Abstract
Respiratory complex I has an L-shaped structure formed by the hydrophilic arm responsible for electron transfer and the membrane arm that contains protons pumping machinery. Here, to gain mechanistic insights into the role of subunit NuoL, we investigated the effects of Mg2+, Zn2+ and the Na+/H+ antiporter inhibitor 5-(N-ethyl-N-isopropyl)-amiloride (EIPA) on proton pumping activities of various isolated NuoL mutant complex I after reconstitution into Escherichia coli double knockout (DKO) membrane vesicles lacking complex I and the NADH dehydrogenase type 2. We found that Mg2+ was critical for proton pumping activity of complex I. At 2 µM Zn2+, proton pumping of the wild-type was selectively inhibited without affecting electron transfer; no inhibition in proton pumping of D178N and D400A was observed, suggesting the involvement of these residues in Zn2+ binding. Fifteen micromolar of EIPA caused up to ∼40% decrease in the proton pumping activity of the wild-type, D303A and D400A/E, whereas no significant change was detected in D178N, indicating its possible involvement in the EIPA binding. Furthermore, when menaquinone-rich DKO membranes were used, the proton pumping efficiency in the wild-type was decreased significantly (∼50%) compared with NuoL mutants strongly suggesting that NuoL is involved in the high efficiency pumping mechanism in complex I.
Keywords: complex I, energy coupling, menaquinone, proton pumping, ubiquinone
Complex I (NADH:quinone oxidoreductase) (E.C.1.6.5.3) is one of the largest multi-subunit membrane protein complex found in mitochondria and many aerobic microorganisms. It plays a central role in cellular energy metabolism, and complex I dysfunction has been implicated in a variety of human mitochondrial diseases including neurodegenerative diseases and aging (1, 2). Complex I transfers two electrons from NADH to ubiquinone (UQ), translocates protons across the membrane, and generates a transmembrane electric potential and proton gradient essential for ATP synthesis and transport processes (3–6). Recent X-ray crystal structures from prokaryotic and eukaryotic complex I confirmed (7–9) that the redox reaction which involves flavin mononucleotide (FMN), a chain of seven Fe/S clusters (4), and UQ, takes place in the hydrophilic peripheral domain of the L-shaped molecule, whereas proton pumping machinery is equipped in the hydrophobic membrane domain far from the catalytic site. The membrane-bound subunits NuoL (Escherichia coli name)/ND5, NuoM/ND4 and NuoN/ND2 are related to subunits of bacterial multiple resistance and pH adaptation (Mrp) family of Na+/H+ antiporters (10), suggesting some similarities in their mechanisms of ion translocation. It has been hypothesized that long-range conformational change triggered by redox energy drives proton translocation through these antiporter-like subunits. However, the molecular details of how the conformational changes are transmitted into the antiporter domains to activate proton pumps are far from being understood.
We have previously shown the functional importance of NuoL/ND5 for redox-linked proton pumping coupling mechanism by mutagenesis study in the E. coli NuoL subunit (11). Although NuoL is situated at the distal end of the membrane domain, specific point mutations in NuoL could change the degree of coupling between electron transfer and proton pumping. Peripheral deamino-NADH (dNADH):K3Fe(CN)6 reductase activities basically remained unchanged in all the NuoL mutants, whereas the proton pumping efficiency (the ratio of H+/e−, the initial pumping rate versus NADH oxidase) was decreased in most NuoL mutants by 30–50%, compared with the wild-type (WT) (11). Especially, the proton pumping efficiency in D178N, D303A and D400A mutants was significantly decreased to 50%, suggesting that the H+/e− stoichiometry became half (2H+/2e−), based on the literature that complex I has the high proton pumping efficiency, a stoichiometry of 4H+/2e−.
In this study, to gain a more detailed mechanistic insight, by using purified the WT and NuoL mutant complex I, we further investigated how mutations of conserved charged amino acids in the entrance and exit of the proposed proton pumping pathways in NuoL affect the proton-pumping machinery of complex I. To facilitate purification of each mutant, we newly generated each NuoL mutation in a strain (MC4100/His9nuoE) containing nine histidine coding sequence inserted upstream of nuoE in the chromosome (12) by homologous recombination. We have shown that the WT complex I was successfully purified via the His-tag. By using this method, we were able to obtain enough amounts of pure and intact E. coli NuoL mutants, regardless of the expression levels of NuoL mutant complex I. Also, we have developed an instant reconstitution method, which is alternative to preparing proteoliposomes (13–16), to measure proton pumping activities of isolated mutant complex I for our screening purpose. Preliminary results (17) showed us the feasibility of reconstituting purified complex I into E. coli double knockout (DKO) membrane vesicles. Our E. coli DKO membrane vesicles contain neither complex I nor the alternative NADH dehydrogenase (NDH-2), showing no NADH-initiated oxidase and H+ pumping activities. Regular traditional preparation of proteoliposomes takes at least 3–4 h, whereas our new membrane reconstitution allows us to analyse proton pumping and NADH oxidase activities of various mutant complex I in 5 min. We found that the results by this DKO reconstitution method qualitatively reflected electron transfer and proton pumping activities of the WT and NuoL mutants, compared with the results with membrane vesicles prepared from the WT and NuoL mutant strains (11).
Using this system, we analysed the effects of inorganic divalent cations, Mg2+ and Zn2+ on proton pumping activities. Mg2+ plays an important role in transmembrane ion movement (18, 19), and Mg2+ binding is suggested to have a structural role in the stabilization of the interface between the two subunits important for proton pumping in cytochrome c oxidase (20). The Zn2+ binding is known to inhibit proton transfer activity in the bacterial reaction centre (21), cytochrome bc1 complex (22) and cytochrome c oxidase (23, 24). Although the effects of Mg2+ and Zn2+ on electron transfer activities (NADH:decylubiquinone (DQ) or NADH:hexaammineruthenium (HAR) or K3Fe(CN)6) have been well studied (13, 25, 26), this study is the first detailed report for their effects on proton pumping activities of complex I. We also studied the effects of 5-(N-ethyl-N-isopropyl)-amiloride (EIPA), a specific inhibitor for Na+/H+ antiporters, and menaquinone (MK), a ‘low potential’ electron acceptor.
Experimental Procedures
Preparation of the knockout NuoL (ΔNuoL) and mutant strains in the (His)9-nuoE MC4100 cells
The (His)9-nuoE MC4100 (F–, araD139, Δ(arg F-lac)U169, ptsF25, relA1, flb5301, rpsL 150.–) strain was generated previously for efficient purification purpose (12). The ΔNuoL [Δ(nuoL::Spc)] and mutant NuoL strains were generated in this strain by employing the same method described previously (11). The presence of mutation in the genomic gene was verified by PCR and DNA sequencing.
Isolation of complex I
Escherichia coli complex I was isolated from the WT, ΔNuoL and NuoL mutant strains by following the procedure published previously (12). Briefly, complex I was extracted from the membrane fraction with dodecyl-β-d-maltoside (DDM) at a final concentration of 1.2% (w/v), isolated using Ni-NTA resin, desalted, and concentrated to 3–8 mg protein/ml. The purified enzyme was quickly frozen in liquid nitrogen and stored at −80 °C until use.
Generation of DKO E. coli strains
There are two types of NADH dehydrogenases in E. coli membranes. One is called, complex I (NADH dehydrogenase type 1 or NDH-1), and the other is NDH-2. To eliminate NADH dehydrogenase activities that interfere with purified complex I proteins in our membrane reconstitution experiments, we decided to generate a DKO strain in the E. coli MC4100 strain, which lacks both complex I and NDH-2 activities. First, we chose a complex I deficient strain, which was previously generated in our lab by knocking out the iron–sulphur cluster N1b. Cluster N1b is located in the NuoG subunit and essential for electron transfer activity of complex I as well as the complex I assembly. The cluster N1b knockout (ΔN1b strain) was generated by homologous recombination from the nuoG knockout strain [Δ(nuoG::Spc)] described in ref. (27), in which all four cysteine residues ligating to cluster N1b were replaced with alanine (C36A, C47A, C50A and C69A), using a gene replacement technique with pKO3 (28). The detailed procedures were published elsewhere (27, 29, 30). In this ΔN1b strain, no dehydrogenase subcomplex (NuoEFG subcomplex) of complex I was detected in the membrane and supernatant fractions. Next, the ΔN1b strain was further used to knock out the ndh gene (encoding NDH-2), yielding a DKO strain by using the same gene replacement technique as used to generate the ΔN1b strain.
Preparation of E. coli DKO membrane vesicles
The standard E. coli DKO membrane vesicles were prepared according to (29). In brief, the cells were grown in 250 ml of Terrific Broth medium (31) until A600 of ∼6 and were then harvested at 5,800 × g for 10 min. The cells were resuspended at 10% (w/v) in a buffer containing 50 mM Bis-Tris (pH 6.0), 1 mM EDTA, 1 mM DTT, 1 mM PMSF and 10% (w/v) glycerol. The cell suspensions were passed once in a French press at 25,000 p.s.i. and centrifuged at 23,400 × g for 10 min. The supernatant was ultracentrifuged at 256,600 × g for 1 h. The pellet was resuspended in a buffer contacting 50 mM Bis-Tris (pH 6.0), 2 mM CaCl2 and 10% glycerol. The resulting membrane vesicles were frozen in liquid nitrogen and stored in small aliquots at −80 °C until use. To prepare UQ-rich and MK-rich membranes, the cells were grown in 280 ml of Terrific Broth medium in a 2.8-l flask at 250 rpm for 5 h, and in 2.5 l of Terrific Broth medium in a 2.8-l flask at 80 rpm for 12 h, respectively.
Reconstitution of purified complex I into DKO membrane
Purified WT and NuoL mutant complex I were mixed with DKO membrane vesicles by tapping in an Eppendorf tube with a total volume of 50 μl at 1:20 ratio (typically, 1:20 mg/ml), centrifuged briefly and then placed on ice right before use. We measured proton pumping and NADH oxidase activities in parallel for each experiment.
Proton translocation activity
The generation of a proton gradient was determined by monitoring the fluorescence quenching of 9-amino-6-chloro-2-methoxyacridine (ACMA, Sigma). Reconstituted membranes, 2.5–5 μg, were added to the assay buffer, 50 mM MOPS pH 7.0 containing 50 mM KCl, 20 mM MgCl2 and 0.2 μM ACMA as a standard condition and incubated at 30 °C for 3 min. In the experiments to investigate the effect of MgCl2 on proton pumping activities, we varied MgCl2 from 0 to 20 mM. The fluorescence was detected with a Fluoromax-4 spectrofluorometer (Horiba) at an excitation wavelength of 430 nm and an emission wavelength of 480 nm. The reaction was started by the addition of 150 μM NADH. The slopes for the initial acidification rates were calculated from the data using a 5-s time window 5 s after the addition of NADH.
Other analytical procedures
NADH-oxidase activity was measured by adding 1–5 μg of reconstituted membranes in the same assay buffer used for proton translocation activity and incubated at 30 °C for 3 min. The absorbance was measured at 340 nm using a Cary 60 spectrophotometer (Agilent). The reaction was initiated by the addition of 150 μM NADH. Extinction coefficient of €340 = 6.22 mM−1 cm−1 for NADH was used for activity calculations. Reported values are the average of three measurements. The extraction and quantification of quinones in DKO membrane vesicles were performed as described in Ref. (12).
Results
Instant reconstitution of purified complex I into E. coli DKO membrane vesicles
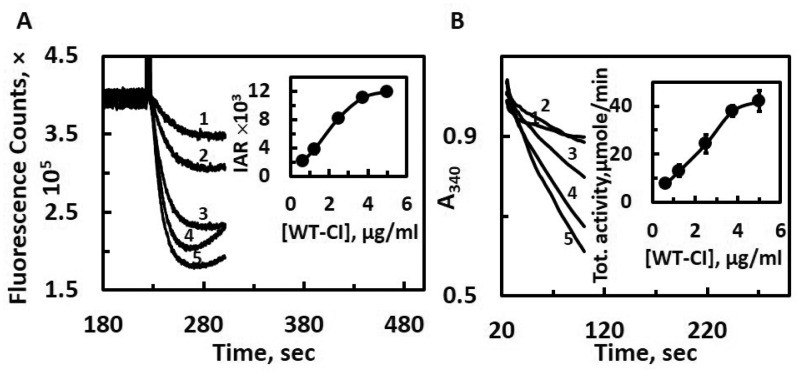
Proteoliposome preparation requires a relatively long processing time to remove detergent molecules effectively (13–16). In addition, the reproducibility of proteoliposome preparations is rather low in terms of both quality and quantity, even though the same lot of proteins and lipids are used. Thus, in this study, we have developed a simple and efficient screening method for proton pumping and electron transfer assay. We prepared membrane vesicles from a DKO E. coli cells, which do not contain both complex I and NDH-2 (32). The DKO membrane vesicles showed no NADH-oxidase activities or NADH-initiated proton pumping activities (32). Through optimization experiments, we found that just briefly mixing by tapping is enough to reconstitute DKO membranes with isolated complex I. The ratio of 1:20 between complex I and DKO membranes typically gave the highest activity per milligram of complex I. Higher detergent (DDM) concentrations (>0.05%) inhibited the activities. Figure 1A shows proton pumping activities of the isolated WT complex I after the instant DKO reconstitution. By using ACMA as a delta-pH indicator, reproducible NADH-linked proton pumping activity was observed after the addition of NADH. The initial rate of proton pumping activities was dose-dependent up to 3.75 μg of DKO reconstituted WT E. coli complex I (Fig. 1A, inset). Similarly, the electron transfer activity of DKO reconstituted WT complex I was also linear up to 3.75 μg of the WT complex I (Fig. 1B, inset). The specific NADH-oxidase activity was in the similar range of the specific NADH:DQ activities obtained from proteoliposome experiments, around 4–6 μmol/min/mg. Therefore, we set the amount of complex I at 2.5 μg/ml for further assays under various conditions. As both proton pumping and NADH-oxidase activities require complex I to be successfully inserted into the membrane, the presence of these activities strongly suggests that a substantial population was inserted in the right orientation. In fact, there was no difference in NADH:ferricyanide activities of these DKO membranes reconstituted with the WT complex I in the presence or absence of 1% DDM. This indicates that we can rule out the possibility that some population of complex I was inserted in the wrong direction, which usually happens for proteoliposomes. We also confirmed that the specific E. coli complex I inhibitor squamotacin completely inhibited the both NADH oxidase and proton pumping activities (data not shown).
Fig. 1.
Proton pumping (A) and NADH-oxidase (B) activities of the WT complex I reconstituted in DKO membranes. The traces from 1 through 5 refer to different WT complex I concentrations in the assay mixture: 0.625, 1.25, 2.5, 3.75 and 5 μg/ml, respectively. The insets in (A) and (B) show the relationship between WT complex I concentrations versus initial acidification rate (IAR) or specific NADH-oxidase activities, respectively. All measurements were carried out in the reaction mixture containing 50 mM MOPS/Na pH 7.0, 50 mM KCl, 20 mM MgCl2, 0.2 μM ACMA and 20 μM DQ. The reaction was initiated by the addition of 150 μM NADH. Both proton pumping and NADH-oxidase activities were measured by using the same reconstituted samples. Averages and standard deviations were calculated from four trials.
Purification of NuoL mutant complex I
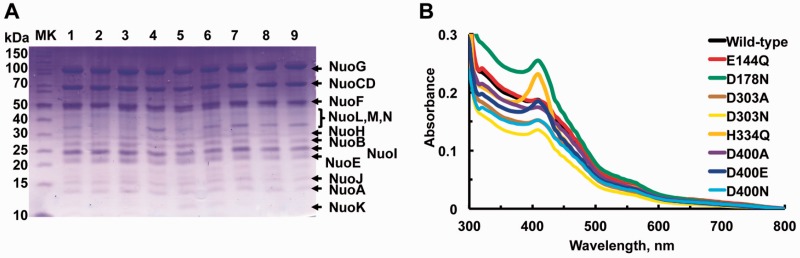
Sequence comparisons previously suggested that NuoL/ND5 of complex I bears striking resemblance to MrpA/MnhA of multisubunit secondary Na+/H+ antiporters (11). In this study, we introduced NuoL point mutations at highly conserved charged residues E144, D178, D303, H334 and D400 (E. coli numbering) in the (His)9-nuoE MC4100 strain for purification, which are located near the entrances and exits of the proposed proton-pumping pathways (33) as shown in Fig. 2. We purified the total of eight NuoL mutant complex I, E144Q, D178N, D303A, D303N, H334Q, D400A, D400E and D400N. Based on the SDS-PAGE patterns (Fig. 2A), these purified NuoL complex I contain all the subunits and have similar purity. The UV-Vis spectra data (Fig. 2B) suggest that they contain roughly similar amount of cofactors, although a trace amount of cytochrome b impurity was noticeable in H334Q and D178N.
Fig. 2.
(A) SDS-PAGE analysis and (B) UV–visible absorption spectra of purified WT and various NuoL mutant proteins. Samples (10 µg) were loaded onto a 10% Tris–Tricine SDS-PAGE gel. lane 1, WT; lane 2, E144Q; lane 3, D178N; lane 4, D303A; lane 5, D303N; lane 6, H334Q; lane 7, D400A; lane 8, D400E; lane 9, D400N. The UV–visible spectra were taken in 50 mM Bis-Tris (pH 6.0) containing 10% glycerol. All the data were normalized at 1 mg/ml.
Mg2+ plays a critical role in operating proton pumping machinery of complex I
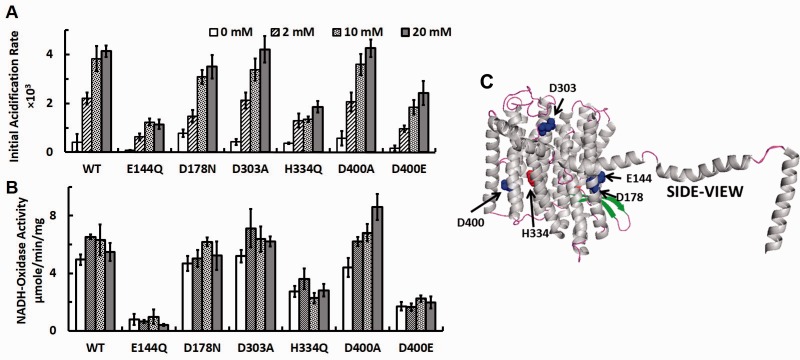
Using this instant reconstitution method with DKO membranes, we first studied effects of Mg2+ on complex I activities. Surprisingly, there were almost no proton pumping activities (measured as initial acidification rates) detected in the absence of Mg2+ in the WT complex I, and proton pumping activities increased in a dose-dependent manner as the concentration of Mg2+ was raised up to 10–20 mM (Fig. 3A). Basically, the similar activation effect by Mg2+ on proton pumping rates was also observed in all NuoL mutant complex I tested including E144Q, in which it has previously been reported that the proton pumping activity (and dNADH oxidase activities) was drastically decreased to ∼10–20% of WT using intact membrane vesicles (11). These data suggest that in order to obtain the maximum proton pumping activities of complex I, at least 10–20 mM Mg2+ was required regardless of point mutation sites (Fig. 3C). Interestingly, however, electron transfer (NADH-oxidase) activities were found to be independent of Mg2+ concentrations and were not affected by much except in the D400A mutant, in which the electron transfer activity was almost doubled at 20 mM compared with 0 mM (Fig. 3B). These results suggest that Mg2+ plays a crucial role in proton pumping function by complex I. In striking contrast, it does not seem to play an essential role in electron transfer activities of E. coli complex I.
Fig. 3.
Effects of Mg2+ on proton-pumping and electron transfer activities in the WT and various NuoL mutant complex I reconstituted into DKO membranes. Initial acidification rates (A) and NADH-oxidase activities (B) of at various Mg2+ concentrations are shown. Values are means ± SD (n = 3–4). (C) The locations of the mutated amino acids are shown in the NuoL subunit structure extracted from the three-dimensional crystal structure of Escherichia coli complex I (PDBID: 3RKO).
Effects of low Zn2+ concentrations and EIPA on proton pumping activities of complex I
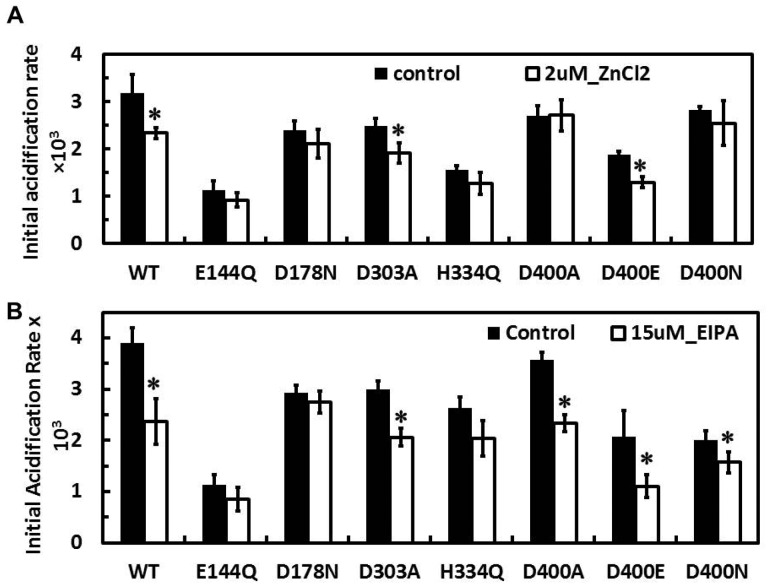
Next, we studied the effects of Zn2+ on complex I activities. It is known that Zn2+ rapidly and tightly binds to the resting state(s) of complex I (25). Reported IC50 values for the Zn2+ inhibition of electron transfer activities by complex I are 10–50 μM (25, 26). As we would like to selectively detect effects of Zn2+ on proton pumping activities, we checked the effect of 10 μM or lower Zn2+ concentrations in our system and decided to use 2 μM Zn2+ for comparison between the WT and NuoL mutants. At this concentration, no inhibition of NADH-oxidase activity by Zn2+ was observed (Table I), whereas proton pumping activity significantly decreased by 24% (Fig. 4A). The similar inhibitory effect of 2 μM Zn2+ on proton pumping activities was observed in the D303A and D400E complex I. In contrast, the inhibitory effect of 2 μM Zn2+ on proton pumping activities likely disappeared in the E144Q, D178N, H334Q, D400A and D400N (Fig. 4A). The contrasting results between D400A/D400N and D400E seem to suggest that D400 could be involved in a Zn2+ binding site.
Table I.
Electron transfer activities (NADH-oxidase) of WT and various NuoL mutants in the absence and presence of 2 µM ZnCl2 (n = 3–4)
| Control | 2 μM ZnCl2 | P values | |
|---|---|---|---|
| WT | 4.62 ± 0.37 | 4.52 ± 0.51 | 0.7738 |
| E144Q | 0.41 ± 0.07 | 0.52 ± 0.23 | 0.4682 |
| D178N* | 3.54 ± 0.27 | 2.90 ± 0.14 | 0.009 |
| D303A* | 2.91 ± 0.60 | 1.90 ± 0.38 | 0.0294 |
| H334Q* | 2.37 ± 0.83 | 1.29 ± 0.15 | 0.0429 |
| D400A* | 3.86 ± 0.04 | 2.99 ± 0.57 | 0.0497 |
| D400E* | 2.22 ± 0.44 | 1.25 ± 0.41 | 0.018 |
| D400N | 1.30 ± 0.27 | 0.85 ± 0.29 | 0.0635 |
*Difference with respect to the control is statistically significant (P < 0.05).
Fig. 4.
Effects of Zn2+ (A) and EIPA (B) on proton-pumping activities in the WT and various NuoL mutant complex I reconstituted into DKO membrane vesicles. Initial acidification rates of proton pumping activities were measured in the absence (solid bars) and presence (open bars) of 2 μM ZnCl2 (A) or 15 M EIPA (B) Values are means ± SD (n = 3–4).
EIPA has been known as a specific inhibitor for Na+-related transporters such as Na+/H+ exchangers and Na+/K+ ATPase (34). It was previously reported that EIPA does not efficiently inhibit NADH-oxidase activity of E. coli complex I in Δndh membranes (IC50 > 100 μM) (35) or NADH:UQ reductase activity of the reconstituted purified E. coli complex I (14). However, we previously reported that lower concentrations of EIPA inhibit proton pumping activity of the WT complex I but not certain NuoL mutants including D178N from E. coli without affecting electron transfer activities in membrane vesicle experiments (11). Thus, we predicted that EIPA might primarily inhibit proton transfer in NuoL and investigated the effects of EIPA on proton pumping activities in the DKO membranes reconstituted with isolated NuoL mutants. In WT, the proton pumping activity was significantly decreased to 60.8% in the presence of 15 μM EIPA (Fig. 4B), where its NADH oxidase activity was not inhibited (Table II). This result can be interpreted as only a proton transfer pathway being blocked without affecting electron transfer pathway. In contrast, no inhibitory effect of EIPA on proton pumping activity was observed in D178N (Fig. 4B). This result is consistent with our previous report using intact membrane vesicles from the D178N strain (11). Otherwise, proton pumping activities in most NuoL mutants were inhibited by 15 μM EIPA (Fig. 4B).
Table II.
Electron transfer activities (NADH-oxidase) of WT and various NuoL mutants in the absence and presence of 15 µM EIPA (n = 3–4)
| Control | 15 μM EIPA | P values | |
|---|---|---|---|
| WT | 4.54 ± 0.14 | 4.75 ± 0.24 | 0.1814 |
| E144Q | 0.47 ± 0.03 | 0.54 ± 0.08 | 0.2289 |
| D178N* | 2.90 ± 0.60 | 4.06 ± 0.52 | 0.0445 |
| D303A* | 3.30 ± 0.72 | 2.08 ± 0.15 | 0.0193 |
| D303N | 1.90 ± 0.76 | 1.97 ± 0.37 | 0.8739 |
| H334Q* | 2.16 ± 0.14 | 3.15 ± 0.48 | 0.0265 |
| D400A* | 2.84 ± 0.44 | 3.96 ± 0.58 | 0.0217 |
| D400E | 1.59 ± 0.21 | 1.71 ± 0.34 | 0.5701 |
| D400N* | 2.10 ± 0.08 | 2.80 ± 0.45 | 0.0221 |
*Difference with respect to the control is statistically significant (P < 0.05).
Characterization of DKO membranes reconstituted with WT complex I
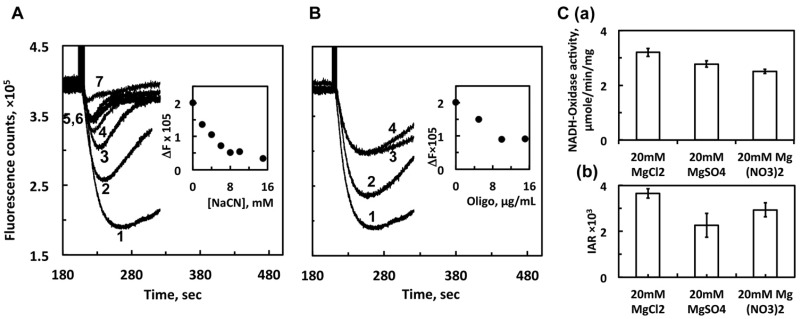
Our results above support that the instant DKO membrane reconstitution method is useful for the functional assessment of complex I and particularly for screening purposes to compare the proton pumping activities between the WT and NuoL mutants. However, in contrast to proteoliposomes, we need to be more careful about the interpretation of the results, as DKO membranes contain other proteins that might affect our results to some extent. Thus, we further studied some characteristics of our DKO membranes. Escherichia coli membranes contain quinol oxidase(s), cytochrome bo3 or/and bd, both proton translocating enzymes, which can oxidize the UQ reduced by complex I. First, we checked the effect of NaCN, an inhibitor for quinol oxidases. Proton pumping activities in the DKO membranes reconstituted with WT were strongly inhibited by NaCN regardless of the presence of externally added DQ (Fig. 5A). This is indeed very consistent with our previous observations that proton pumping activity of complex I is very sensitive to quinol, which we and others (13) previously observed with intact WT membrane vesicles. Probably, rapid accumulation of quinol caused by the inhibition of quinol oxidase reverses the complex I reaction backward, as it is known that complex I catalyse the ΔμH+-dependent NAD+ reductase reaction (36). Next, as the rate of proton pumping could be underestimated by ATP synthase, which decreases ΔpH, we investigated the effect of oligomycin, an inhibitor for ATP synthase. To our surprise, oligomycin inhibited NADH-initiated proton pumping activities by up to 50% (Fig. 5B). Interestingly, we observed that oligomycin also inhibited on quinol-initiated proton pumping activities (by quinol oxidase) in the DKO membrane itself (data not shown). Third, it is possible that the Mg2+ effect shown in Fig. 2 could be the result of the increase in chloride added with Mg2+ rather than Mg2+ itself. Thus, we measured proton pumping activities with other magnesium salts (Fig. 5C). Compared with the chloride, sulphate and nitrate salts gave slightly lower proton pumping and electron transfer activities. Seemingly, the effect of anions on proton pumping is likely through the decreasing effect of electron transfer activities. This confirms that the increase observed in proton pumping activities with increasing MgCl2 concentrations (Fig. 3A) is solely due to Mg2+.
Fig. 5.
Effects of NaCN (A), oligomycin (B) and various counter anions of magnesium salts (C) on proton-pumping activities of DKO membrane reconstituted with WT complex I. (A) Proton-pumping activity of WT-DKO membrane was measured in the presence of 20 μM DQ, 150 μM NADH and various NaCN concentrations. Trace 1 represents the control with no NaCN. Traces 2 through 7 represent NaCN concentrations at 2, 4, 6, 8, 10 and 15 mM, respectively. (B) Proton-pumping activity of WT-DKO membrane was measured in the presence of 20 μM DQ, 150 μM NADH and various oligomycin at 0, 5, 10 and 15 μg/ml. Traces 1–4 correspond increasing oligomycin concentrations. The plots of amplitudes of acidification (ΔF) versus NaCN or oligomycin concentrations are shown in the insets in Fig. 4A and B, respectively. (C) Proton-pumping activity (IAR, initial acidification rate) of WT-DKO membrane was measured in 20 mM of the various magnesium salts. Averages and standard deviations were calculated from four trials. The asterisk symbols on the top of the bars indicate that the difference with respect to the control is statistically significant (P < 0.05).
Proton pumping and electron transfer activities in UQ-rich and MK-rich DKO membranes reconstituted with E. coli complex I
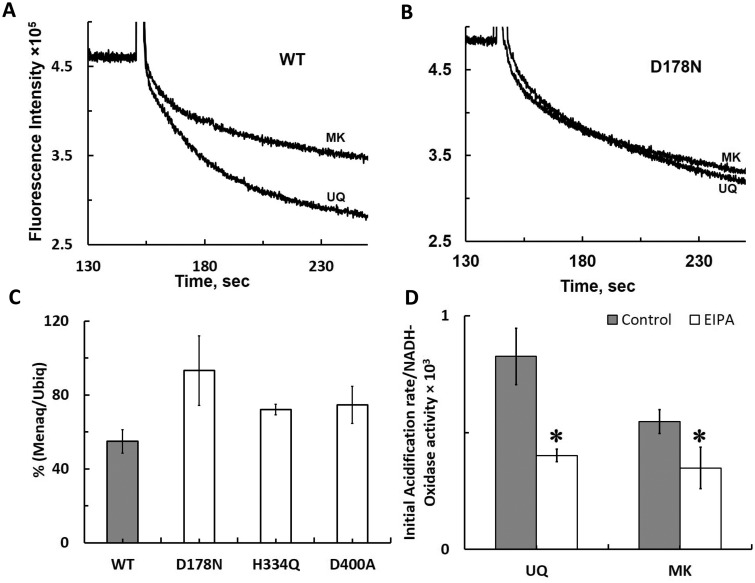
MK is known to work as an electron acceptor in E. coli anaerobic respiratory chain. To investigate how redox potential difference between UQ and MK affects proton pumping activities of complex I, we prepared UQ-rich and MK-rich DKO membrane vesicles from E. coli cells grown aerobically and anaerobically, respectively. Our membrane vesicles contained ∼70 μmol of total quinone per mg of membranes, and typically, MK was 2.5 times higher than UQ in MK-rich membranes, UQ was 2.5 times higher than MK in UQ-rich membranes. We found that the initial proton pumping rate was almost half when the WT complex I was reconstituted into MK-rich DKO membrane vesicles compared with that with UQ-rich membrane vesicles (Fig. 6A). In contrast, no significant difference was observed in initial proton pumping activities between the UQ-rich and MK-rich DKO membranes reconstituted with the D178N complex I (Fig. 6B). Interestingly, we noticed that electron transfer activities were always the same or even higher with MK-rich DKO membranes vesicles, indicating somewhat more effective insertion of complex I into a lipid bilayer with MK-rich DKO membranes vesicles. Thus, we repeated at least three different sets of experiments (Table III) and calculated the coupling efficiency ratio after dividing initial proton pumping rates by electron transfer activities for each experiment (Table I), to normalize deviations derived from DKO reconstitution efficiency between UQ-rich and MK-rich DKO membrane vesicles. The efficiency of electron transfer coupling to proton pumping of the WT reconstituted into MK-rich membrane vesicles decreased to 54.9% compared with that with UQ-rich membranes, whereas the coupling efficiency of D178N reconstituted into MK-rich membranes was retained similar to that with UQ-rich membranes (Fig. 6C). The coupling efficiency of other NuoL mutants H334Q and D400A decreased to ∼75% (Fig. 6C). These data suggest that MK might be insufficient to induce conformational changes needed for a full operating mode of proton pumping machinery, leading to a lower proton/electron coupling efficiency in WT. However, D178N already lost the high coupling efficiency part of proton pumping operating mechanism, thus, no difference was observed between the coupling efficiency between UQ-rich and MK-rich DKO membranes. As it was previously suggested that low concentrations of EIPA lowers a high coupling efficiency mechanism in WT by probably blocking a part of proton pumping pathway (11), we compared the effects of EIPA between the UQ-rich and MK-rich DKO membrane vesicles reconstituted with WT. The coupling efficiency was reduced to 47% in the UQ-rich membranes and 64.5% in the MK-rich membranes by 15 μM EIPA (Fig. 6D). The effect of EIPA in the MK-rich membranes was significantly smaller, compared with UQ-rich membranes.
Fig. 6.
Proton pumping activities of the WT and selected NuoL mutant complex I reconstituted in UQ-rich and MK-rich DKO membranes. UQ-rich and MK-rich DKO membranes were prepared from E. coli cells cultured under our regular aerobic and semi-anaerobic conditions, respectively. Representative data of proton pumping traces for the WT (A) and D178N mutant (B) are shown. (C) Percentages of proton pumping efficiency in MK-rich versus UQ-rich DKO membranes calculated from the average of three separate experiments is shown. For each experiment, we carried out at least three measurements (n = 3–5). (D) Effect of EIPA on proton pumping activities of the WT complex I reconstituted UQ-rich and MK-rich DKO membranes. The data were obtained from three separate experiments as described in (C); 15 μM EIPA was used. The asterisk symbols on the top of the bars indicate that the difference with respect to the control is statistically significant (P < 0.05).
Table III.
Proton pumping and NADH-oxidase activities of the NuoL mutant complex I reconstituted into UQ- and MK-rich DKO membranes
| Sample | Trial | UQ-rich DKO membranes |
MK-rich DKO membranes |
% (Menaq/Ubiq) | ||||
|---|---|---|---|---|---|---|---|---|
| Initial acidification rate (IAR) | NADH-oxidase activity (NOA) | IAR/NOA | Initial acidification rate (IAR) | NADH-Oxidase activity (NOA) | IAR/NOA | |||
| WT | 1 | 3,474.9 | 3.28 | 1,058.2 | 2,156.9 | 4.28 | 503.5 | 47.6 |
| 2 | 2,682.9 | 5.01 | 535.9 | 1,748.1 | 5.66 | 308.6 | 57.6 | |
| 3 | 4,139.2 | 3.97 | 1,043.2 | 3,313.1 | 5.35 | 619.5 | 59.4 | |
| D178N | 1 | 2,491.4 | 3.06 | 814.7 | 2,541.9 | 2.98 | 854.2 | 104.8 |
| 2 | 2,529.7 | 4.46 | 566.6 | 2,756.0 | 4.71 | 585.2 | 103.3 | |
| 3 | 3,392.0 | 3.10 | 1,095.3 | 3,628.2 | 4.64 | 782.2 | 71.4 | |
| H334Q | 1 | 2,095.5 | 2.94 | 713.9 | 2,309.3 | 4.21 | 549.0 | 76.9 |
| 2 | 1,974.7 | 2.78 | 710.1 | 2,178.0 | 4.17 | 521.8 | 73.5 | |
| 3 | 1,965.4 | 2.86 | 686.1 | 1,901.2 | 4.20 | 452.7 | 66.0 | |
| D400A | 1 | 2,456.7 | 4.19 | 586.3 | 2,621.8 | 5.35 | 490.1 | 83.6 |
| 2 | 2,579.5 | 4.13 | 624.6 | 2,658.6 | 5.68 | 468 | 74.9 | |
| 3 | 2,729.6 | 4.13 | 660.9 | 2,990.7 | 5.29 | 565.3 | 85.5 | |
The values are the average of n = 3–4 measurements.
Discussion
Investigating the proton pumping mechanism of complex I is one of the most challenging bioenergetic problems, due to the structural/functional complexity and the difficulty of accounting for the exceptionally high proton pumping stoichiometry of 3–4H+/2e− (proton/electron) (37–41). In this study, we focused on the most distal membrane subunit NuoL/ND5 more than 150 Å away from the quinone reducing site (8). As it is commonly believed that acidic residues are required near the orifice of proton pathways to facilitate proton uptake, we investigated roles of highly conserved charged amino acids in NuoL for proton pumping mechanism of E. coli complex I, using isolated NuoL mutant complex I. To monitor proton pumping activities of isolated complex I, purified proteins have to be reconstituted into proteoliposomes. However, preparing proteoliposomes requires relatively large amount of purified proteins and long processing time, and has a problem in reproducibility. Therefore, we first developed an alternative and convenient method, in vitro reconstitution using E. coli DKO membrane vesicles, which have no NADH-initiated proton pumping activities or NADH oxidase activities. This membrane reconstitution method allows us to analyse proton pumping activities of various mutant complex I faster (5 min versus 3–4 h) and with less protein (50 versus 250 μg) than the traditional proteoliposome method. The additional advantage of using DKO membrane vesicles is that DKO membrane preserves natural membrane conditions such as containing native E. coli lipids that are necessary for the stabilization and effective insertion of complex I into a lipid bilayer, and for optimal activity. The problem with using DKO membranes could be the interpretations of the results, which could be complicated by other proteins contained such as quinol oxidases and ion transporters. However, as shown in Fig. 1, there is a linear relationship between the amount of complex I and proton pumping and electron transfer activities. Thus, as long as the same lot of DKO membranes is used, these background effects applied to all the samples, thus, qualitative and relative comparisons between the WT and mutants are still possible and valid.
We previously reported that proton pumping activities in membrane vesicles prepared from the E144Q, D178N and D400A strains are ∼5%, ∼45% and 45% of the WT, respectively (11). However, in the DKO reconstitution experiments, proton pumping activities of E144Q, D178N and D400A are ∼35%, ∼75% and 85% of the WT, respectively (Fig. 3A). The differences between the WT and mutants are much smaller. This apparent discrepancy could be explained by the followings. First, the amount of complex I present in the membrane vesicles is varied even from the WT strain, because complex I levels really depend on cell growth phases. The highest expression levels are usually seen in early stationary phase, but the specific activity of complex I could still be varied by up to 50% from batch to batch. Usually, mutants grow slower compared with the WT, so it is more difficult to judge the right time to harvest cells. In addition, mutations tend to destabilize the complex I assembly. So, the amounts of fully assembled complex I remained in the membrane are also varied. Therefore, the observed low activities are not necessarily to be interpreted by the direct effect of mutation in the important residues and could be caused simply by low levels of complex I proteins in the membrane. Second, the discrepancy could also originate from differences in the nature of samples between membrane vesicles and our reconstituted DKO membranes. We have recently discovered that the product QH2 is extremely inhibitory towards complex I reactions, especially the proton pumping activity. Low concentrations QH2 (like 3 μM) can shut down proton pumping activity instantaneously like an uncoupler (unpublished observations). In membrane vesicles, the generated QH2 by complex I is promptly removed by the quinol oxidase bo3 in the membrane. However, in the DKO membranes reconstituted with complex I, it seems that the product QH2 by complex I is not efficiently consumed by bo3. Because, the DKO membranes themselves have shown very strong proton pumping activity by bo3 after the addition of 6 μM of QH2, and this activity can be completely inhibited in the presence of NaCN (data not shown). Compared with this proton pumping activity by bo3, NADH-initiated proton pumping activity by complex I in DKO-WT reconstituted membranes is much slower, suggesting almost no contribution from bo3 for the NADH-initiated proton pumping activity, although substantial QH2 is produced during the complex I reaction based on NADH-oxidase activity. Therefore, we speculated that the connection between complex I and bo3 is very loose unlike intact membrane vesicles. Thus, the initial maximum acidification phase is very short especially for the WT and proton pumping is immediately inhibited by QH2. We cannot eliminate a possibility that we underestimated the proton pumping activity from the WT, because the WT has the highest complex I activity and generated QH2 at the fastest, and its proton pumping activity is inhibited faster than those of other mutants. As a result, proton pumping activities of mutant complex I became relatively much higher.
It has been not explicitly but generally noticed that Mg2+ is required to measure proton pumping activities. However, this study is the first report on the Mg2+ effect on proton pumping function of complex I by varying Mg2+ concentrations in the assay buffer. We have observed that 0.4–1 mM Mg2+ in the reaction mixture helps measure reproducibly the maximum proton pumping activities in proteoliposomes and/or E. coli intact membrane vesicles. However, in the DKO reconstitution system, the effect of Mg2+ turned to be drastic. Surprisingly, there was almost no proton pumping activity detected in the absence of Mg2+, and 10–20 mM of Mg2+ was necessary to obtain the maximum protonpumping activities. In addition, an interesting thing is that electron transfer activities were not affected by Mg2+ concentrations (Fig. 3B). It is known that Mg2+ interacts with the head-groups of phospholipids in membranes (42). Also, a bound Mg2+ ion found in cytochrome c oxidases was suggested to have a structural role in the stabilization of the interface between Subunits 1 and 2 (20). Therefore, perhaps, Mg2+ facilitates complex I self-integration into membrane vesicles by stabilizing and aligning membrane subunits in a right direction to secure proton pathways upon conformational changes triggered by quinone reduction. As there was no difference in the Mg2+ effect between the WT and NuoL mutants, it can be suggested that those residues are not directly involved in this Mg2+ role.
Zn2+ binding may disrupt many different components of the mechanism, including proton transfer pathways (proton transfer to bound quinone or proton translocation). Zn2+ is believed to be one of the most potent inhibitor of proton channels. The inhibitory action of Zn2+ is derived from the metal ion binding to histidine residues, and also to the thiol group of the cysteine residues (43). In fact, it has been reported that Zn2+ does not inhibit NADH oxidation at the flavin site or intramolecular electron transfer in complex I, but it does inhibit proton transfer to bound quinone or proton translocation (25). Although the reported IC50 value for the Zn2+ inhibition of NADH:DQ activities in isolated E. coli complex I is 50 μM (26), in our study, E. coli complex I was much more sensitive to Zn2+ after the instant DKO reconstitution. This suggests that when complex I is embedded in the membrane, the conformation of complex I is different compared with the conformation as isolated in solution. At 2 μM, we finally did not see any inhibitory effect of Zn2+ on NADH-oxidase activity, whereas Zn2+ inhibited proton pumping activity in the WT. The results that D400A and D400N but not D400E was unaffected by 2 μM Zn2+ suggest that D400 is possibly involved in a Zn2+ binding site that involves proton translocation and could be the exit of proton transfer pathway in NuoL (Fig. 3C). Indeed, our data are consistent with the previous results by FT-IR spectroscopy that carboxylic acid residues in a hydrophobic environment were perturbed by the presence of Zn2+, which was assumed to be conserved charged amino acids located in proton pathways (26). Also it is interesting to note that E494, which is located in a close proximity to D400, could help in Zn2+ binding.
EIPA, like other amiloride derivatives, has been known to inhibit Na+/H+ antiporters (34, 44). Previously, we have shown that EIPA inhibits mitochondrial and bacterial complex I, and we have reported that the IC50 values of EIPA for NADH oxidase activities of bovine SMP and E. coli NDH-1 are 17 μM and >100 μM, respectively (35). Although E. coli complex I is more resistant to EIPA, later, we found that low concentrations of EIPA (50 μM or less) primarily inhibit the proton transfer in NuoL (11). In this study, we observed E. coli complex I became more sensitive to EIPA after reconstitution into DKO membranes also in proteoliposome experiments (MN and ENO, unpublished observation). However, by lowering the EIPA concentration to 15 μM, we were able to confirm that low concentrations of EIPA selectively inhibits only the pumping activity in WT by 40% without affecting the NADH oxidase activity, whereas EIPA had no effect on the proton pumping rate in the D178N mutant that seemingly lost the high affinity EIPA binding site (Fig. 4B). The EIPA-binding site(s) has been expected to be in NuoL/ND5, based on the several facts: (1) high sequence similarity between NuoL/ND5 and MrpA/MnhA, although D178 is only conserved in homologs of NuoL (11); (2) Na+ transport by E. coli complex I but not by E. coli complex I devoid of NuoL (45); (3) heterogeneously overexpressed E. coli NuoL subunit is capable of supporting the growth of the ΔMrpA mutant (46); (4) EIPA inhibition of the sodium uptake by C-terminally truncated NuoL reconstituted in proteopliposomes (47); and (5) the blocking effect of EIPA on the ND5 labelling with a fenpyroximate photoaffinity analogue (48). Therefore, all together, our results suggest that D178 could be a possible EIPA binding site, although we cannot rule out the possibility that D178A mutation indirectly affected the EIPA binding site.
One advantage to use DKO membranes in our case was the possibility that we could modify quinone compositions in DKO membranes by changing growth conditions to study MK effects on complex I. It is generally believed that MK can serve as an electron carrier only for backward electron transfer in eukaryotic mitochondria under anaerobic conditions (49). In contrast, for E. coli complex I, MK could work as an alternative substrate (50). To gain new insight on how the redox potential difference between UQ and MK affects proton pumping activities, we prepared MK-rich DKO membranes and measured the proton pumping activities in reconstituted DKO membranes with the WT and selected NuoL mutants. As the midpoint redox potential of MK/MKH2 is −80 mV, much lower than that of UQ/UQH2 (+110 mV), we hypothesized that we might be able to observe a lower proton pumping coupling efficiency with the ‘low redox potential’ electron acceptor MK, compared with UQ. We successfully observed that the decreased proton pumping efficiency and much less additional inhibitory effect by EIPA in the WT reconstituted with MK-rich DKO membranes (Fig. 6C and D, respectively). Our results strongly suggest that ‘low redox potential’ MK cannot operate the high proton pumping capacity mechanism. This can also be supported by the contrasting result of D178N that no difference in proton pumping coupling efficiency between UQ- and MK-rich membranes was detected (Fig. 6C), as D178N has already lost the high efficiency proton pump coupling mechanism (11). It is tempting to speculate that the redox energy released upon MK reduction with NADH by complex I might not be enough for the full operation of proton pumping machinery. Although we cannot rule out a possibility that some unknown differences in membrane components contributed the difference in proton pumping efficiency between UQ- and MK-rich DKO membranes, this possible background effects apply to the results with NuoL mutants, which did not display drastic differences. Thus, it is highly likely that the redox energy released upon MK reduction with NADH by complex I might not be enough for the full operation of proton pumping machinery. Previously, based on the report using E. coli cells grown anaerobically, the H+/e− stoichiometry for bacterial complex I was calculated to be 1.5 (41), whereas it is established that the H+/e− stoichiometry of mitochondrial complex I is 2. The lower stoichiometry of E. coli complex I in this report might be explained by the involvement of MK in the membranes vesicles they used.
In conclusion, our results strongly suggest that D178 in NuoL plays a key role in the indirect high efficiency proton pumping coupling in complex I. The other conserved residues E144, H334 and D400 also seem to be involved in proton pumping and/or coupling mechanisms, as local conformational changes triggered by single mutations in these residues led to the reduction of proton pumping activities and proton pump coupling efficiency and changed sensitivities towards Zn2+ and EIPA. Further studies of the role of conserved residues in NuoL are necessary for the understanding of complex I coupling mechanisms.
Acknowledgements
The authors would like to thank Dr Carolina Sepulveda Nunez for discussion and Ms Diana Orlandi for her technical assistance.
Funding
This work was supported by National Institute of Health General Medicine grant R01GM097409 to E.N.-O. and American Heart Association grant 11SDG5560001 to E.N.-O.
Conflict of Interest
None declared.
Glossary
Abbreviations
- DDM
dodecyl-β-d-maltoside
- DKO
double knockout
- dNADH
deamino-NADH
- DQ
decylubiquinone
- EIPA
5-(N-ethyl-N-isopropyl)-amiloride
- HAR
hexaammineruthenium
- MK
menaquinone
- Mnh
multisubunit Na+/H+ antiporter
- Mrp
multiple resistance and pH adaptation antiporter
- NDH-2
NADH dehydrogenase type 2
- Q
quinone
- ROS
reactive oxygen species
- SQ
semiquinone
- UQ
ubiquinone
- WT
wild-type
References
- 1.Schapira A.H. (1998). Human complex I defects in neurodegenerative diseases. Biochim. Biophys. Acta 1364, 261–270 [DOI] [PubMed] [Google Scholar]
- 2.Hur J.H., Stork D.A., Walker D.W. (2014). Complex-I-ty in aging. J. Bioenerg. Biomembr. 46, 329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yagi T., Matsuno-Yagi A. (2003). The proton-translocating NADH-quinone oxidoreductase in the respiratory chain: the secret unlocked. Biochemistry 42, 2266–2274 [DOI] [PubMed] [Google Scholar]
- 4.Sazanov L.A. (2007). Respiratory complex I: mechanistic and structural insights provided by the crystal structure of the hydrophilic domain. Biochemistry 46, 2275–2288 [DOI] [PubMed] [Google Scholar]
- 5.Brandt U. (2006). Energy converting NADH:quinone oxidoreductase (complex I). Annu. Rev. Biochem. 75, 69–92 [DOI] [PubMed] [Google Scholar]
- 6.Hirst J. (2013). Mitochondrial complex I. Annu. Rev. Biochem. 82, 551–575 [DOI] [PubMed] [Google Scholar]
- 7.Zickermann V., Wirth C., Nasiri H., Siegmund K., Schwalbe H., Hunte C., Brandt U. (2015). Structural biology. Mechanistic insight from the crystal structure of mitochondrial complex I. Science 347, 44–49 [DOI] [PubMed] [Google Scholar]
- 8.Baradaran R., Berrisford J.M., Minhas G.S., Sazanov L.A. (2013). Crystal structure of the entire respiratory complex I. Nature 494, 443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinothkumar K.R., Zhu J., Hirst J. (2014). Architecture of mammalian respiratory complex I. Nature 515, 80–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathiesen C., Hagerhall C. (2002). Transmembrane topology of the NuoL, M and N subunits of NADH:quinone oxidoreductase and their homologues among membrane-bound hydrogenases and bona fide antiporters. Biochim. Biophys. Acta 1556, 121–132 [DOI] [PubMed] [Google Scholar]
- 11.Nakamaru-Ogiso E., Kao M.C., Chen H., Sinha S.C., Yagi T., Ohnishi T. (2010). The membrane subunit NuoL(ND5) is involved in the indirect proton pumping mechanism of Escherichia coli complex I. J Biol Chem 285, 39070–39078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narayanan M., Gabrieli D.J., Leung S.A., Elguindy M.M., Glaser C.A., Saju N., Sinha S.C., Nakamaru-Ogiso E. (2013). Semiquinone and cluster N6 signals in His-tagged proton-translocating NADH:ubiquinone oxidoreductase (complex I) from Escherichia coli. J. Biol. Chem. 288, 14310–14319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verkhovskaya M., Knuuti J., Wikstrom M. (2011). Role of Ca(2+) in structure and function of complex I from Escherichia coli. Biochim. Biophys. Acta 1807, 36–41 [DOI] [PubMed] [Google Scholar]
- 14.Stolpe S., Friedrich T. (2004). The Escherichia coli NADH:ubiquinone oxidoreductase (complex I) is a primary proton pump but may be capable of secondary sodium antiport. J. Biol. Chem. 279, 18377–18383 [DOI] [PubMed] [Google Scholar]
- 15.Drose S., Galkin A., Brandt U. (2005). Proton pumping by complex I (NADH:ubiquinone oxidoreductase) from Yarrowia lipolytica reconstituted into proteoliposomes. Biochim. Biophys. Acta 1710, 87–95 [DOI] [PubMed] [Google Scholar]
- 16.Roberts P.G., Hirst J. (2012). The deactive form of respiratory complex I from mammalian mitochondria is a Na+/H+ antiporter. J. Biol. Chem. 287, 34743–34751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamaru-Ogiso E., Narayanan M., Sakyiama J.A. (2014). Roles of semiquinone species in proton pumping mechanism by complex I. J. Bioenerg. Biomembr. 46, 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jahnen-Dechent W., Ketteler M. (2012). Magnesium basics. Clin. Kidney J. 5, i3–i14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith R.L., Maguire M.E. (1998). Microbial magnesium transport: unusual transporters searching for identity. Mol. Microbiol. 28, 217–226 [DOI] [PubMed] [Google Scholar]
- 20.Abramson J., Svensson-Ek M., Byrne B., Iwata S. (2001). Structure of cytochrome c oxidase: a comparison of the bacterial and mitochondrial enzymes. Biochim. Biophys. Acta 1544, 1–9 [DOI] [PubMed] [Google Scholar]
- 21.Axelrod H.L., Abresch E.C., Paddock M.L., Okamura M.Y., Feher G. (2000). Determination of the binding sites of the proton transfer inhibitors Cd2+ and Zn2+ in bacterial reaction centers. Proc. Natl Acad. Sci. U. S. A. 97, 1542–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee D.W., El Khoury Y., Francia F., Zambelli B., Ciurli S., Venturoli G., Hellwig P., Daldal F. (2011). Zinc inhibition of bacterial cytochrome bc(1) reveals the role of cytochrome b E295 in proton release at the Q(o) site. Biochemistry 50, 4263–4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson A.L., Hogbom M., Carlsson J., Gennis R.B., Brzezinski P. (2013). Role of aspartate 132 at the orifice of a proton pathway in cytochrome c oxidase. Proc. Natl Acad. Sci. U. S. A. 110, 8912–8917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martino P.L., Capitanio G., Capitanio N., Papa S. (2011). Inhibition of proton pumping in membrane reconstituted bovine heart cytochrome c oxidase by zinc binding at the inner matrix side. Biochim. Biophys. Acta 1807, 1075–1082 [DOI] [PubMed] [Google Scholar]
- 25.Sharpley M.S., Hirst J. (2006). The inhibition of mitochondrial complex I (NADH:ubiquinone oxidoreductase) by Zn2+. J. Biol. Chem. 281, 34803–34809 [DOI] [PubMed] [Google Scholar]
- 26.Schulte M., Mattay D., Kriegel S., Hellwig P., Friedrich T. (2014). Inhibition of Escherichia coli respiratory complex I by Zn(2+). Biochemistry 53, 6332–6339 [DOI] [PubMed] [Google Scholar]
- 27.Nakamaru-Ogiso E., Yano T., Yagi T., Ohnishi T. (2005). Characterization of the iron-sulfur cluster N7 (N1c) in the subunit NuoG of the proton-translocating NADH-quinone oxidoreductase from Escherichia coli. J. Biol. Chem. 280, 301–307 [DOI] [PubMed] [Google Scholar]
- 28.Link A.J., Phillips D., Church G.M. (1997). Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179, 6228–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao M.C., Di Bernardo S., Perego M., Nakamaru-Ogiso E., Matsuno-Yagi A., Yagi T. (2004). Functional roles of four conserved charged residues in the membrane domain subunit NuoA of the proton-translocating NADH-quinone oxidoreductase from Escherichia coli. J. Biol. Chem. 279, 32360–32366 [DOI] [PubMed] [Google Scholar]
- 30.Torres-Bacete J., Nakamaru-Ogiso E., Matsuno-Yagi A., Yagi T. (2007). Characterization of the NuoM (ND4) subunit in Escherichia coli NDH-1: conserved charged residues essential for energy-coupled activities. J. Biol. Chem. 282, 36914–36922 [DOI] [PubMed] [Google Scholar]
- 31.Tartoff K.D., Hobbs C.A. (1987). Improved media for growing plasmid and cosmid clones. Bethesda Res. Lab. Focus 9, 12 [Google Scholar]
- 32.Elguindy M.M., Nakamaru-Ogiso E. (2015). Apoptosis inducing factor (AIF) and its family member, AMID, are rotenone-sensitive NADH:ubiquinone oxidoreductases (NDH-2). J. Biol. Chem. 290, 20815–20826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Efremov R.G., Baradaran R., Sazanov L.A. (2010). The architecture of respiratory complex I. Nature 465, 441–445 [DOI] [PubMed] [Google Scholar]
- 34.Masereel B., Pochet L., Laeckmann D. (2003). An overview of inhibitors of Na(+)/H(+) exchanger. Eur. J. Med. Chem. 38, 547–554 [DOI] [PubMed] [Google Scholar]
- 35.Nakamaru-Ogiso E., Seo B.B., Yagi T., Matsuno-Yagi A. (2003). Amiloride inhibition of the proton-translocating NADH-quinone oxidoreductase of mammals and bacteria. FEBS Lett. 549, 43–46 [DOI] [PubMed] [Google Scholar]
- 36.Vinogradov A.D. (1998). Catalytic properties of the mitochondrial NADH-ubiquinone oxidoreductase (complex I) and the pseudo-reversible active/inactive enzyme transition. Biochim. Biophys. Acta 1364, 169–185 [DOI] [PubMed] [Google Scholar]
- 37.Ripple M.O., Kim N., Springett R. (2013). Mammalian complex I pumps 4 protons per 2 electrons at high and physiological proton motive force in living cells. J. Biol. Chem. 288, 5374–5380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandt U. (2011). A two-state stabilization-change mechanism for proton-pumping complex I. Biochim. Biophys. Acta 1807, 1364–1369 [DOI] [PubMed] [Google Scholar]
- 39.Wikstrom M. (1984). Two protons are pumped from the mitochondrial matrix per electron transferred between NADH and ubiquinone. FEBS Lett. 169, 300–304 [DOI] [PubMed] [Google Scholar]
- 40.Galkin A.S., Grivennikova V.G., Vinogradov A.D. (1999). H+/2e- stoichiometry in NADH-quinone reductase reactions catalyzed by bovine heart submitochondrial particles. FEBS Lett. 451, 157–161 [DOI] [PubMed] [Google Scholar]
- 41.Bogachev A.V., Murtazina R.A., Skulachev V.P. (1996). H+/e- stoichiometry for NADH dehydrogenase I and dimethyl sulfoxide reductase in anaerobically grown Escherichia coli cells. J. Bacteriol. 178, 6233–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilschut J., Duzgunes N., Papahadjopoulos D. (1981). Calcium/magnesium specificity in membrane fusion: kinetics of aggregation and fusion of phosphatidylserine vesicles and the role of bilayer curvature. Biochemistry 20, 3126–3133 [DOI] [PubMed] [Google Scholar]
- 43.Kawai K., Nagata N. (2012). Metal-ligand interactions: an analysis of zinc binding groups using the Protein Data Bank. Eur. J. Med. Chem. 51, 271–276 [DOI] [PubMed] [Google Scholar]
- 44.Kleyman T.R., Cragoe E.J., Jr. (1988). Amiloride and its analogs as tools in the study of ion transport. J. Membr. Biol. 105, 1–21 [DOI] [PubMed] [Google Scholar]
- 45.Marreiros B.C., Batista A.P., Pereira M.M. (2014). Respiratory complex I from Escherichia coli does not transport Na(+) in the absence of its NuoL subunit. FEBS Lett. 588, 4520–4525 [DOI] [PubMed] [Google Scholar]
- 46.Moparthi V.K., Kumar B., Al-Eryani Y., Sperling E., Gorecki K., Drakenberg T., Hagerhall C. (2014). Functional role of the MrpA- and MrpD-homologous protein subunits in enzyme complexes evolutionary related to respiratory chain complex I. Biochim. Biophys. Acta 1837, 178–185 [DOI] [PubMed] [Google Scholar]
- 47.Steuber J. (2003). The C-terminally truncated NuoL subunit (ND5 homologue) of the Na+-dependent complex I from Escherichia coli transports Na+. J. Biol. Chem. 278, 26817–26822 [DOI] [PubMed] [Google Scholar]
- 48.Nakamaru-Ogiso E., Sakamoto K., Matsuno-Yagi A., Miyoshi H., Yagi T. (2003). The ND5 subunit was labeled by a photoaffinity analogue of fenpyroximate in bovine mitochondrial complex I. Biochemistry 42, 746–754 [DOI] [PubMed] [Google Scholar]
- 49.Tielens A.G., Van Hellemond J.J. (1998). The electron transport chain in anaerobically functioning eukaryotes. Biochim. Biophys. Acta 1365, 71–78 [DOI] [PubMed] [Google Scholar]
- 50.Tran Q.H., Bongaerts J., Vlad D., Unden G. (1997). Requirement for the proton-pumping NADH dehydrogenase I of Escherichia coli in respiration of NADH to fumarate and its bioenergetic implications. Eur. J. Biochem. 244, 155–160 [DOI] [PubMed] [Google Scholar]