, resulted in improved understanding of research consent forms by study participants. However, other studies that compared a standard consent form with a form incorporating plain language features (e.g., use of active voice, larger font size, use of section headers) and found no significant difference in comprehension between the forms[8, 25, 26].
Based on prior research, some have proposed supplementing consent forms with additional written materials to improve comprehension of the information[19, 27]. Decision aids and informational booklets have increased participants’ understanding of research trials[28, 29]. One study found that a bulleted fact sheet used with a feedback session resulted in higher comprehension compared with a standard consent form[30]. Use of plain language strategies in developing supplemental material may be important in improving comprehension. For example, Campbell and colleagues[28] found that use of a supplemental informational handbook written at a 7th grade reading level improved comprehension among VA and hospital outpatients considering participating in a randomized control trial.
The use of plain language supplemental written materials in the informed consent process may be particularly important for informed consent to participate in a biobank, a process that poses substantial communication challenges. Storage of donated biospecimens in a biobank allows researchers to address research questions unplanned at the time of biospecimen donation, yet comprehension of information about a biobank may be particularly challenging for research participants during the consent process[31, 32]. Ormond and colleagues[33] found that participants had particular difficulty understanding the research goals of a biobank, as well as the experimental nature, privacy issues, and lack of direct personal benefit to participants. Other researchers have found that participants have difficulty understanding that consent for a biobank is a two-step process including consent to both the storage of biospecimens in the biobank and also possible future use of that stored biospecimen in other research projects[34].
Beskow and colleagues[35] used a Delphi process to create a plain language biobank consent form to address the communication and comprehension issues related to biobank consent. This consent form adhered to regulatory guidelines while also including information that potential participants considered important. The researchers used plain language principles to improve the understandability and readability of a biobank consent form, including use of short sentences with common language, a clear format, and sufficient white space and margins throughout the form[35]. However, because institutional review boards may not allow substitution of their standard consent form with this plain language consent form, there is a need to develop plain language supplemental material to assist in the biobank informed consent process. To address this issue, we developed plain language materials (i.e. brochures) designed to supplement the informed consent process for a biobank and conducted cognitive interviews to examine responses to the brochures with a diverse sample of women.
METHODS
Brochures development
We developed three plain language supplemental brochures for use in a randomized experiment to examine the effect of model of consent on participation in a hypothetical research study that also generates biospecimen samples for a biobank. In the experiment, participants were asked to consider participating in this research study to identify risk factors for cancers affecting women. They reviewed (1) a consent form that explained the research study, including the biobank; and (2) a plain language supplemental brochure that described the biobank component in more detail. We developed one brochure for each of three models of consent for secondary use of biospecimens stored in a biobank: notice (i.e., participants are notified that their samples may be used in research); broad (i.e., participants are asked to prospectively agree to allow use of their biospecimen in a broad range of studies); and study-specific (i.e., participants are asked for consent prior to each future study for which their biospecimen will be used)[36].
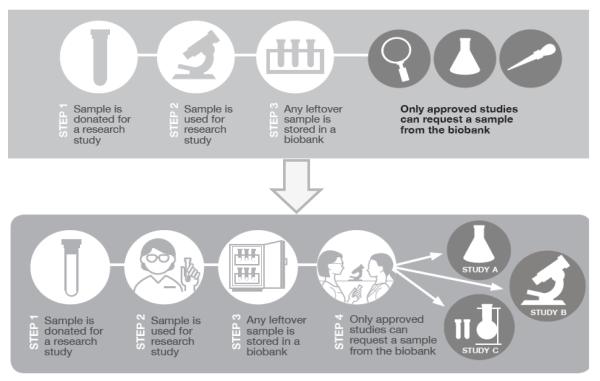
We worked with a graphic designer to develop the three supplemental brochures. We developed the content based on prior research by ourselves and others highlighting particularly difficult topics related to biobanks[33-35, 37] and integrated our prior findings about difficult terms in developing the wording of the text[37]. Each of the three brochures contained the following elements: Title page introducing the topic of the brochure; back page with information on the affiliated institution; a Frequently Asked Questions section; a graphic illustrating what happens when a sample is donated to a biobank (Figure 1); and sections describing the purpose of the research, the process of biospecimen donation, timing of consent process, and information about future research that might be conducted using stored biospecimens (Table 1).
Figure 1.
Modifications to graphic based on cognitive interviews
Table 1.
Content of supplemental consent brochures for a cancer biobank.
| Uniform content across the three supplemental brochures | |
| Name of section | Content |
| Title Page/Cover | A photo of middle-aged women of diverse ethnicities and statement of the brochure’s purpose. |
| Why am I being asked to donate a sample for research? |
Specific information to explain why individuals are being approached to participate in a research study. |
| How do researchers use samples? |
Statement conveying that future studies may use the donated biospecimen to test whether a particular treatment works or to try to prevent disease. The statement explains that some research may involve genes. |
| Graphic of biospecimen donation process |
A sequential graphic with both illustrations and text displaying 5 steps that would occur if a sample were donated for research. |
| How will my privacy be protected? |
Component of the frequently asked questions section that outlines how participant information will be encoded and kept private. |
| Will I find out the results of the research using my blood? |
Part of the frequently asked questions section that gives a direct answer and explanation of why participants should not expect to receive personal or general research results. |
| Back Page | The name and logo of the affiliated research institution. |
| Content that varies across supplemental brochures according to model of consent | |
| Name of section | Content |
| What happens when I donate a sample for research? |
Explanation of the process of donation to a biobank according to the specific model of consent. |
| What is a biobank? | Part of the frequently asked questions section that describes a biobank. The information provided also states whether active permission is needed for donation to the biobank according to the model of consent. |
| Will I be told when my sample is used in other future research studies? |
Part of the frequently asked questions section that describes whether participants will know when their sample is used in a future research study according to the model of consent. |
All of the brochures used the same title page and back page. In addition, much of the other content was consistent across the three brochures. The information that differed between the three brochures was specific to a particular model of consent for a biobank: processes of donation based on a specific model of consent; whether a specific permission step is needed for use of stored biospecimens in future research or whether consent is implied by participation in the original research study; and whether or not participants will know if their biospecimen is used in a future research study (Table 1).
We based all three brochures on best practices in plain language and health literacy[10, 19, 20, 38-41]. We used short sentences, active voice, common language and provided definitions for technical words (i.e., “gene,” “biobank”), headers before each section, presentation of context first, use of visual aids (e.g., boxes) to highlight key content, and font greater than 12 point when possible. We also avoided writing sentences using only all capital letters or using distracting color schemes.
During the development process, the brochures were reviewed multiple times by the full investigator team, which included researchers with expertise in bioethics, informed consent, health disparities, health literacy, and community-based participatory research. As described below, review by the research team and testing of brochures through cognitive interviews was conducted in an iterative process.
Cognitive interviews
During the brochures development process, we conducted qualitative, semi-structured, individual, in-person cognitive interviews to examine participants’ perceptions of the brochures. Each participant provided feedback regarding the understandability, graphics and layout, and cultural appropriateness of the brochures. Cultural appropriateness was based on participants’ perceptions of what was culturally appropriate and not a pre-determined definition of cultural appropriateness[39].
Participants
All participants in the cognitive interviews were female because they were the target audience for the brochures; the brochures were intended to be used in a randomized experiment focused on women considering participation in a research study and biobank[37]. Recruitment for this study was stratified between Black and White females because we found in a prior study that participants’ perceptions of various models of consent differed by race[37]. Women were eligible to participate in the cognitive interviews if they self-identified as either non-Hispanic White or Black; we recruited 7 Black women and 7 White women. All participants had the ability to speak and read English and were eighteen years of age or older. Participants also had to have utilized breast health services (e.g., mammogram, breast biopsy, breast cancer care) prior to the interview based on planned recruitment for the randomized experiment in which the brochures would be used.
We recruited participants through two mechanisms. The first was sending letters to participants in an existing biobank, the Women’s Health Repository (WHR), a cohort of women who sought breast health services at a clinic affiliated with a comprehensive cancer center. WHR participants had agreed to be re-contacted for research purposes. Nine women from WHR completed an interview. We also recruited 5 women who had not previously participated in the WHR biobank by distributing flyers at the clinic. In total, we conducted cognitive interviews with 14 women, at which point we reached saturation of qualitative themes[42].
Cognitive interview procedures
Each participant in a cognitive interview reviewed two of the three plain language supplemental consent brochures in order to limit participant burden and allow us to collect data comparing reactions to different brochures. We randomly assigned the brochures viewed within strata defined by race (i.e., Black / White). All cognitive interviews were conducted by trained masters-level research staff. Interviews were digitally recorded and lasted 40 minutes on average. Participants received a $50 gift card to a local grocery store as thanks for their time and cooperation. All study procedures were approved by the Washington University Institutional Review Board.
Cognitive interview participants were asked a series of open-ended questions by interviewers using a semi-structured interview guide to ensure consistency of topic, although they could vary the order. Participants were first asked to read one of the brochures and were then asked questions focused on understandability, acceptability of graphics and layout, and cultural appropriateness. For understandability, participants were asked to “teach-back” the content of key sections, stating in their own words the meaning of the information so that we could assess its clarity[43]. For graphics and layout, participants were asked their perceptions of the design of the brochure (e.g., font, color scheme, visuals). For cultural appropriateness, participants were asked whether they felt the brochure was appropriate for people like them. Participants were also asked what changes might be incorporated to improve the brochure. They then reviewed the second brochure and were asked a series of open-ended questions to assess whether the differences between the two brochures were clear (e.g., “What would you say are the differences between these two ways of donating samples to research?”).
Feedback from cognitive interview participants was used to revise the brochures using an iterative process. The study team completed four separate blocks of cognitive interviews. After each block of interviews, the study team reviewed participants’ responses and edited the brochures. The full investigator team then reviewed the content and design edits while focusing on examining the accuracy of the presented information. After each round of revision was completed, the next block of participants reviewed the revised brochures during their interview. Revisions affected both the design and content of the brochures. The cognitive interviews continued until saturation of themes was reached[42].
Analysis of cognitive interview data
Interviewers recorded participants’ responses using an interview comment form. The form was divided into two columns; interviewers recorded participants’ likes and dislikes about each section separately and also overall comments on other aspects of the brochures (i.e., appearance, graphics, layout). Within 24 hours, the interviewer summarized key observations and shared them with the research team. A master list was compiled for each of the three brochures, organizing participants’ likes and dislikes for each section and their responses regarding appearance, graphics and layout. Participants’ comments were then re-categorized for analysis based on the 3 domains (i.e., understandability, graphics and layout, and cultural appropriateness). To increase validity, a second trained graduate student indexed participants’ comments using the interview comment form. These analyses formed the basis for research team discussions and revisions to the brochures.
RESULTS
Understandability
We found that participants had good understanding of many of the concepts presented in text in the brochures. For example, participants generally understood the concept of a biobank, how their privacy would be protected, that they would not receive individual results from future research studies conducted with their biospecimen, and that their sample would be coded to not contain identifying information such as their name.
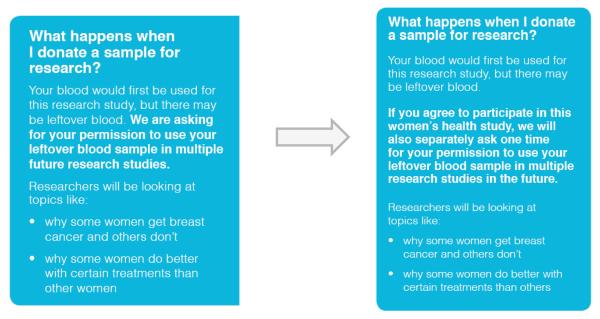
We found that other concepts were less understandable in early drafts of the brochures. In particular, information regarding the process of consent to a biobank proved difficult to convey clearly. This was information that varied between models of consent. We found that distinguishing between the notice model (with passive consent to a biobank) and broad model (with active general consent to a biobank) was particularly challenging. For example, the broad model of consent was originally described as: “We are asking for your permission to use your leftover blood sample in multiple future research studies.” The notice model of consent was described as: “We are letting you know that your leftover blood sample may be used in multiple research studies in the future.” In the initial cognitive interviews, the distinction between these models was unclear to participants. Participant 1 stated, “The words are different, but it’s saying the same thing.” In order to differentiate these two models of consent from one another, the research team revised the wording for the broad model of consent to: “If you agree to participate in this women’s health study, we will also separately ask one time for your permission to use your leftover blood sample in multiple research studies in the future.” (see Figure 2). The new explanation included the phrase “separately ask one time” to help clarify that participants would be asked for permission for future use of their biospecimen separately from being asked for consent to participate in the original research study. This wording change greatly improved comprehension of this concept.
Figure 2.
Changes to biospecimen donation section in brochure based on the broad model of consent.
In addition, we clarified one aspect of the notice model of consent. Many participants thought that the researcher would be required to explicitly ask for their permission before future use of a biospecimen, even though the notice model presumes consent for future uses of a biospecimen if the participant does not object upon initial collection. We observed that even when participants understood this information in the brochure, they still believed that this explicit permission step would take place. We addressed this issue in our revisions by removing the word “again”. We changed our original wording “We will not notify you or ask you for permission again when your sample is used in future research studies” to “We will not notify you or ask you for permission when your sample is used in future research studies.” This revision to the language clarified that researchers are notifying participants that their biospecimens may be used in the future but that they will not ask for permission for this use in a separate step.
Graphics and layout
Participants said that they liked many of the design elements of the brochures and these elements were therefore left largely unchanged during the revision process. For example, participants like the use of bold font, boxes to highlight key material, the blue background, font size, and sans serif font. Participant 4 mentioned that the “font is easy on the eyes” and that it was “easy to read.”
We changed the graphic illustrating what happened once a sample was donated extensively based on participant feedback (see Figure 1). Some participants liked the graphic and found it to be helpful. Participant 4 noted that the “diagram at the bottom is very helpful… I’m drawn to that” and Participant 8 noted that it was helpful because “it gives you step by step instructions.” Participant 4 also noted that the diagram “guided you through, kinda cartoony…and shows step by step what's going on.” However, while some participants liked the lack of text in the initial version of the graphic, others requested more explanatory text. In addition, some of the elements of the graphic were unclear. For example, some participants thought that a separate image of a test tube was confusing because it was not clear if the test tube was empty or if it contained a sample.
We therefore added explanatory text and replaced images that were found to be confusing. An image of test tubes was replaced with a refrigerator storing samples to show that leftover biospecimens will be stored in a biobank. We also better illustrated the concept that a donated biospecimen would be used in a research study by displaying a test tube full of liquid. This graphic showed that the blood sample had been collected and would be used in the future. We changed the icons of a magnifying glass and pipette to a flask and a microscope because participants more readily associated these icons with scientific research. We also added brief, explanatory captions to each step of the graphic. These modifications clarified the meaning of the graphic for participants.
We made a number of other changes to the brochures based on comments about graphics and layout. We made color changes in a number of places. For example, we changed a bright orange background color because participants felt that it had poor contrast with the text. Participant 1 noted that the font color was “it’s probably too like the orange background”. We also revised the layout of the FAQ section to reduce what participants perceived as blank space in one of the panels in the original versions.
Cultural appropriateness
Many participants highlighted the plain language design of the brochure favorably, saying it seemed to have been designed with them in mind. For example, Participant 5 noted that the brochure was “short and sweet and to the point and gets to every question that a person like me… might ask.” Participant 3 added that she appreciated that the brochure did not use jargon and stated that “big words confuse people.”
We made a number of changes to increase the appropriateness of brochures for our target audience. For example, participants suggested that we include more information on why they were contacted. Participant 12 stated, “It don’t make you feel comfortable on what you’re donating your blood for…it's missing more information on the reason why they're taking the sample”. In response, we changed the statement of the purpose of contact from “You are being asked to donate a blood sample because you are receiving breast health care” to “You are being asked to donate a blood sample for a study on women’s health.”
Participants noted several ways that the graphics and design could be a better fit to other women like them. For example, several participants did not like the original front page, which depicted a cartoon image of a magnifying glass; many wanted a picture of real women. Participant 1 noted that “I would want to enhance the appeal of the front and back [of the brochure]”. We therefore eliminated the cartoon illustration and instead used a picture of an ethnically diverse group of women of middle and older age. In addition, several participants felt that it was important to add information about the affiliation of the researchers and contact information. Participant 4 stated, “[I would like to see] a more prominent logo of [the institution] on the brochure.” We therefore added our institutional and contact information.
DISCUSSION
Participant input during the cognitive interview process led us to make substantial changes to improve the understandability, graphics and layout, and cultural appropriateness of plain language brochures to supplement an informed consent process for a biobank based on different models of consent. Participants generally liked the language, which was based on plain language and clear communication recommendations[20, 39]. However, we improved understandability in a number of different ways based on results from the cognitive interviews. We found that participants generally understood information on topics such as privacy and return of individual results. These topics were the subjects of the most common queries related to secondary research use of biospecimens found in our previous research[37]. We also found that topics such as possible risks of the use of biospecimens were least understood, which was similarly found in a prior study by Klima and colleagues[34]. We likewise found that a participant’s prior beliefs affect how they interpreted information about biobanks, particularly whether researchers would be able to use her biospecimen without a separate consent or permission step. Addressing this issue explicitly in the brochures proved to be important.
In terms of graphics and layout, participants generally liked the use of bolded text and boxed sections to draw their attention to important information. We found that our revised graphic helped participants understand the concept of a biobank, consistent with prior research showing the value of graphics and illustrations in informational brochures[22, 44-46]. We also found that explanatory captions used within the graphic improved comprehension of the information, consistent with guidelines from Doak and colleagues[39]. Participants highlighted some issues of poor contrast between background and text, which we revised consistent with plain language guidelines[39].
Work by other investigators suggests that individuals with limited health literacy and those from racial and ethnic minority groups have different informational needs and a greater mistrust of research[14, 47, 48]. As we intend to disseminate these brochures to diverse populations, we assessed whether both Black and White women found the brochures to be culturally appropriate. We made a number of revisions to improve participants’ perceptions of the appropriateness of the brochures for women similar to them. Our main modifications were replacing the front cover image from a cartoon magnifying glass to a picture of a diverse group women who appeared to be members of the target audience, as well as providing an institutional affiliation.
Best Practices
We based the development and revision of the plain language brochures on existing recommendations and guidelines. Central to plain language guidelines is communicating in a way that is straightforward and utilizes conversational, commonly used vocabulary[18]. Consistent with plain language guidelines[19, 20, 38-41], participants in this study liked the lack of medical jargon and the use of common words.
Incorporating another strategy to make our graphics clear, we developed a graphic with brief, explanatory captions to clarify biobank concepts and procedures[22, 39]. We found that using cognitive interviews to assess the graphic was important, as particular images did not resonate with participants and required revisions. Participants also liked the formatting of the brochures overall but wanted a balance between text, graphics, and white space, leading to revisions.
There are additional insights to guide other researchers and practitioners developing educational brochures related to cancer biobanks. For example, we found that participants believed that they would be asked for permission at some time in the future under the notice model of consent, even if they understood the statement that they were only being notified. Adding an explicit statement that they would not be contacted for permission helped to clarify this issue. We would recommend those developing consent forms or brochures to explicitly address the timing and details regarding when and how participants will be notified or asked for permission for future research uses of biospecimens.
Cognitive interview participants were Black and White women aged 18 years of age and older who were associated with a comprehensive cancer center. Although these participants were representative of the target audience for the brochures under development, the findings may not generalize to other populations. It would certainly be important to test the brochures with other target audiences defined by race/ethnicity, gender, or setting before using the brochures more broadly. However, our findings demonstrate that these methods may be used to tailor consent form brochures, such as the ones developed here, to other populations. In addition, future research is needed to investigate the effects of the brochures on participants’ responses and donation to an actual biobank. We are currently conducting a randomized trial to examine comprehension of the brochures and intentions to donate to a cancer-related biobank.
Educational Implications
Our main goal was to provide plain language information about donation processes to a cancer biobank to supplement an informed consent form. We developed and conducted cognitive testing with supplemental brochures that clearly communicated information about three different models for consent to future use of biospecimens. This study therefore adds to our understanding of how best to present content to help women from two different racial groups make informed decisions about participation in a cancer biobank. This is particularly critical as participation of all population subgroups in biobanks is essential to translational research goals in cancer[49, 50].
Previous research shows that participants consider a multitude of topics important when reviewing consent forms and making a decision to participate in research studies[11, 14, 51, 52]. Our findings demonstrate the importance of utilizing plain language guidelines to clearly communicate information to participants and using brochures to improve participants’ understanding of all aspects of the study. Research study staff interacting with prospective participants should be trained to verbally communicate consent information in plain language in order to supplement traditional consent processes and plain language brochures. Finally, we recommend working with institutional review boards to advance the use of plain language consent brochures and forms in all studies. The universal usage of plain language guidelines and brochures can increase informed decision making in the consent process.
Acknowledgements
This project was supported by grant number U54CA153460-03S1, a supplement to the Program for the Elimination of Cancer Disparities grant from the National Cancer Institute. Washington University School of Medicine, the Barnes-Jewish Hospital Foundation, and Siteman Cancer Center. We thank all of our community partners, The Breakfast Club, Inc., Siteman Cancer Center and Women’s Health Repository. We thank the 60 women who participated in the study and shared their opinions.
Contributor Information
Bettina F. Drake, Division of Public Health Sciences, Washington University in St. Louis School of Medicine, St. Louis, MO 63110 United States, Alvin J. Siteman Cancer Center, St. Louis, MO 63110.
Katherine M. Brown, Division of Public Health Sciences, Washington University in St. Louis School of Medicine, St. Louis, MO 63110 United States.
Sarah Gehlert, Division of Public Health Sciences, Washington University in St. Louis School of Medicine, St. Louis, MO 63110 United States, Alvin J. Siteman Cancer Center, St. Louis, MO 63110 United States.
Leslie E. Wolf, Center for Law Health and Society, Georgia State University College of Law, 85 Park Place NE, Atlanta, GA 30303.
Joann Seo, Division of Public Health Sciences, Washington University in St. Louis School of Medicine, St. Louis, MO 63110 United States.
Hannah Perkins, Division of Public Health Sciences, Washington University in St. Louis School of Medicine, St. Louis, MO 63110 United States.
Melody S. Goodman, Division of Public Health Sciences, Washington University in St. Louis School of Medicine, St. Louis, MO 63110 United States, Alvin J. Siteman Cancer Center, St. Louis, MO 63110 United States.
Kimberly A. Kaphingst, Department of Communication, University of Utah, 255 S. Central Campus Dr., Salt Lake City, UT 84112-0491, Huntsman Cancer Institute, 2000 Circle of Hope Drive, Salt Lake City, UT 84112.
REFERENCES
- 1.Servies', U.D.o.H.a.H. Human Subjects Research Protection: Enhancing Protections for Research Subjects and Reducing Burden, Delay and Ambiguity for Investigators. 2011.
- 2.Services', U.D.o.H.a.H. Code of Federal Regulations: 45 C.F.R. § 46. 2009 Retrieved from http://www.hhs.gov/ohrp/policy/ohrpregulations.pdf.
- 3.Cambon-Thomsen A. The social and ethical issues of post-genomic human biobanks. Nat Rev Genet. 2004;5(11):866–73. doi: 10.1038/nrg1473. [DOI] [PubMed] [Google Scholar]
- 4.Hansson MG, et al. Should donors be allowed to give broad consent to future biobank research? Lancet Oncol. 2006;7(3):266–9. doi: 10.1016/S1470-2045(06)70618-0. [DOI] [PubMed] [Google Scholar]
- 5.Services', U.D.o.H.a.H. Federal Policy for the Protection of Human Subjects. Docket Number: HHS-OPHS-2015-0008. Federal Register. 2015;80(173) [Google Scholar]
- 6.Falagas ME, et al. Informed consent: how much and what do patients understand? Am J Surg. 2009;198(3):420–35. doi: 10.1016/j.amjsurg.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Paasche-Orlow MK, Taylor HA, Brancati FL. Readability standards for informed-consent forms as compared with actual readability. N Engl J Med. 2003;348(8):721–6. doi: 10.1056/NEJMsa021212. [DOI] [PubMed] [Google Scholar]
- 8.Davis TC, et al. Informed consent for clinical trials: a comparative study of standard versus simplified forms. J Natl Cancer Inst. 1998;90(9):668–74. doi: 10.1093/jnci/90.9.668. [DOI] [PubMed] [Google Scholar]
- 9.Sheridan SL, et al. Interventions for individuals with low health literacy: a systematic review. J Health Commun. 2011;16(Suppl 3):30–54. doi: 10.1080/10810730.2011.604391. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen-Bohlman L, Panzer A, Kindig D, editors. Health Literacy: A Prescription to End Confusion. National Academies Press; Washington, DC: 2004. [PubMed] [Google Scholar]
- 11.Chen DT, et al. Research with stored biological samples: what do research participants want? Arch Intern Med. 2005;165(6):652–5. doi: 10.1001/archinte.165.6.652. [DOI] [PubMed] [Google Scholar]
- 12.Helft PR, et al. Cancer patients' attitudes toward future research uses of stored human biological materials. J Empir Res Hum Res Ethics. 2007;2(3):15–22. doi: 10.1525/jer.2007.2.3.15. [DOI] [PubMed] [Google Scholar]
- 13.Huber J, et al. Two decades' experience with a prospective biobank for urologic oncology: research, clinical care, and the patients' view. Urol Oncol. 2013;31(7):990–6. doi: 10.1016/j.urolonc.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Pentz RD, Billot L, Wendler D. Research on stored biological samples: views of African American and White American cancer patients. Am J Med Genet A. 2006;140(7):733–9. doi: 10.1002/ajmg.a.31154. [DOI] [PubMed] [Google Scholar]
- 15.Luque JS, et al. Formative research on perceptions of biobanking: what community members think. J Cancer Educ. 2012;27(1):91–9. doi: 10.1007/s13187-011-0275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donovan-Kicken E, et al. Health literacy, self-efficacy, and patients' assessment of medical disclosure and consent documentation. Health Commun. 2012;27(6):581–90. doi: 10.1080/10410236.2011.618434. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services Healthy People 2010. 2000 Retrieved from http://www.healthypeople.gov/2010/
- 18.Eagleson R. Short Definition of Plain Language. Improving Communication from the Federal Government to the Public. 2014 from http://www.plainlanguage.gov/whatisPL/definitions/eagleson.cfm. [Google Scholar]
- 19.Jefford M, Moore R. Improvement of informed consent and the quality of consent documents. Lancet Oncol. 2008;9(5):485–93. doi: 10.1016/S1470-2045(08)70128-1. [DOI] [PubMed] [Google Scholar]
- 20.Ridpath JR, Wiese CJ, Greene SM. Looking at research consent forms through a participant-centered lens: the PRISM readability toolkit. Am J Health Promot. 2009;23(6):371–5. doi: 10.4278/ajhp.080613-CIT-94. [DOI] [PubMed] [Google Scholar]
- 21.Services', U.D.o.H.a.H. Guidelines for the conduct of research involving human subjects at the National Institutes of Health. Washington DC: 2013. Retrieved from http://ohsr.od.nih.gov/ohsr/public/SOP_12_v3_3-12-14_508.pdf. [Google Scholar]
- 22.Schnitzer A, Rosenzweig M, Harris B. Health literacy: A survey of the issues and solutions. Journal of Consumer Health on the Internet. 2011;15(2):164–179. [Google Scholar]
- 23.Walters K, Hamrell M. Consent forms, lower reading levels, and using Flesch-Kincaid readability software. Drug Information Journal. 2008;42(4):385–394. [Google Scholar]
- 24.Tait A, et al. Informing the uninformed: optimizing the consent message using a fractional factorial design. JAMA pediatrics. 2013;167(7):640–646. doi: 10.1001/jamapediatrics.2013.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enama ME, et al. Randomization to standard and concise informed consent forms: development of evidence-based consent practices. Contemp Clin Trials. 2012;33(5):895–902. doi: 10.1016/j.cct.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coyne CA, et al. Randomized, controlled trial of an easy-to-read informed consent statement for clinical trial participation: a study of the Eastern Cooperative Oncology Group. J Clin Oncol. 2003;21(5):836–42. doi: 10.1200/JCO.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 27.McGraw S, et al. Clarity and appeal of a multimedia informed consent tool for biobanking. IRB: A Review of Human Subjects Reserach. 2012;34(1):9–19. [PubMed] [Google Scholar]
- 28.Campbell H, et al. Impact of a clinical trials information handbook on patient knowledge, perceptions, and likelihood of participation. IRB: Ethics & Human Research. 2008:6–14. [PubMed] [Google Scholar]
- 29.Juraskova I, et al. Improving informed consent: pilot of a decision aid for women invited to participate in a breast cancer prevention trial (IBIS-II DCIS) Health Expect. 2008;11(3):252–62. doi: 10.1111/j.1369-7625.2008.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kass NE, et al. A pilot study of simple interventions to improve informed consent in clinical research: feasibility, approach, and results. Clin Trials. 2015;12(1):54–66. doi: 10.1177/1740774514560831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mancini J, et al. Consent for biobanking: assessing the understanding and views of cancer patients. J Natl Cancer Inst. 2011;103(2):154–7. doi: 10.1093/jnci/djq498. [DOI] [PubMed] [Google Scholar]
- 32.Robinson JO, et al. Participants' recall and understanding of genomic research and large-scale data sharing. J Empir Res Hum Res Ethics. 2013;8(4):42–52. doi: 10.1525/jer.2013.8.4.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ormond KE, et al. Assessing the understanding of biobank participants. Am J Med Genet A. 2009;149A(2):188–98. doi: 10.1002/ajmg.a.32635. [DOI] [PubMed] [Google Scholar]
- 34.Klima J, et al. Understanding of informed consent by parents of children enrolled in a genetic biobank. Genet Med. 2014;16(2):141–8. doi: 10.1038/gim.2013.86. [DOI] [PubMed] [Google Scholar]
- 35.Beskow LM, et al. Developing a simplified consent form for biobanking. PLoS One. 2010;5(10):e13302. doi: 10.1371/journal.pone.0013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mello MM, Wolf LE. The Havasupai Indian tribe case--lessons for research involving stored biologic samples. N Engl J Med. 2010;363(3):204–7. doi: 10.1056/NEJMp1005203. [DOI] [PubMed] [Google Scholar]
- 37.Brown KM, et al. Differences in preferences for models of consent for biobanks between Black and White women. J Community Genet. 2015 doi: 10.1007/s12687-015-0248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The Plain Language Action and Information Network (PLAIN) Federal Plain Language Guidelines. Washington D.C.: 2011. Retrieved from http://www.plainlanguage.gov/howto/guidelines/bigdoc/fullbigdoc.pdf. [Google Scholar]
- 39.Doak CC, Doak LG, Root JH. Teaching Patients with Low Literacy Skills. 2nd ed J.B. Lippincott Company; Philadelphia: 1996. [Google Scholar]
- 40.Institute', N.C. Clear & Simple: Developing Effective Print Materials for Low-Literate Readers. 2003.
- 41.Prevention', C.f.D.C.a. Simply put: A guide for creating easy-to-understand materials. Strategic and Proactive Communication Branch Retrieved; Atlanta, Georgia: 2009. from http://www.cdc.gov/healthliteracy/pdf/Simply_Put.pdf. [Google Scholar]
- 42.Strauss A, J C. Basics of Qualitative Research: Second Edition: Techniques and Procedures for Developing Grounded Theory. Sage Publications, Inc; 1998. [Google Scholar]
- 43.Schillinger D, et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med. 2003;163(1):83–90. doi: 10.1001/archinte.163.1.83. [DOI] [PubMed] [Google Scholar]
- 44.Delp C, Jones J. Communicating information to patients: the use of cartoon illustrations to improve comprehension of instructions. Acad Emerg Med. 1996;3(3):264–70. doi: 10.1111/j.1553-2712.1996.tb03431.x. [DOI] [PubMed] [Google Scholar]
- 45.Houts PS, et al. The role of pictures in improving health communication: a review of research on attention, comprehension, recall, and adherence. Patient Educ Couns. 2006;61(2):173–90. doi: 10.1016/j.pec.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Mansoor LE, Dowse R. Effect of pictograms on readability of patient information materials. Ann Pharmacother. 2003;37(7-8):1003–9. doi: 10.1345/aph.1C449. [DOI] [PubMed] [Google Scholar]
- 47.Pentz RD, Theriault RL. Research Ethics: Clinical trial abuse and the public trust. Breast diseases: A year book quarterly. 2001;12:141–144. [Google Scholar]
- 48.Randall V. Slavery, Segregation and Racism: Trusting the Health Care Sysmtem Ain't Always Easy--An African American Perspective on Bioethics. St. Louis University Public Law Review. 1995;15:191. [PubMed] [Google Scholar]
- 49.Branson RD, Davis K, Jr., Butler KL. African Americans' participation in clinical research: importance, barriers, and solutions. Am J Surg. 2007;193(1):32–9. doi: 10.1016/j.amjsurg.2005.11.007. discussion 40. [DOI] [PubMed] [Google Scholar]
- 50.Field LA, et al. Identification of differentially expressed genes in breast tumors from African American compared with Caucasian women. Cancer. 2012;118(5):1334–44. doi: 10.1002/cncr.26405. [DOI] [PubMed] [Google Scholar]
- 51.Murphy J, et al. Public Perspectives on Informed Consent for Biobanking. Am J Public Health. 2009;99(12):2128–2134. doi: 10.2105/AJPH.2008.157099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Platt J, et al. Public preferences regarding informed consent models for participation in population-based genomic research. Genet Med. 2014;16(1):11–8. doi: 10.1038/gim.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]