Abstract
Objective(s):
Activation of acid-sensing ion channel 1a (ASIC1a) is responsible for tissue injury caused by acidosis in nervous systems. But its physiological and pathological roles in nucleus pulposus cells (NPCs) are unclear. The aim of this study is to investigate whether ASIC1a regulates the survival of NPCs in the acidic environment of degenerated discs.
Materials and Methods:
NPCs were isolated and cultured followed by immunofluorescent staining and Western-blot analysis for ASIC1a. Intracellular calcium ([Ca2+]i) was determined by Ca2+-imaging using Fura-2-AM. Cell necrosis, apoptosis, and senescence following acid exposure were determined using lactate dehydrogenase (LDH) release assay, annexin V-fluorescein isothiocyanate/propidium iodide dual-staining and cell cycle analysis, respectively, followed by Western-blot analysis for apoptosis-related proteins (Bax, Bcl-2, and caspase-3) and senescence-related proteins (p53, p21, and p16). Effects of treatment with psalmotoxin-1 (PcTX1, blocker of ASIC1a) on [Ca2+]i and cell survival were investigated.
Results:
ASIC1a was detected in healthy NPCs, and its expression was significantly higher in degenerated cells. When NPCs were treated with PcTX1, acid-induced increases in [Ca2+]i were significantly inhibited. PcTX1 treatment also resulted in decreased LDH release, cell apoptosis and cell cycle arrest in acid condition. Acid exposure decreased the expression of Bcl-2 and increased the expression of Bax, cleaved caspase-3 and senescence-related proteins (p53, p21, and p16), which was inhibited by PcTX1.
Conclusion:
The present findings suggest that further understanding of ASIC1a functionality may provide not only a novel insight into intervertebral disc biology but also a novel therapeutic target for intervertebral disc degeneration.
Keywords: Acidic, Acid-sensing ion channel 1a, Apoptosis, Intervertebral disc, degeneration, Nucleus pulposus cells, Senescence
Introduction
Low back pain (LBP) is one of the most debilitating conditions, resulting in substantial socioeconomic and health-care consequences (1). The most frequent cause of LBP is lumbar intervertebral disc degeneration (IVDD) (2). Various biological therapies, including growth factor injection, cell transplantation, and gene therapy, have attempted to prevent or to reverse disc degenerative process (3). Among these therapies, increasing the number of functional nucleus pulposus cells (NPCs) can be a key to rebuilding the function of IVD (4). However, the biochemical and biophysical changes in a degenerated IVD will present a harsh microenvironment for both the endogenous and transplanted cells.
The disc microenvironment is characterized by low pH, reduced oxygen supply, and reduced nutria- tion as well as high osmolarity (5). Wuertz et al (5) previously demonstrated that the most challenging chemical condition in this microenvironment is matrix acidity, which has a potentially negative effect on cell viability and function. Several factors contribute to the acidic pH. Firstly, the anaerobic glycolysis of NPCs and the slow diffusion of lactic acid across dense matrix result in elevated acidity in the disc (5-8). Secondly, negatively charged proteo-glycans attract large amounts of cations, such as H+ ions, creating a low pH environment (9, 10). Thirdly, pro-inflammatory cytokines in thedegene-rated disc increase the rate of lactic acid production markedly (9, 11-13). These mechanisms are all thought to maintain the extracellular environment in the normal IVD at pH 7.0–7.2 (9, 14). However, in seriously degenerated discs, the pH usually reduces to 6.5, although values as low as 5.5–5.6 have been reported for diseased tissue removed at surgery (15, 16).
Acid-sensing ion channels (ASICs) are H+-gated voltage-insensitive ion channels and belong to the degenerin/epithelial Na+ channel superfamily. The ASIC family has six subunit proteins (ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4) that are encoded by four genes (ASIC1, ASIC2, ASIC3, and ASIC4). Homomeric ASIC1a is Na+ and Ca2+ permeable, whereas other combinations are only permeable to Na+ (17-19). ASICs are the primary acid sensors in the neurons of the mammalian central and peripheral nervous systems and are involved in physiologic and pathologic processes (20). Activation of Ca2+-permeable ASIC1a is responsible for acidosis-mediated ischemic brain injury caused by Ca2+ influx in neurons (21). Intracellular Ca2+ ([Ca2+]i) represents a prevalent signaling mechanism and is involved in processes such as cell division and injury (22). Evidence indicates that the ASIC subunit proteins are also associated with physiologic and pathologic processes in a diverse range of species and cell types. An excessive accumulation of cytosolic Ca2+ may also trigger apoptosis in chondrocytes (14).
However, it is uncertain whether ASIC1a mediates directly Ca2+ influx and increased [Ca2+]i in human NPCs. It is also uncertain whether ASIC1a contri-butes to NPCs injury through an acidosis-evoked increase in [Ca2+]i. Therefore, the aim of the present study was to confirm if ASIC1a could alter [Ca2+]i, and whether ASIC1a activation regulated the survival of NPCs in the acidic environment of degenerated discs.
Materials and Methods
Patient samples
Degenerated human IVDs were obtained from patients receiving surgical procedures for treatment of LBP due to IVDD (Table 1). The IVDD was measured as grade III (6 donors), IV (19 donors), and V (9 donors) according to the Pfirrmann scale of IVDD (23). In addition, ten patients (8 males and 2 females; age range 51–63 years; L1-L2=5, L2-L3=2, L3-L4=2, L4-L5=1) with acute spine damage but without IVDD according to the Pfirrmann scale were also included as the healthy cases. The IVDs were collected and divided into several segments containing only NP tissue and kept in sterile tubes.
Table 1.
Sex, age, operated lumbar segment, and corresponding grade of degeneration
| Donor | Sex | Age | Segment | Pfirrmann scale | Degeneration |
|---|---|---|---|---|---|
| 1 | M | 51 | L1-L2 | I | N |
| 2 | M | 53 | L3-L4 | II | N |
| 3 | M | 54 | L1-L2 | I | N |
| 4 | F | 55 | L2-L3 | II | N |
| 5 | M | 55 | L3-L4 | I | N |
| 6 | M | 57 | L1-L2 | I | N |
| 7 | F | 58 | L4-L5 | I | N |
| 8 | M | 59 | L1-L2 | II | N |
| 9 | M | 61 | L1-L2 | II | N |
| 10 | M | 63 | L2-L3 | II | N |
| 11 | M | 54 | L3-L4 | IV | |
| 12 | M | 66 | L4-L5 | IV | D |
| 13 | M | 59 | L3-L4 | V | D |
| 14 | F | 58 | L5-S1 | III | D |
| 15 | M | 69 | L4-L5 | V | D |
| 16 | F | 72 | L5-S1 | V | D |
| 17 | F | 56 | L5-S1 | IV | D |
| 18 | M | 64 | L3-L4 | IV | D |
| 19 | M | 57 | L3-L4 | III | D |
| 20 | M | 56 | L3-L4 | III | D |
| 21 | M | 58 | L3-L4 | IV | D |
| 22 | M | 55 | L3-L4 | IV | D |
| 23 | M | 70 | L4-L5 | I | D |
| 24 | M | 70 | L4-L5 | II | D |
| 25 | M | 67 | L5-S1 | I | D |
| 26 | F | 68 | L5-S1 | II | D |
| 27 | F | 66 | L5-S1 | I | D |
| 28 | M | 65 | L4-L5 | I | D |
| 29 | F | 64 | L3-L4 | I | D |
| 30 | M | 65 | L3-L4 | II | D |
| 31 | M | 68 | L5-S1 | II | D |
| 32 | M | 55 | L4-L5 | II | D |
| 33 | M | 63 | L4-L5 | IV | D |
| 34 | M | 61 | L5-S1 | IV | D |
| 35 | M | 65 | L5-S1 | V | D |
| 36 | M | 64 | L4-L5 | III | D |
| 37 | F | 68 | L5-S1 | V | D |
| 38 | F | 59 | L5-S1 | V | D |
| 39 | F | 64 | L5-S1 | IV | D |
| 40 | M | 58 | L4-L5 | IV | D |
| 41 | M | 58 | L5-S1 | III | D |
| 42 | F | 71 | L5-S1 | III | D |
| 43 | M | 64 | L4- L5 | IV | D |
| 44 | M | 68 | L4- L5 | IV | D |
M: Male; F: female; N: normal; D: degenerated
All of the tissues used in this study were obtained in compliance with Chinese Law and the guidelines of the Helsinki Declaration II.
Isolation and culture of NPCs
NP tissues obtained from the 44 patients were digested using collagenase II at a concentration of 0.2% for 6 hr. The isolated NPCs were harvested and grown in Dulbecco’s modified Eagle’s medium with low glucose (DMEM-LG; Gibco, USA), supplemented with 10% (v/v) fetal bovine serum (FBS; Wisent Inc., Canada) and incubated at 37 °C in a humidified atmosphere with 5% CO2 with regular replenishment of medium every three days. When they reached 80–90% confluence, the cells were sub-cultured using 0.25% trypsin/0.02% ethylenediaminetetraacetate (EDTA; Gibco, USA) and designated passage 1 (P1).
Toluidine blue staining
After being fixed in 4% paraformaldehyde for 20 min, NPCs on poly-L-lysine-coated coverslips were stained with 1% toluidine blue staining for 4 hr and were rinsed with distilled water. The samples were observed using a microscope (Olympus, Japan).
Exposure of NPCs to low pH
Culture media with different pH levels were prepared by adding an appropriate amount of sterilized HCl (1 mol/l) and NaOH (1 mol/l) into DMEM and monitoring using a commercial pH microelectrode (Thermo, USA). Four media were prepared at pH 7.4 (standard condition), pH 7.0 (representing the normal IVD), pH 6.5 (mildly degenerated IVD), and pH 6.0 (severely degenerated IVD). The medium and the cells were harvested 24 hr after exposure to different conditions.
Western blot analysis
NPCs were rinsed with ice-cold phosphate buffered saline (PBS) and lysed in lysis buffer containing a mixture of protease inhibitors. Total protein was extracted from six samples and protein content was quantified using a bicinchoninic acid quantification kit (Thermo, USA). Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto polyvinylidene fluoride membranes (Millipore, USA), and blocked for 1 hr with 5% (w/v) bovine serum albumin in Tris-buffered saline-0.1% Tween. After blocking, membranes were incubated with rabbit anti-ASIC1a (1:500, Alomone Labs, Israel), anti-Bcl-2 (1:500, Abcam, UK), anti-Bax (1:500, Abcam, UK), anti-caspase-3 (1:200, Abcam, UK), anti-p53 (1:1000, Abcam, UK), anti-p21 (1:1000, Abcam, UK), anti-p16 (1:1000, Abcam, UK), and anti-α-tubulin (1:3000; KeyGEN, China) at 4 °C with gentle shaking, over-night. Next, the membranes were incubated with secondary antibodies coupled to horseradish peroxidase (goat anti-rabbit IgG, 1:2500; KeyGEN, China) for 1 hr at room temperature. Protein was observed using the enhanced chemiluminescence method according to the manufacturer’s instructions (Millipore, USA). The density of the immunoblot bands was quantified using a Bio-Rad calibrated densitometer.
Immunofluorescent staining
After being fixed in 4% paraformaldehyde for 20 min, NPCs on poly-L-lysine-coated coverslips were blocked in a buffer with 5% bovine serum albumin and 0.1% Triton X-100 in PBS for 20 min. Then, the cells were washed three times and incubated with rabbit anti-ASIC1a (1:200, Alomone Labs, Israel) and anti-type II collagen (COL2; 1:300, Abcam, USA) at 4 °C overnight. Next, the cells were washed and incubated with secondary antibodies (Alexa Fluor 594 goat anti-rabbit, 1:1000; Invitrogen, USA) diluted in PBS for 2 hr at room temperature. Cell nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, Ireland) for 15 min at room temperature and observed using a fluorescence microscope (Olympus, Japan).
ASIC1a-mediated intracellular calcium ([Ca2+]i)
Intracellular calcium imaging was performed as described previously (24). Cells were pre-treated with psalmotoxin-1 (PcTX1, 100 ng/ml; Alomone Labs, Israel), a selective blocker of ASIC1a. Cells on coverslips were washed with D-Hanks’ solutions and incubated with 5 μM Fura-2-AM (Sigma, Ireland) for 30 min at room temperature. After incubation, the cells were washed with D-Hanks’ solutions to remove the extracellular Fura-2-AM. The fluore-scence of intracellular Fura-2-AM was quantitated using a laser scanning confocal microscope (Olym-pus, Japan) with excitation at 488 nm and emission at 525 nm. The recording continued when the acidic solution (pH 6.0) was added and continued for approximately 10 min. Six cells were measured each time, and the mean value of the fluorescence of each cell was calculated. The intensity of the fluorescence in individual cells was measured using the LSM 5 Image software.
Lactate dehydrogenase (LDH) release assay
Acid-induced cell death was assessed by quanti-tatively measuring the LDH released from membrane-damaged cells. Cells were pre-treated with PcTX1. LDH release was detected in culture medium using a chromatometry assay kit (Jiancheng, China) according to the manufacturer’s instructions after cells were incubated in the acidic solution for 24 hr. After the reaction, absorbance was measured at 440 nm on a spectrophotometric microplate reader. At the completion of each experiment, the maximum LDH content of each sample was obtained by incubating with 1% Triton X-100 for 20 min. The percentage of specific lysis was expressed as a ratio of LDH released/(LDH released+LDH Triton X extracted).
Detection of apoptosis and senescence by flowcytometry
Apoptosis was determined using the annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) dual-staining kit in accordance with the manufacturer’s instructions (KeyGen, China). In brief, cells were washed in cold PBS and stained with 5 µl of annexinV-FITC and 5 µl of PI in 500 µl of binding buffer for 15 min in the dark at 37 °C. Stained cells were quantified by flowcytometry (FACS Caliber; BD Biosciences, USA).
Cell cycle was analyzed by flowcytometry. Cells were harvested and fixed in 70% ethanol in cold PBS overnight at 4°C. After centrifugation, cells were washed with cold PBS twice, incubated with RNase A (0.1 mg/ml) for 1 hr at 37 °C, and then stained with PI (0.1 mg/ml) for 30 min in the dark. The distribution of cells in the cell cycle was determined by flowcytometry. In addition, the percentage of cells in each phase of the cell cycle was evaluated using the ModFit software.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software. All values were expressed as mean±SD. The comparison among different groups was done using one-way analysis of variance (ANOVA) and unpaired student’s t-test, multiple comparison was done using the Dunnett test. P-values less than 0.05 were considered statistically significant.
Results
Morphology and function characterization of primary cultured human NPCs
NPCs isolated from discs were polygonal, with multiple cytoplasmic processes and granular cyto-plasm. Immunofluorescent analysis showed that the cytoplasm of these cells contained the COL2 protein. ACAN, another marker for human NPCs, was significantly expressed in the cytoplasm as observed by toluidine blue staining (Figures 1 A, B, C).
Figure 1.
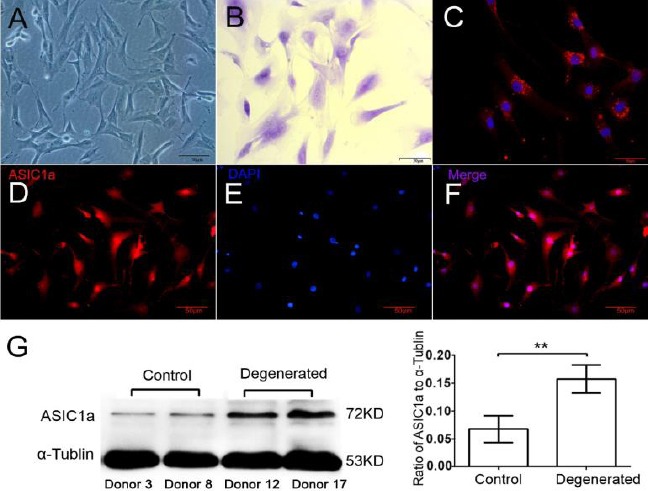
Characterization of primary cultured human NPCs and the expression of ASIC1a. (A) Phase contrast Microscope. (B) Toluidine blue staining (ACAN). (C) Immunofluorescent staining (COL2 and DAPI). (D, E, F) Immunofluorescent staining (ASIC1a and DAPI). (G) Quantified ASIC1a expression in healthy (donors 3, 8) and degenerated (donors 12, 17) NPCs. Compared to the healthy NPCs, ASIC1a expression was significantly higher in degenerated cells (P<0.01, n=10). Pictures are representative of n=10 donors (donors 1–10, donors 11–20)
Human NPCs expressed ASIC1a
In primary cultured human NPCs, ASIC1a expression was determined using immunofluo-rescent analysis. ASIC1a expression was quantified in NPCs from healthy (donors 1–10) IVDs and degenerated IVDs (donors 11–20) by Western-blot analysis. Compared to the healthy NPCs, ASIC1a expression was significantly higher in degenerated cells (P<0.01, n=10 donors) (Figures 1 E, D, F, G).
Blockade of ASIC1a inhibited the increase of [Ca2+]i induced by acidic stimulation in NPCs
The [Ca2+]i in NPCs obtained from donors 21–26 was investigated using Fura-2-AM fluorescent Ca2+-imaging following the treatment with extracellular acid. Inhibitors of voltage-gated Ca2+ channels and glutamate receptors were included in the treatment solutions to inhibit possible secondary activation of these channels/receptors. Ca2+-imaging demonstrated a significant increase in intracellular Ca2+ in response to the acidic extracellular solution (pH 6.0) containing Ca2+. However, acidic incubation failed to increase [Ca2+]i in the presence of PcTX1, and this observation supported the involvement of Ca2+-permeable ASIC1a in acid-mediated NPCs injury (Figure 2). The cells in this experiment were obtained from six donors (donors 21–26) suffering an IVD degeneration.
Figure 2.
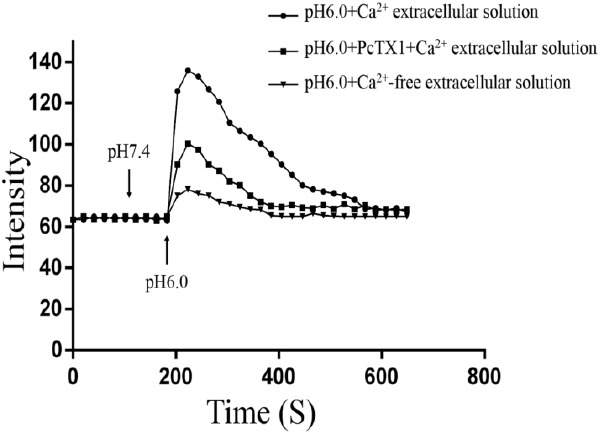
Ca2+-imaging demonstrated a significant increase in intracellular Ca2+ in response to the acidic extracellular solution (pH 6.0) containing Ca2+. However, acidic incubation failed to increase [Ca2+]i in the presence of PcTX1. n=6 donors (donors 21–26)
Acid-induced activity of ASIC1a was involved in NPCs necrosis
The LDH release assay was used to assess the degree of acid-induced cell injury. Compared to NPCs incubated in pH 7.4, acidosis induced a significant increase in LDH release (n=6 donors, donor 21–26, P<0.01). However, the acidosis-mediated LDH release decreased with the presence of PcTX1 (n=6 donors, donor 21–26, P<0.01) (Figure 3).
Figure 3.
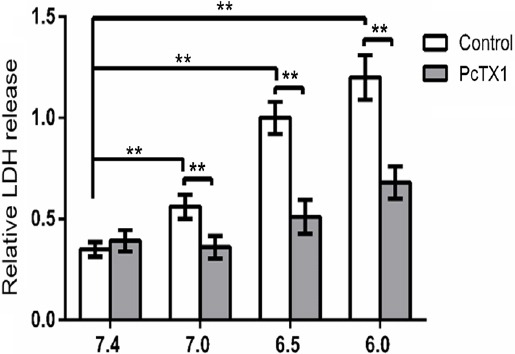
Acid exposure significantly induced LDH release, which was inhibited by PcTX1. n=6 donors (donor 21–26), ** P<0.01
Acid-induced activity of ASIC1a was involved in NPCs apoptosis
To determine whether extracellular acidosis is associated with NPCs apoptosis, annexin-V-FITC/PI double staining was used to quantify the percentage of apoptotic cells at pH 6.0, 6.5, 7.0, and 7.4 for 24 hr (Figure 4A). The results showed that the percentage of apoptotic cells significantly increased in pH 6.0, 6.5, and 7.0, respectively, compared to pH 7.4. A one-way ANOVA resulted in F=58.32, P<0.01 (n=6).
Figure 4.
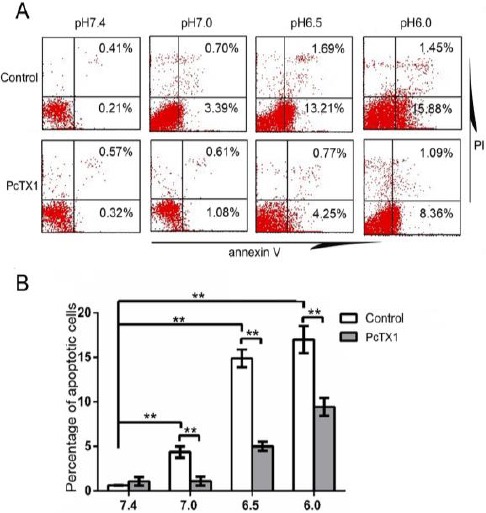
Acid exposure significantly induced NPCs apoptosis, which was inhibited by PcTX1. (A) Flowcytometry. Apoptotic cells= early apoptotic cells (lower right) + late apoptotic cells (upper right). (B) Cell apoptosis was inhibited by PcTX1 in acid. Pictures are representative of n=6 donors (donors 27–32), ** P<0.01
Further Dunnett tests revealed that the differences in the rate of apoptosis between pH 7.0 and pH 7.4, between pH 6.5 and pH 7.4, and between pH 6.0 and pH 7.4 were statistically significant (P<0.01, P<0.01, P<0.01, respectively; n=6) (Figure 4B). To further investigate whether the acid-induced activity of ASIC1a plays a role in acidosis-mediated
NPCs apoptosis, the cells were treated with PcTX1 before and during incubation in acidic conditions. Results showed that the apoptotic rate in acid-induced NPCs decreased in PcTX1 treated groups compared with the control groups (Figure 4). The cells in this experiment were obtained from six donors (donors 27–32) suffering IVDD.
To investigate the effect of PcTX1 on apoptosis-related protein expression, Western-blot analysis was used to quantify the expression of Bax, Bcl-2, and cleaved caspase-3 in the cells. It was shown that acidic incubation (pH 6.0, 6.5, and 7.0) induced increased protein expression of Bax and cleaved caspase-3 and decreased protein expression of Bcl-2 compared to the pH 7.4 group. However, PcTX1 treatment decreased Bax and cleaved caspase-3 expression, and increased Bcl-2 expression compar-ed to cells that had been treated under acidic conditions (pH 6.0, 6.5, and 7.0) (Figure 5). The cells in this experiment were obtained from six donors (donors 33–38) suffering IVDD.
Figure 5.
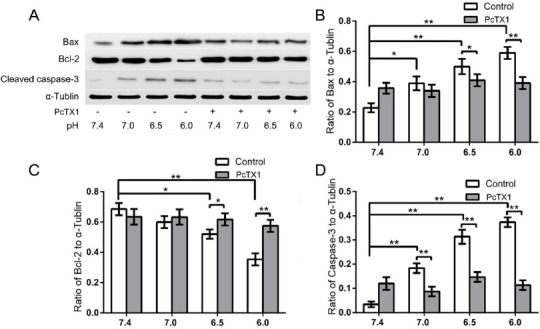
(A) Western blot analysis of apoptosis-related proteins. (B, C, D) Acidic incubation (pH 6.0, 6.5, and 7.0) induced increased protein expression of Bax and cleaved caspase-3 and decreased protein expression of Bcl-2 compared to the pH 7.4 group. However, PcTX1 treatment decreased Bax and cleaved caspase-3 expression, and increased Bcl-2 expression compared to cells that had been treated with acidic conditions (pH 6.0, 6.5, and 7.0). Pictures are representative of n=6 donors (donors 33–38), * P<0.05, ** P<0.01
Acid-induced activity of ASIC1a was involved in stress-induced premature senescence of NPCs
To determine whether extracellular acidosis is associated with NPCs senescence, the cell cycle assay was used to quantify the percentage of cells that had entered the G1 phase after they were cultured in pH 6.0, 6.5, 7.0, and 7.4 for 24 hr followed by 3 days of normal DMEM-LG. In cells exposed to acidic conditions, cell cycle analysis demonstrated that the acidity had induced the cells to accumulate in the sub-G1 phase and arrest in the G2 phase. The results showed that the percentage of cells in the G1 phase significantly increased in pH 6.0, 6.5, and 7.0, compared to pH 7.4 (Figure 6A). A one-way ANOVA resulted in F=26.63, P<0.01 (n=6). Further Dunnett tests revealed that the differences in the senescence rate between pH 7.0 and pH 7.4, between pH 6.5 and pH 7.4 and between pH 6.0 and pH 7.4 were statistically significant (P<0.05, P<0.01, P<0.01, respectively) (Figure 6B). To further investigate whether the acid-induced activity of ASIC1a plays a role in acidosis-mediated NPCs senescence, the cells were treated with PcTX1 before and during incubation in acidic conditions. Results showed that the senescence rate in acid-induced NPCs decreased in PcTX1 treated groups compared with control groups (Figure 6). The cells in this experiment were obtained from six donors (donors 39–44) suffering IVDD.
Figure 6.
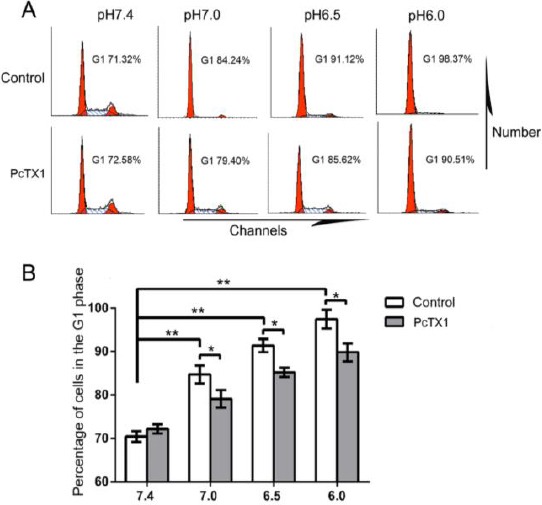
Acid exposure significantly induced NPCs senescence, which was inhibited by PcTX1. (A) Cell cycle analysis using flowcytometry. (B) Cell senescence was inhibited by PcTX1 in acid. Pictures are representative of n=6 donors (donors 39–44), * P<0.05, ** P<0.01
To investigate the effect of PcTX1 on senescence-related protein expression, Western-blot analysis was used to quantify the expression of p53, p21, and p16 in the cells. It was shown that acidic incubation (pH 6.0, 6.5, and 7.0) induced increased protein expression of p53, p21, and p16 compared to the pH 7.4 group. However, PcTX1 treatment decreased p53, p21, and p16 expression compared to cells that had been treated with acidic conditions (pH 6.0, 6.5, and 7.0) (Figure 7). The cells in this experiment were obtained from six donors (donors 33–38) suffering from IVDD.
Figure 7.
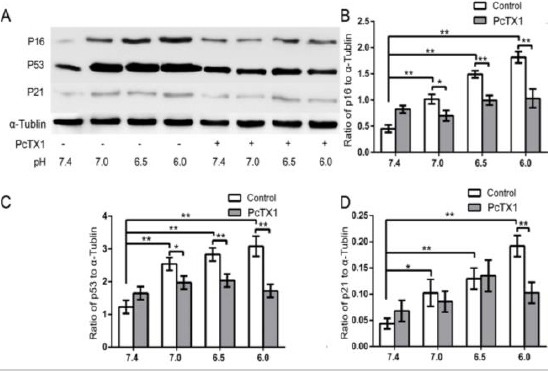
(A) Western blot analysis of senescence-related proteins. (B, C, D) Acidic incubation (pH 6.0, 6.5, and 7.0) induced increased protein expression of p53, p21, and p16 compared to the pH 7.4 group. However, PcTX1 treatment decreased p53, p21, and p16 expression compared to cells that had been treated with acidic conditions (pH 6.0, 6.5, and 7.0). Pictures are representative of n=6 donors (donors 33–38), * P<0.05, ** P<0.01
Discussion
In this study, we examined whether drugs such as PcTX1 that block ASIC1a could attenuate acid-induced injury to NPCs. We investigated the expression of ASIC1a in normal and degenerated human NPCs and the role of ASIC1a in acid-induced Ca2+ influx and NPCs death. Three main findings are noted in this study. First, human NPCs expressed ASIC1a protein, and the expression of ASIC1a in degenerated NPCs was significantly higher than the healthy ones. Second, acid exposure activated ASIC1a in NPCs, leading to an increase of Ca2+ influx. While the Ca2+ influx was significantly reduced in the presence of PcTX1 or in Ca2+ free extracellular solution. Third, acid-induced NPCs necrosis, apopto-sis, and stress-induced premature senescence were inhibited by PcTX1. These findings suggest that ASIC1a may be involved in intervertebral disc degeneration, and play a role in acidosis-mediated NPCs injury.
In order to compare the expression of ASIC1a in human healthy and degenerated IVDs, ten donors (donors 11–20) with IVDD and another ten donors (donors 1–10) with acute spine damage but without IVDD according to the Pfirrmann scale were included. The results showed that ASIC1a expression was significantly higher in cells from degenerated disc. Thus, in the following experiments, we cultured degenerated cells (cells from donors 21–44) to investigate whether ASIC1a could regulate the survival of NPCs in the acidic environment of degenerated discs. Because of the relatively hypo-cellular environment in intervertebral discs and the limited amount of NP tissue, it is difficult to complete all the experiments using the NPCs from the same donor. Usually, expansion in vitro is the feasible way to obtain enough cells. But due to the special characteristics of NPCs, expansion in vitro may lead to phenotype alteration and replicative senescence (25). Thus, we used the NPCs from different donors in different experiments. The NPCs in all the experiments were cultured in vitro no more than 2 weeks and no more than three passages.
Our study showed a significant increase of ASIC1a expression in degenerated NP. This increased expression of ASIC1a was probably due to the inflammatory response in the NP during the process of degeneration. Pro-inflammatory mediators such as IL-1 and nitric oxide (NO) could increase the expression of ASIC1a (26, 27). IL-1 up-regulates ASIC1a mRNA expression by increasing its half-life (26), while NO acts as a strong enhancer of ASICs, including ASIC1a, and potentiated the activity of ASIC1a (27).
Furthermore, we found that the ASIC1a expressed in NPCs was activated in pH 6.0 by Ca2+-imaging analysis. This was consistent with previous findings regarding activation of ASIC1a in articular chondro-cyte, endplate chondrocyte and mesenchymal stem cell (5, 20, 28). Even a transient Ca2+ signal overload activates a cascade of cytotoxic events, leading to a long-term activation of mitochondrial metabolism, and contributes to cell apoptosis (29). The excessive [Ca2+]i in the cell activates calcineurin, which in turn results in Bcl-2-antagonist of cell death (BAD) protein dephosphorylated to induce translocation and further induces cytochrome C release (20, 30). The release of cytochrome C from mitochondria begins the activation of downstream caspases, leading to the activation of caspase-9 and in turn activates caspase-3. Increased [Ca2+]i could also result in mitochondrial dysfunction characterized by elevated glutamine consumption, citric acid cycle flux, oxygen consumption, and reactive oxygen species (ROS) accumulation (31, 32). Cellular Ca2+ overload and mitochondrial function have been proposed to have a crucial role in ROS generation. Many studies have supported a causal role for mitochondrial dysfunction and ROS generation in senescent cells (32, 33). According to the free-radical theory, ROS might be one of the main candidates responsible for stress-induced premature senescence (34).
In this study, we found evidence of NPCs apoptosis and stress-induced premature senescence in acidic conditions mimicking degenerative inter-vertebral discs. Studies focused on the relationship between apoptosis and disc degeneration indicate that apoptosis plays an important role in disc degeneration and suggest that potential therapy for disc degeneration should prevent NPCs from undergoing apoptosis (35). In addition, previous in vivo studies have shown that there was an accumulation of senescent human NPCs in degenerated discs, especially in cell clusters in herniated NP (36). Senescent NPCs proliferated more slowly in vitro and had an altered phenotype, which was characterized by reduced extracellular matrix synthesis and enhanced catabolic metabolism (35). The imbalance between the catabolic and anabolic processes of the extracellular matrix, especially COL2 and ACAN, was involved in IVDD. What is more, we found that acid-induced NPCs apoptosis and stress-induced premature senescence were inhibited by blocking ASIC1a. Thus, the present study suggests that blocking ASIC1a may slow down the process of intervertebral disc degeneration by the inhibition of acid-induced NPCs apoptosis and stress-induced premature senescence.
We demonstrated that NPCs respond to a reduction in extracellular pH by increasing [Ca2+]i levels, which was inhibited by ASIC1a blockade with PcTX1. These findings support an essential role for ASIC1a and Ca2+ signal in acid-induced NPCs dying. Thus, ASIC1a regulated the survival of NPCs in the acidic environment of degenerated intervertebral discs via a Ca2+ influx-mediated mechanism. This finding is in agreement with a previous report that ASIC1a-mediated Ca2+ influx regulates apoptosis of endplate chondrocytes in intervertebral discs (20).
There are some limitations to the present study that must be acknowledged. First, this is a preliminary study which suggests that ASIC1a may be involved in intervertebral disc degeneration, and play a role in acidosis-mediated NPCs injury. Although this study provides a novel insight into IVD biology, further research is necessary to strengthen this topic. Secondly, there is more to inhibiting degeneration than merely stopping the cells from dying. It is recommended that in future studies, the effect of ASIC1a blockade on matrix synthesis, degradation, and other catabolic processes be examined. Third, this study examined only ASIC1a although a previous study showed that other subunits were also expressed in NPCs (18). ASIC1a is the only subunit permeable to Ca2+, which is responsible for acidosis-mediated cell injury. However, another study on chondrocyte suggested that the activation of ASIC3 in acidic conditions increased the expression of the matrix within the joint tissue. Thus, in future studies, ASIC1a-mediated cell injury and ASIC3 mediated matrix production should be considered together.
Conclusion
In summary, we have demonstrated that ASIC1a activation induced the increased [Ca2+]i of NPCs, which resulted in cell apoptosis and stress-induced premature senescence. Although further experi-ments are needed to elucidate the function of ASIC1a in vivo, the current research suggests that further understanding of ASIC1a functionality may provide not only a novel insight into IVD biology but also a novel therapeutic target for IVD degeneration.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (81272035; 81572190; 81572170; 81572109; 81201423), the 51st China Postdoctoral Science Foundation 2012 (2012M511800), the Fundamental Research Funds for the Central Universities (KYLX_0202) and the Jiangsu Provincial Special Program of Medical Science (BL20122004).
Conflict of interest
The authors confirm that there is no conflict of interest to disclose.
References
- 1.Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–664. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 2.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Sakai D, Grad S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv Drug Deliv Rev. 2015;84:159–171. doi: 10.1016/j.addr.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Leung VY, Chan D, Cheung KM. Regeneration of intervertebral disc by mesenchymal stem cells:potentials, limitations, and future direction. Eur Spine J. 2006;15:S406–413. doi: 10.1007/s00586-006-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wuertz K, Godburn K, Iatridis JC. MSC response to pH levels found in degenerating intervertebral discs. Biochem Biophys Res Commun. 2009;379:824–829. doi: 10.1016/j.bbrc.2008.12.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stairmand JW, Holm S, Urban JP. Factors influencing oxygen concentration gradients in the intervertebral disc. A theoretical analysis. Spine. 1991;16:444–449. doi: 10.1097/00007632-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17:829–835. doi: 10.1002/jor.1100170607. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Liang C, Tao Y, Zhou X, Li F, Chen G, et al. Acidic pH conditions mimicking degenerative intervertebral discs impair the survival and biological behavior of human adipose-derived mesenchymal stem cells. Exp Biol Med. 2012;237:845–852. doi: 10.1258/ebm.2012.012009. [DOI] [PubMed] [Google Scholar]
- 9.Razaq S, Wilkins RJ, Urban JP. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur Spine J. 2003;12:341–349. doi: 10.1007/s00586-003-0582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray ML, Pizzanelli AM, Grodzinsky AJ, Lee RC. Mechanical and physiochemical determinants of the chondrocyte biosynthetic response. J Orthop Res. 1988;6:777–792. doi: 10.1002/jor.1100060602. [DOI] [PubMed] [Google Scholar]
- 11.Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine. 1998;23:2538–2544. doi: 10.1097/00007632-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 12.Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Evans CH. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976) 1996;21:271–277. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 13.Stefanovic-Racic M, Stadler J, Georgescu HI, Evans CH. Nitric oxide and energy production in articular chondrocytes. J Cell Physiol. 1994;159:274–280. doi: 10.1002/jcp.1041590211. [DOI] [PubMed] [Google Scholar]
- 14.Ichimura K, Tsuji H, Matsui H, Makiyama N. Cell culture of the intervertebral disc of rats:factors influencing culture, proteoglycan, collagen, and deoxyribonucleic acid synthesis. J Spinal Disord. 1991;4:428–436. doi: 10.1097/00002517-199112000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Diamant B, Karlsson J, Nachemson A. Correlation between lactate levels and pH in discs of patients with lumbar rhizopathies. Experientia. 1968;24:1195–1196. doi: 10.1007/BF02146615. [DOI] [PubMed] [Google Scholar]
- 16.Kitano T, Zerwekh JE, Usui Y, Edwards ML, Flicker PL, Mooney V. Biochemical changes associated with the symptomatic human intervertebral disk. Clin Orthop Relat Res. 1993:372–377. [PubMed] [Google Scholar]
- 17.Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci U S A. 2004;101:6752–6757. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuesta A, Del Valle ME, Garcia-Suarez O, Vina E, Cabo R, Vazquez G, et al. Acid-sensing ion channels in healthy and degenerated human intervertebral disc. Connect Tissue Res. 2014;55:197–204. doi: 10.3109/03008207.2014.884083. [DOI] [PubMed] [Google Scholar]
- 19.Wang YZ, Xu TL. Acidosis, acid-sensing ion channels, and neuronal cell death. Mol Neurobiol. 2011;44:350–358. doi: 10.1007/s12035-011-8204-2. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Wu FR, Xu RS, Hu W, Jiang DL, Ji C, et al. Acid-sensing ion channel 1a-mediated calcium influx regulates apoptosis of endplate chondrocytes in intervertebral discs. Expert Opin Ther Targets. 2014;18:1–14. doi: 10.1517/14728222.2014.859248. [DOI] [PubMed] [Google Scholar]
- 21.Ye JH, Gao J, Wu YN, Hu YJ, Zhang CP, Xu TL. Identification of acid-sensing ion channels in adenoid cystic carcinomas. Biochem Biophys Res Commun. 2007;355:986–992. doi: 10.1016/j.bbrc.2007.02.061. [DOI] [PubMed] [Google Scholar]
- 22.Berridge MJ, Bootman MD, Lipp P. Calcium--a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 23.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 24.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, et al. Neuroprotection in ischemia:blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 25.Ciapetti G, Granchi D, Devescovi V, Leonardi E, Greggi T, Di Silvestre M, et al. Ex vivo observation of human intervertebral disc tissue and cells isolated from degenerated intervertebral discs. Eur Spine J. 2012;21:S10–19. doi: 10.1007/s00586-012-2234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22:10662–10670. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yingjun G, Xun Q. Acid-sensing ion channels under hypoxia. Channels (Austin) 2013;7:231–237. doi: 10.4161/chan.25223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan FL, Chen FH, Lu WG, Li X, Wu FR, Li JP, et al. Acid-sensing ion channel 1a mediates acid-induced increases in intracellular calcium in rat articular chondrocytes. Mol Cell Biochem. 2010;340:153–159. doi: 10.1007/s11010-010-0412-y. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald G, Shi L, Vande Velde C, Lieberman J, Greenberg AH. Mitochondria-dependent and -independent regulation of Granzyme B-induced apoptosis. J Exp Med. 1999;189:131–144. doi: 10.1084/jem.189.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 31.Egnatchik RA, Leamy AK, Jacobson DA, Shiota M, Young JD. ER calcium release promotes mitochondrial dysfunction and hepatic cell lipotoxicity in response to palmitate overload. Mol Metabol. 2014;3:544–553. doi: 10.1016/j.molmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007;5:e110. doi: 10.1371/journal.pbio.0050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Kim JS, Kim EJ, Kim HJ, Yang JY, Hwang GS, Kim CW. Proteomic and metabolomic analysis of H2O2-induced premature senescent human mesenchymal stem cells. Exp Gerontol. 2011;46:500–510. doi: 10.1016/j.exger.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Zhao CQ, Wang LM, Jiang LS, Dai LY. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6:247–261. doi: 10.1016/j.arr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Gruber HE, Ingram JA, Norton HJ, Hanley EN., Jr Senescence in cells of the aging and degenerating intervertebral disc:immunolocalization of senescence-associated beta-galactosidase in human and sand rat discs. Spine (Phila Pa 1976) 2007;32:321–327. doi: 10.1097/01.brs.0000253960.57051.de. [DOI] [PubMed] [Google Scholar]


