Abstract
Objective(s):
In food industry, the inhibition of tyrosinase is very important, because this enzyme catalyzes the oxidation of phenolic compounds found in fruits and vegetables into quinones, which contribute in undesirable color and taste of fruits and vegetables. Teucrium polium L. var. gnaphalodes (Lamiaceae), a wild-growing flowering plant that has many applications in food preparations and traditional medicine. In Persian language, this medicinal herb is called Kalpoureh.
Materials and Methods:
1D- and 2D-NMR experiments were used to determine the chemical structures of the isolated compounds. Antioxidant and tyrosinase inhibitory activities of the isolated compounds were evaluated using DPPH, FRAP and mushroom tyrosinase inhibition assays.
Results:
In this research, we isolated two phenylpropanoid glycosides including verbascoside and poliumoside and two flavonoids including jaranol and isorhoifolin using chromatographic techniques. We found promising antioxidant and anti-tyrosinase compounds from Teucrium polium L. var. gnaphalodes.
Conclusion:
To date, different compounds have been isolated and characterized from T. polium including terpenoids and flavonoids. But no phytochemical study has been reported from T. polium var. gnaphalodes. Poliumoside and jaranol showed promising antioxidant and tyrosinase inhibitory activities, respectively.
Keywords: DPPH, FRAP, Isorhoifolin, Jaranol, Lamiaceae, Mushroom tyrosinase -, inhibition assay, Poliumoside, Teucrium polium var.- gnaphalodes
Introduction
Tyrosinase is a kind of oxidase enzyme that involves in reactions of melanin synthesis. In food industry, the inhibition of tyrosinase is very important, because this enzyme catalyzes the oxidation of phenolic compounds found in fruits and vegetables into quinones, which contribute in undesirable color and taste of fruits and vegetables. In addition, quinones reduce the bioavail-ability of essential amino acids. Arbutin, kojic acid, and hydroquinones have been reported to have tyrosinase inhibitory activity. Also, they have been used in cosmetic industry as whitening composition (1, 2).
The genus Teucrium consists of more than 340 species widely distributed around the world (South-west of Asia, North of Africa, and South and North-east of Iran.). Twelve species occur in Iran including three endemic species. The main compounds reported from the genus Teucrium include terpenoids and flavonoids (3, 4). Teucrium is called Kalpoureh in Persian language. It has been long used in Iranian traditional medicine to treat stomach disorders, malabsorption, grippe, cold, and was reputable for having hypoglycemic, anti-hyperlipi-demia (3), diuretic, analgesic, antipyretic, anti-spasmodic, anti-inflammatory and anti-hypertensive properties. All species of this genus have been showed considerable hypoglycemic and anti-hyperlipidemia properties (5).
A literature review shows that there are a large number of phytochemical studies on the genus Teucrium including T. polium. Different parts of plants from the genus Teucrium have been reported to have monoterpenes (6), sesquiterpenes (7), polyphenols and flavonoids such as apigenin and rutin (8, 9) and some fatty acids and steroids such as β-sitosterol and stigmasterol. But there is no.
phytochemical study on T. polium var. gnaphalodes. Considering pharmacological benefits of T. polium var. gnaphalodes and its traditional applications, that are similar to those of T. polium, we aimed to characterize its components and primarily evaluate their antioxidant and anti-tyrosinase properties.
In this study, we reported the isolation and structure elucidation of the main constituents of T. polium var. gnaphalodes, and antioxidant (FRAP and DPPH test) and anti-tyrosinase (mushroom tyrosinase inhibition assay) activities of the main compounds.
Materials and Methods
General experimental procedures
Preparative HPLC-DAD was performed on a KNAUER liquid chromatograph system consisting of a quaternary pump (Smartline Pump 1000). Detection was carried out using UV/Vis diode array detector (Smartline DAD 2800), and data were processed using EZ Chrom Elite software. The fractions were subjected to reverse-phase HPLC using a gradient method of 20-100 % methanol in water as the eluent including 0.05 % trifluoroacetic acid.
The 1H-NMR spectra of the isolated compounds were recorded at 30 °C on a Varian 600 1H-NMR spectrometer. Samples were dissolved in DMSO-d6. The 1H-NMR chemical shifts were referenced to the solvent peaks at σH 2.50 ppm.
Plant material
The aerial parts of T. polium var. gnaphalodes were collected in October from north of Iran, Firoozkouh, Alborz Mountains, 2200 meters height. The plant material was identified by Mohammad Reza Joharchi. A voucher specimen (No. 11377) has been deposited at the herbarium of School of Pharmacy, Mashhad University of Medical Sciences.
Extraction and isolation
Aerial parts have been dried in room temperature and then have been finely powdered by a miller. The powdered aerial parts (250 g) were extracted in 500 ml of methanol at room temperature for three times each for 24 hr by maceration method. After concentration of extracts with a rotary evaporator and completion of drying of them with a freeze dryer, the obtained extract (37 g) has been conserved in the refrigerator.
10 g of the dried extract was loaded on silica gel column chromatography (5 × 50 cm, normal phase). The column has then been eluted by hexane and then gradual adding of ethyl acetate and methanol to increase mobile phase polarity. The obtained fractions (200 mL each) were compared by TLC and those giving similar spots were combined. Three fractions (A-C) were finally obtained. Then fractions A, B and C were subjected to more purification via HPLC apparatus (C-18 reversed phase with methanol: water solvent system) to obtain pure compound of 1 (21 mg), 2 (9.3 mg), 3 (101.4 mg) and 4 (10.4 mg). Purification of fractions was carried out using an ACE 5 C18 (5 μM, 250 × 21.2 mm) at a flow rate 9 ml/min and linear gradient conditions of 20 % - 100 % MeOH (0.05 % TFA) within 20 min, followed by an isocratic condition of MeOH (0.05 % TFA) for 5 min.
Antioxidant activity
DPPH free radical scavenging assay
DPPH is a stable radical that is used in a popular method for screening free radical-scavenging ability of compounds or antioxidant activity of plant extracts (10).
Free radical scavenging activity was evaluated by measuring the scavenging activity of the compounds in the solution of 2, 2-diphenyl-1-picrylhydrazyl (DPPH Briefly, a 0.3 mM solution of DPPH in ethanol was prepared. An aliquot (50 μl) of samples (at four different concentrations (µg/ml) were added to 150 μl of the DPPH solution in each well of a 96-well plate. For blank, only 50 μl of solvent was added to the DPPH solution. The decrease in absorbance was measured at 515 nm after 30 min of incubation at 37 °C using the BioTek micro plate reader (Synergy H4, USA). All tests were performed in triplicate (11) and the data presented as mean of the three values. When a solution of DPPH is mixed with that of a substance, this gives rise to the reduced form diphenylpicrylhydrazyl with the loss of this violet color.
The IC50 values were calculated as the concentration of extracts causing a 50% inhibition of DPPH radical. A lower IC50 value corresponds to a higher antioxidant activity of sample.
Ferric reducing antioxidant potential (FRAP) assay
The FRAP assay was performed according to a previous work (11). FRAP reagent was prepared by adding 10 mL of acetate buffer 300 mM, pH 3.6 (3.1 g sodium acetate trihydrate), to 1.0 ml of ferric chloride hexahydrate 20 mM (dissolved in distilled water) and 1.0 ml of 2,4,6-tri-(2-pyridyl)-s-triozine (TPTZ) 10 mM (dissolved in HCl 40 mM). In a well of a 96-well plate, an aliquot (10 μl) of sample (at five different concentrations (µg/ml) was added to 190 μl of the FRAP solution. After 30 min of incubation at 37 °C, absorbance of the reaction mixture was measured at 593 nm using BioTek micro plate reader (Synergy H4, USA). All tests were carried out in triplicate.
Mushroom tyrosinase inhibition assay
Melanin, which is secreted by melanocyte cells, is the major pigment for color of human skin. It may be overproduced with chronic sun exposure or other hyperpigmentation diseases. Tyrosinase, a copper-containing monooxygenase, is a key enzyme that catalyzes melanin synthesis in melanocytes. Inhibiting this enzyme may be the least invasive procedure for maintaining skin whiteness (10).
Mushroom tyrosinase inhibition assay was performed in the 96-well micro plates. The L-DOPA oxidation activity of tyrosinase was measured by spectrophotometry as described previously with some modifications. Briefly, 160 µl of 5 µM L-DOPA (in 100 µM sodium phosphate buffer pH 6.8) and 20 µl of the same buffer with and without the test sample were placed in the wells of a 96 micro plate, and then 20 µl of mushroom tyrosinase (200 units/ml) were mixed into each well at 37°C over 30 min. The amount of dopachrome produced in the reaction mixture was measured at 475 using the micro plate reader. Kojic acid was used as positive control of tyrosinase inhibitor.
The 50 % inhibition of tyrosinase activity (IC50) was calculated by the use of Prism Graph pad software.
Results
Normal-phase column chromatography of the methanol extract of aerial parts, followed by semi-preparative HPLC, afforded four pure known compounds including isorhoifolin (1), jaranol (2), poliumoside (3) and verbascoside (4). We evaluated antioxidant and tyrosinase inhibitory activity of the purified compounds. Jaranol showed the highest tyrosinase inhibitory activity. On the other hand, poliumoside was the best antioxidant among the tested compounds.
Compound 1 was obtained as pale yellow amorphous powder. It was clear from the 1H and 13C NMR data (Table 1) that compound 1 consisted of a flavone nucleus and two sugar moieties.
Table 1.
1H-NMR and 13C-NMR data of compounds 1 and 2 (600 MHz, DMSO-d6)
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 2 | - | 164.8 | - | 164.5 |
| 3 | 6.83 (s) | 103.5 | - | 132.3 |
| 4 | - | 182.4 | - | 182.4 |
| 5 | - | 161.6 | - | 153 |
| 6 | 6.43 | 100 | 6.90 (s) | 92 |
| 7 | - | 163.3 | - | 159 |
| 8 | 6.75 | 95.2 | 6.82 (s) | 103.1 |
| 9 | - | 157.3 | - | 152.5 |
| 10 | - | 105.8 | - | 105.5 |
| 1’ | 5.04 (d, J=7.8) | 121.4 | - | 121.5 |
| 2’,6’ | 7.93 (d, J=8.8) | 129 | 7.94 (d, J=8) | 128.9 |
| 3’,5’ | 6.94 (d, J=8.8) | 116.5 | 6.91 (d, J=8) | 116.4 |
| 4’ | - | 161.7 | - | 161.7 |
| Glc: 1" | 5.04 (d, J=7.8) | 100.3 | 60.4 | |
| 2" | 3.28 | 73.5 | 56.8 | |
| 3" | 3.26 (m) | 76.7 | - | |
| 4" | 3.14 (m) | 70 | ||
| 5" | 3.58 (m) | 76 | ||
| 6" | 3.84(d),3.41 (m) | 66.5 | ||
| Rha: 1’" | 4.54 (s) | 100.9 | ||
| 2’" | 3.64 (bs) | 70.7 | ||
| 3’" | 3.41 (m) | 71.2 | ||
| 4’" | 3.13 (m) | 72.5 | ||
| 5’" | 3.44 (m) | 68.7 | ||
| 6’" | 1.06 (d, J=6) | 18.2 | ||
| 3-OMe | - | - | 3.72 | 60.4 |
| 7-OMe | - | - | 3.91 | 56.8 |
| 5-OH | - | - | 12.9 | - |
| 4’-OH | - | - | 10.36 | - |
The 1H-NMR spectrum revealed the presence of glucose and rhamnose. The glycoside structure and its connectivity were confirmed by a HMBC experiment, which showed long-range correlations between the signals of H-1” and C-7 and H-1’” and C-6”. The β-configuration of the anomeric glucose unit was assigned on the basis of its typical coupling constant, J = 7.8 Hz. The spectrum showed four aromatic protons appearing as two doublets at δ 7.93 with (J= 8.8 Hz) and δ 6.94 with (J= 8.8 Hz) due to ortho-coupled protons, that were assigned to H-2’, 6’ and H-3’, 5’, respectively (Figure 1). Also a singlet at δ 6.83 ppm corresponding to H-3, two protons broad singlet at δ 6.75 and 6.43 ppm, assigned to H-8 and H-6, respectively. From these data and by comparing them with the published data (12), compound 1 was identified as isorhoifolin.
Figure 1.
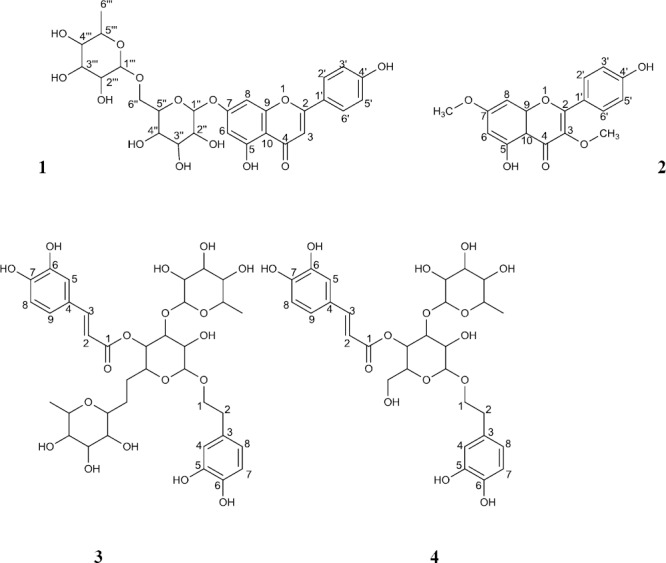
Chemical structures of compounds 1-4 isolated from T. polium var. gnaphalodes
Compound 2 was also obtained as yellow amorphous powder. The 1H and 13C NMR data of 2 showed a bi-methoxylated flavone structure for compound 2 (Table 1). The aromatic part of the 1H NMR spectra of compound 2 suggested the presence of a flavone structure. Typical 1H NMR signals of two methoxy groups at δ 3.72 and 3.91 correlated in the HMBC spectrum to the 13C NMR signals at δ 132.3 (C-3) and 159.0 (C-7), respectively, suggesting that methoxyl groups were located at C-3 and C-7. The spectrum also showed four aromatic protons appearing as two doublets at δ 7.94 with (J = 8 Hz) and δ 6.91 with (J = 8 Hz) due to ortho-coupled protons, that were assigned to H-2’, 6’ and H-3’, 5’, respectively (Figure 1). The meta position protons of ring A were also appeared as two singlets in 6.82 and 6.90 ppm. The other 1H and 13C NMR data of compound 2 were in agreement with those of jaranol (Syn. = kumatakenin) reported in the literature (13, 14).
Compound 3 was obtained as brownish amorphous powder. 1H and 13C-NMR data indicated the presence of three sugar moieties, due to the presence of signals at δC 102.8 with δH 4.35 (d, J = 7.9 Hz) for β-glucose moiety and at δC 101.6 with δH 5.01 (d, J = 1) and δC 100.9 with δH 4.47 (d, J = 1) for rhamnose moieties. The 1H NMR spectral data also revealed the presence of trans-olefinic protons attached to tri-substituted benzene ring along with the carbonyl carbon (trans-caffeoyl moiety). The presence of trans-caffeoyl moiety was indicated from the signals at δH 7.44 (H-3, J = 15.7 Hz) and δH 6.17 (H-2, J = 15.7 Hz) for trans double bond and δH 7.01 (H-5), 6.73 (H-8) and 6.95 (H-9) for the tri-substituted benzene ring, which was supported by the 13C NMR spectrum. From the above mentioned data and by comparing these data with the published data (15-17), compound 3 was identified as poliumoside.
Compound 4 was obtained as yellow amorphous powder. The 1H-NMR and 13C-NMR spectral data of 4 were very similar to those of 3 (Table 2) except for the loss of one of rhamnose moieties. Therefore, compound 4 was assumed to have a disaccharide structure. By comparing these data with the published data (18), this compound was also identified as verbascoside. The NMR data of the compounds 1-4 has been summarized in tables 1 and 2. The structure assignments of four compounds were also supported by other 2D NMR experiments including HSQC and HH-COSY.
Table 2.
1H-NMR and 13C-NMR data of compounds 3 and 4 (600 MHz- DMSO-d6)
| Position | 3 | 4 | |
|---|---|---|---|
| δH | δC | δH | |
| Glc: 1 | 4.35 (d, J=7.9) | 102.8 | 4.33 (d, J=7.9) |
| 2 | 3.21 (dd) | 74.8 | 3.21* |
| 3 | 3.77(dd) | 79.3 | 3.79 (m) |
| 4 | 4.71 (t, J=9.6) | 69.3 | 4.69 (t, J=9.6) |
| 5 | 3.65 (m) | 73.3 | 3.68 (m) |
| 6 | 3.35-3.51 (m) | 66.3 | 3.51,3.35* |
| Rha: 1 | 4.47 (d, J=1) | 100.9 | 4.3 |
| 2 | 3.60 (dd) | 71 | 3.6 |
| 3 | 3.40 (dd) | 70.9 | 3.28* |
| 4 | 3.13 (t) | 72.1 | 3.10 (t) |
| 5 | 3.35 (m) | 68.8 | 3.34* |
| 6 | 1.02 (d, J=6) | 18.2 | 0.94 (d, J=6) |
| Rha’: 1’ | 5.01 (d, J=1) | 101.6 | |
| 2’ | 3.68 (dd) | 70.8 | |
| 3’ | 3.28 (dd) | 70.7 | |
| 4’ | 3.10 (t) | 72.3 | |
| 5’ | 3.34 (m) | 69.1 | |
| 6’ | 0.94 (d, J=6) | 18.5 | |
| Caf: 1 | - | 166 | - |
| 2 | 6.17 (d, J=15.7) | 113.8 | 6.17 (d, J=15.7) |
| 3 | 7.44 (d, J=15.7) | 145.9 | 7.43 (d, J=15.7) |
| 4 | - | 125.9 | - |
| 5 | 7.01 (d, J=1.8) | 115.1 | 7.09 (s) |
| 6 | - | 145.9 | - |
| 7 | - | 148.9 | - |
| 8 | 6.73 (d, J=8.1) | 116.3 | 6.74 (d, J=8.1) |
| 9 | 6.95 (dd, J=8.1,1.8) | 121.8 | 6.95 (d, J=8.1) |
| 6-OH | 9.11 (s) | - | |
| 7-OH | 9.54 (s) | - | |
| DPE: 1 | 3.61 (m) | 70.8 | 3.79 (m), 3.67 (m) |
| 3.81 (m) | |||
| 2 | 2.64 (m) | 35.5 | 2.68 (m) |
| 3 | - | 129.6 | - |
| 4 | 6.60 (d, J=2) | 116.7 | 6.61 (s) |
| 5 | - | 145.3 | - |
| 6 | - | 143.8 | - |
| 7 | 6.47 (dd, J=8,2) | 115.9 | 6.47 (d, J=8) |
| 8 | 6.61 (d, J=8) | 119.9 | 6.61 (d, J=8) |
| 5-OH | 8.64 (s) | - | |
| 6-OH | 8.58 (s) | - | |
Discussion
As shown in Table 3, poliumoside (3) possessed the highest antioxidant capacity in DPPH test with an IC50 of 4.23 μg/ml. In FRAP assay, poliumoside showed the highest activity. It should be pointed out, however, all four compounds, but not jaranol, showed antioxidant activity comparable to the positive control α-tocopherol. Jaranol exhibited the lowest activity among the tested compounds due to the blockage of its two active hydroxyl groups with methyl groups. Hydroxyl groups typically play important role in antioxidant and radical scavenging activity of phenolics. In contrast, jaranol showed the most tyrosinase inhibitory activity with an IC50 value of 0.04 mM, comparable with the IC50 of kojic acid as positive control (Table 4). The other compounds exhibited weaker mushroom tyrosinase inhibitory activity than that of jaranol. Totally, it can be concluded that the methanol extract of T. polium var. gnaphalodes possessed antioxidant compounds with anti-tyrosinase activity, particularly jaranol as potent tyrosinase inhibitor.
Table 3.
Antioxidant activities of compounds 1-4 using FRAP and DPPH radical-scavenging activity
| Compound | FRAP (mmol/g) | IC50 (DPPH radical scavenging activity, µg/ml) |
|---|---|---|
| Poliumoside | 14.32 | 0.042 |
| Isorhoifolin | 7.67 | 1.37 |
| Verbascoside | 7.49 | 0.53 |
| Jaranol | 1.5 | 12 |
| α- tocopherol | 1.99 | 0.41 |
Table 4.
Mushroom anti-tyrosinase activity of compounds 1-4
| Compound | Verbascoside | Jaranol | Poliumoside | Isorhoifolin | Kojic acid |
|---|---|---|---|---|---|
| IC50 (mM) | 0.324 | 0.04195 | 0.5026 | 0.6701 | 0.0205 |
During last decade, much interest has been att-racted to natural and synthetic phenylpropanoids for medicinal use as antioxidant, UV screens, anticancer, antiviral, anti-inflammatory, wound healing, and antibacterial agents. They are of great interest for cosmetic and perfume industries as active natural ingredients (19).
Phenylethanoid glycosides (or phenylpropanoid glycosides) such as verbascoside and poliumoside are structurally characterized with a hydroxyl-phenylethyl moiety attached to a β- glucopyranose through glycosidic linkage. The core structures are often inundated with substituents such as aromatic acids like cinnamic and caffeic acids, and various sugars such as rhamnose, xylose and arabinose attached to the glucose residue through ester or glycosidic linkages. Several pharmacological studies have shown that these compounds possess a broad spectrum of biological activities including antibacterial, antitumor, antiviral, anti-inflammatory, antioxidant and tyrosinase inhibitory actions (20). Studies have also demonstrated that both the number and position of the phenolic hydroxyls play an important role in the antioxidative activity of phenylpropanoid glycosides (21). The length of methylene-chain connected to the benzene ring and the type of phenylpropanoid acids and sugar connected to the aglycone moiety, are major factors affecting the overall activity (20, 22, 23).
Overproduced free radicals can often lead to oxidative stress that may result in oxidative injury and diseases such as cardiovascular diseases, retinal ischemia and neurodegenerative diseases (20). Together with searching new antioxidants, there has been increasing interest in replacing synthetic antioxidants with natural ones for safety concern (24).
Verbascoside [first isolated from Verbascum sinuatum (Scrophulariaceae) in 1963 (25)] showed considerable antibacterial activities against of S. aureus strains with the minimum inhibitory concentration (MIC) values ranging from 64 µg/l to 256 µg/l (26). Poliumoside, has also significantly attenuated glutamate-induced neurotoxicity at concentrations ranging from 0.1 to 10 µM. These compounds, having a caffeoyl moiety, showed stronger neuroprotective activity than those of unsubstituted phenylethanoid glycosides (27).
The role of melanin is to protect the skin against UV light damage by absorbing UV sunlight and removing reactive oxygen species. The key enzyme that is responsible for melanin production is tyrosinase. Hyperpigmentation of the skin occurs due to overactivity of tyrosinase enzyme (28). Many tyrosinase inhibitors find applications in cosmetic products for whitening and depigmentation after sunburn and in the treatment of dermatological disorders related to melanin hyperpigmentation. Due to the harmful effects of commercial skin whitening, natural compounds have been noticed. Among them, phenolic compounds seem to be potent agents (29). Flavonoids are a large class of natural phenolics with various biological activities that occur in many fruits and vegetables (30-34). The studies showed that the presence of the 3, 3’ and 4’-hydroxyl group in flavonoids was essential for high anti-tyrosinase activity, whereas, the presence of a methoxyl group at the C-4’ and C-7 position tended to reduce the anti-tyrosinase activity. Since flavones showed higher antityrosinase activities than flavanones, it was concluded that the presence of the C2–C3 double bond is also essential for tyrosinase inhibitory ability (35). The mechanism of tyrosinase inhibition of flavonoids might also be due to chelating with copper in the active center of tyrosinase enzyme (30). The inhibitory activity of phenylethanoid glycosides could be attributed to the presence of ortho-hydroxyls on the phenolic rings, which give them a property to chelate with metals (29).
Conclusion
Teucrium polium var. gnaphalodes is a traditional medicinal plant that is used for various ailments. No phytochemical study has been conducted on this variety of T. polium. In this study, we have isolated and characterized four compounds including two phenylpropanoid glysosides (verbascoside and poliumoside) and two flavonoids (jaranol and isorhoifolin) from this plant for the first time.
We evaluated antioxidant and tyrosinase inhibitory activity of the purified compounds. Jaranol showed the highest tyrosinase inhibitory activity. On the other hand, poliumoside was the best antioxidant among the tested compounds. Taken together, our findings revealed that T. polium var. gnaphalodes contained potent antioxidant and tyrosinase inhibitors. Tyrosinase inhibitors of this plant, particularly jaranol, have a potential of application like other tyrosinase inhibitors in cosmetic and food industries.
Acknowledgment
This research was financially supported by grants from the Mashhad University of Medical Sciences Research Council (Grant No. 910421). This study was also a part of the dissertation of Mrs. Zahra Boghrati for the degree of Doctor of Pharmacy submitted to the School of Pharmacy, Mashhad University of Medical Sciences.
Conflict of interest
The authors declared no conflict of interest.
References
- 1.Parvez S, Kang M, Chung HS, Bae H. Naturally occurring tyrosinase inhibitors:mechanism and applications in skin health, cosmetics and agriculture industries. Iran J Pharm Res. 2007;21:805–816. doi: 10.1002/ptr.2184. [DOI] [PubMed] [Google Scholar]
- 2.Chang T-S. An updated review of tyrosinase inhibitors. Int J Mol Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahramikia S, Yazdanparast R. Phytochemistry and medicinal properties of Teucrium polium L.(Lamiaceae) Iran J Pharm Res. 2012;26:1581–1593. doi: 10.1002/ptr.4617. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian V. A dictionary of Iranian plant names:Latin, English, Persian:Farhang Mo’aser; 1996 [Google Scholar]
- 5.Pacifico S, D’Abrosca B, Scognamiglio M, D’Angelo G, Gallicchio M, Galasso S, et al. NMR-based metabolic profiling and in vitro antioxidant and hepatotoxic assessment of partially purified fractions from Golden germander (Teucrium polium L.). methanolic extract. Food Chem. 2012;135:1957–1967. doi: 10.1016/j.foodchem.2012.06.071. [DOI] [PubMed] [Google Scholar]
- 6.Bruno M, Bondì ML, Rosselli S, Maggio A, Piozzi F, Arnold NA. Neoclerodane diterpenoids from Teucrium montbretii Subsp libanoticum and their absolute configuration. J Nat Prod. 2002;65:142–146. doi: 10.1021/np010303m. [DOI] [PubMed] [Google Scholar]
- 7.Bruno M, Maria C, Rodríguez B, Omar AA. Guaiane sesquiterpenes from Teucrium leucocladum . Phytochemistry. 1993;34:245–247. [Google Scholar]
- 8.Rizk A, Hammouda F, Rimpler H, Kamel A. Iridoids and flavonoids of Teucrium polium herb. Planta Med. 1986;52:87–88. [PubMed] [Google Scholar]
- 9.Kawashty S, El-Din EG, Saleh N. The flavonoid chemosystematics of two Teucrium species from Southern Sinai, Egypt. Biochem Syst Ecol. 1999;27:657–660. [Google Scholar]
- 10.Wang K-H, Lin R-D, Hsu F-L, Huang Y-H, Chang H-C, Huang C-Y, et al. Cosmetic applications of selected traditional Chinese herbal medicines. J Ethnopharmacol. 2006;106:353–359. doi: 10.1016/j.jep.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Sulaiman SF, Sajak AAB, Ooi KL, Seow EM. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J Food Comp Anal. 2011;24:506–515. [Google Scholar]
- 12.Kokotkiewicz A, Luczkiewicz M, Sowinski P, Glod D, Gorynski K, Bucinski A. Isolation and structure elucidation of phenolic compounds from Cyclopia subternata Vogel (honeybush) intact plant and in vitro cultures. Food Chem. 2012;133:1373–1382. [Google Scholar]
- 13.Zhang X, Li B, Zhou M, Yuan X, Zhang G. Chemical constituents of Buddleja brachystachya diels. Chin J Appl Environ Biol. 2006;12:338. [Google Scholar]
- 14.Shaker KH, Morsy N, Zinecker H, Imhoff JF, Schneider B. Secondary metabolites from Calotropis procera (Aiton) Phytochem Lett. 2010;3:212–216. [Google Scholar]
- 15.El-Mawla A, Ahmed A, Ibraheim Z, Ernst L. Phenylethanoid glycosides from Barleria cristata L. callus cultures. Bull Pharm Sci, Assiut University. 2005;28:199. [Google Scholar]
- 16.Andary C, Wylde R, Heitz A, Rascol J, Roussel J, Laffite C. Poliumoside, a caffeic glycoside ester from Teucrium belion. Phytochemistry. 1985;24:362–364. [Google Scholar]
- 17.De Marino S, Festa C, Zollo F, Incollingo F, Raimo G, Evangelista G, et al. Antioxidant activity of phenolic and phenylethanoid glycosides from Teucrium polium L. Food Chem. 2012;133:21–28. [Google Scholar]
- 18.Singh N, Shukla N, Singh P, Sharma R, Rajendran S, Maurya R, et al. Verbascoside isolated from Tectona grandis mediates gastric protection in rats via inhibiting proton pump activity. Fitoterapia. 2010;81:755–761. doi: 10.1016/j.fitote.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Korkina L. Phenylpropanoids as naturally occurring antioxidants:from plant defense to human health. Cell Mol Biol. 2007;53:15–25. [PubMed] [Google Scholar]
- 20.Fu G, Pang H, Wong YH. Naturally occurring phenylethanoid glycosides:potential leads for new therapeutics. Curr Med Chem. 2008;15:2592–2613. doi: 10.2174/092986708785908996. [DOI] [PubMed] [Google Scholar]
- 21.Shi L, Cao Y, Chen H, Dong JP. Isolation and identification of two new secoiridoids of water-soluble chemical constituents from the fruits of Ligustrum lucidum. Ait. Acta Pharm Sin B. 1997;32:442–446. [PubMed] [Google Scholar]
- 22.Chen Z-Y, Wong IYF, Leung MWS, He Z-D, Huang Y. Characterization of antioxidants present in bitter tea (Ligustrum pedunculare) J Agric Food Chem. 2002;50:7530–7535. doi: 10.1021/jf0206421. [DOI] [PubMed] [Google Scholar]
- 23.Okawa M, Kinjo J, Yang C-R, NONAKA G, NOHARA T. Antioxidative substances in the leaves of Ligustrum purpurascens. Nat Med. 2001;55:209–212. [Google Scholar]
- 24.Chang L-W, Juang L-J, Wang B-S, Wang M-Y, Tai H-M, Hung W-J, et al. Antioxidant and antityrosinase activity of mulberry (Morus alba L.) twigs and root bark. Food Chem Toxicol. 2011;49:785–790. doi: 10.1016/j.fct.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 25.Jimenez C, Riguera R. Phenylethanoid glycosides in plants:structure and biological activity. Nat Prod Rep. 1994;11:591–606. doi: 10.1039/np9941100591. [DOI] [PubMed] [Google Scholar]
- 26.Nazemiyeh H, Rahman MM, Gibbons S, Nahar L, Delazar A, Ghahramani M-A, et al. Assessment of the antibacterial activity of phenylethanoid glycosides from Phlomis lanceolata against multiple-drug-resistant strains of Staphylococcus aureus. J Nat Med. 2008;62:91–95. doi: 10.1007/s11418-007-0194-z. [DOI] [PubMed] [Google Scholar]
- 27.Koo KA, Sung SH, Park JH, Kim SH, Lee KY, Kim YC. In vitro neuroprotective activities of phenylethanoid glycosides from Callicarpa dichotoma. Planta Med. 2005;71:778. doi: 10.1055/s-2005-871213. [DOI] [PubMed] [Google Scholar]
- 28.Mapunya MB, Nikolova RV, Lall N. Melanogenesis and antityrosinase activity of selected South African plants. J Evid Based Complementary Altern Med. 2012;2012 doi: 10.1155/2012/374017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karioti A, Protopappa A, Megoulas N, Skaltsa H. Identification of tyrosinase inhibitors from Marrubium velutinum and Marrubium cylleneum. Bioorg Med Chem Lett. 2007;15:2708–2714. doi: 10.1016/j.bmc.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 30.Iranshahi M, Rezaee R, Parhiz H, Roohbakhsh A, Soltani F. Protective effects of flavonoids against microbes and toxins:The cases of hesperidin and hesperetin. Life Sci. 2015;137:125–132. doi: 10.1016/j.lfs.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin – A mini-review. Life Sci. 2014;113:1–6. doi: 10.1016/j.lfs.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 32.Roohbakhsh A, Parhiz F, Soltani F, Rezaee R, Iranshahi M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015;124:64–74. doi: 10.1016/j.lfs.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 33.Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin:an updated review of their molecular mechanisms and experimental models. Phytother Res. 2015;29:323–331. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 34.Saeidi I, Hadjmohammadi MR, Peyrovi M, Iranshahi M, Barfi B, Babaei AB, Mohammaddoost A. HPLC determination of hesperidin, diosmin and eriocitrin in Iranian lime juice using polyamide as an adsorbent for solid phase extraction. J Pharm Biomed Anal. 2011;56:419–422. doi: 10.1016/j.jpba.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Saewan N, Koysomboon S, Chantrapromma K. Anti-tyrosinase and anti-cancer activities of flavonoids from Blumea balsamifera DC. J Med Plants Res. 2011;5:1018–1025. [Google Scholar]


