Abstract
Objective(s):
Tuberculosis is one of the most important infectious diseases with high mortality rates worldwide, especially in developing countries. Interleukin17 (IL-17) is an important acquired immunity cytokine, which is mainly produced by CD4+TH17 cells. It can recruit neutrophils and macrophages to the infected site in the lungs. IL-23 is one of the most important inducers of IL-17. In the present study, the expressions of IL-23 and IL-17 were examined in the pathogenesis of tuberculosis.
Materials and Methods:
Peripheral blood mononuclear cells (PBMCs) were isolated from subjects with latent tuberculosis infection (LTB) and newly diagnosed active tuberculosis patients (ATB). PBMCs were activated with purified protein derivative (PPD) for 72 hr. Activated cells were harvested, RNA was extracted, and cDNA was synthesized. IL-17 and IL-23 mRNA expressions were evaluated by real-time PCR. The frequency of Th17 cells was examined by flowcytometry.
Results:
The expressions of IL-17 and IL-23 mRNA were lower in patients than subjects with LTB (P<0.05). The frequency of IL-17 producing CD4+ T cells in patients with active TB was lower than LTB subjects (P<0.05).
Conclusion:
The results of the present study might suggest that IL-17 and IL-23 play critical roles in the immune response against TB.
Keywords: Flowcytometry, Interleukin 23, Mycobacterium – tuberculosis, Purified protein – derivative, Th17
Introduction
Tuberculosis is one of the most important infectious diseases with high mortality rates worldwide, especially in developing countries. According to the World Health Organization, one-third of the world’s population is infected with Mycobacterium tuberculosis (Mtb), however only 5–10% of infected individuals develop the active TB disease with clinical symptoms, whilst most of the infected individuals remain asymptomatic (1-3).
Latent tuberculosis is defined as the presence of live Mtb within an infected host without causing disease. It is characterized by a positive response to purified protein derivative (PPD) (4). Latent tuberculosis (TB) can be maintained for the lifetime of the individual unless shifting occurs in the immunologic balance between host and pathogen, resulting in reactivation of Mtb and activation of the disease (5).
CD4+ Th1 immune cells respond by secreting interferon gamma (IFN-γ), which plays a critical role in protective immunity against Mtb(6, 7). It has been shown that low Th1 and high Th2 activity are associated with the failure of an immune response against Mtb (8-10).
IL-17 producing T cells (Th17) have been identified as a CD4+ T cell subset that is distinct from Th1 and Th2 subsets; Th17 cells have significant pro-inflammatory functions via production of the cytokines, IL-17A, and IL-17F (11). Th17 cells have been reported to play a central role, not only in the development of autoimmune and inflammatory diseases (6, 7, 12) but also in protection against intracellular pathogens (13, 14). Th17 cells are antagonized by-products of the Th1 and Th2 cytokines such as IL-12, IFN-γ, and IL-4 (6, 7). Differentiation of Th17 cells from naïve T cells is controlled by the lineage-specific transcription factors ROR-γt and ROR-α (13, 14). This issue is promoted by an IL-21-autocrine loop triggered by a+ transforming growth factor beta (TGF-β), IL-6, and IL-23 (15, 16). IL-23 has key roles in the induction of IL-17 producing antigen-specific CD4+ T cells (Th17) (11). Mycobacterial peptide vaccination induces IL-17 production, which is necessary for recruitment of IFN-γ-producing cells. IL-17 is capable of increasing the concentration of the chemokines such as CXCL9, 10, and 11, which recruit IFN-γ-producing cells to the site of inflammation (17). More recently it has been demonstrated that IL-23 induced a protective Th1 and Th17 response following Mycobacterium bovis BCG vaccination (18). Paidipally et al reported that IL-23 contributes to Mtb-induced IL-17 production by CD4+ cells from healthy tuberculin reactors (19).
The role of Il-17 and IL-23 in Mtb infection has not been yet fully understood. It has been suggested that CD4+ T cells from tuberculosis patients produced less IL-17 in response to Mtb antigens compared to CD4+ T cells from healthy controls and healthy tuberculin reactors (20); While other studies reported that patients with active tuberculosis exhibit high Th17-cell responses (21) that are associated with the severity of disease (22). Research showed no difference in terms of IL-17 production among healthy controls, latently infected individuals, and patients with active TB (23). In the present study, we examined the genes expression of IL-17 and IL-23 in patients with active TB disease and latent TB to clarify the role of these factors in the outcome of TB infection.
Materials and Methods
Study population
A cross-sectional study was conducted on 28 patients newly diagnosed with pulmonary TB who were referred to Ghaem Hospital, Mashhad University of Medical Sciences, Mashhad, Iran, between September 2011 to March 2012 and 26 latent TBs. The diagnoses of the patients were based on positive TB smear tests, positive culture, and clinical and radiological features. Subjects who as a newborn were administered with the BCG vaccination and had no history of TB were selected as the control group. A positive PPD test result was defined as an induration at the site of inoculation of at least 12 mm in diameter. Patients and controls were interviewed using structured questionnaire requesting information related to the inclusion and exclusion criteria. Subjects with the following conditions were excluded from the study: pregnancy, acute and chronic liver disease, renal diseases, and other active inflammatory conditions Subjects infected with human immunodeficiency (HIV) or human T-lymphotropic virus type I (HTLV-I) were also excluded from the study. The study was approved by Ethics Committee of Mashhad University of Medical Sciences and an informed consent was obtained from all the participants. Blood samples were collected from all case and control groups.
Peripheral blood mononuclear cells (PBMCs) cell culture
Ten ml blood samples were collected in tubes containing ethylenediaminetetraacetic acid (EDTA), and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient centrifugation in lymphocyte separation medium (Ficoll-Paque plus; Cederlane, Canada). Freshly PBMCs were cultured in 12-well plates at 2×106 cells/well in RPMI 1640 (Gibco,USA); supplemented with 2 mmol/ml glutamine (Fermentas,-Germany), 12% heat-inactivated fetal bovine serum (Gibco,USA), and 50 μg/ml penicillin (Sigma, Germany) in the presence of PPD antigen (0.75 TU/ml) (SPAN Diagnostic, India) for 72 hrs at 37 °C in a humidified 5% CO2 atmosphere. After 72 hr, cells were collected and mRNA expressions of Il-17 and IL-23 were evaluated in cells before and after stimulation with PPD. Interstellar IL-17 expression was evaluated by flowcytometry.
Intracellular cytokine staining and flowcytometric analysis
To measure intracellular IL-17, PBMCs after culturing with PPD were stimulated with PMA (50 ng/ml) plus ionomycin (1 μg/ml) and monensin (3 μM Monensin) (BD, USA) for 4 hr in 1 ml RPMI 1640 media containing 10% fetal calf serum (FCS). For surface staining, FITC anti-CD4 was added. Cells were washed with PBS containing 2% FCS and were fixed/permeabilized with %2 fixation/permeabilization buffer and stained with the anti-human IL-17PE antibody (BD, USA). After staining, cells were analyzed using CellQuest flowcytometer (BD. USA).
RNA extraction and complementary DNA (cDNA) synthesis
Total RNA was extracted from PBMCs using Tripure (Roche, Germany) according to manufacturer’s instruction and then treatment with DNase I was performed to remove DNA contamination. Reverse transcriptions were performed by random hexamers using a RevertAid™ H minus First strand cDNA Synthesis Kit (Fermentas, Germany). Reverse transcription was carried out at 42 °C for 60 min followed by RT inactivation at 70 °C for 5 min. cDNA was kept at – 20 °C before it was used.
Real-time reverse-transcription polymerase chain reaction (RT-PCR)
Beacon designer’s program V 7.0 was used to design primers and probes of IL-23 and IL-17. For IL-23 real-time PCR, the TaqMan probe method and for IL-17, the real-time PCR Syber green method was carried out using Q 6000 Machine (Qiagen, Germany).
The forward and reverse primers and probe for IL-23 were:
5’GCCTTCTCTGCTCCCTGAT-AG3’
5’TGG-GACTGAGGCTTGGAATC3’
5’TCTCC CAGTGGTG-ACCCTCAGGCT3’
respectively. All samples were normalized to the amount of beta 2 micro-globulin (β2M) transcript present in each sample and the forward and reverse and probe for β2M were:
5’TTGTCTTTCAGCAAGGACTGG3
5’CCACTTAACTA-TCTTGGGCTGTG3’ 5’TCACATGGTTCACACGGCA-GGCAT3’
respectively. For IL-17 the forward and reverse primers were:
5’GTCAACCTGAA CATCCATA-ACCG3’
5’ACTTTGCCTCCCAGATCAC-AG3’ respectively. The forward and reverse primers for β2M were:
5’AATTGAAAAAGTGGAGCATTCAGA3’ 5’GGCTGTGACAAAGTCACATGGTT3’ respect-tively. The optimization process was performed to obtain the best concentrations of primers, probes, and Master Mix reagents. Relative transcripts of IL-23 and IL-17 mRNA were normalized to β2M.
Statistical analysis
A one-sample Kolmogorov-Smirnov test was used to check whether data was normally distributed. Mean and standard deviation (SD) are used for reporting normally distributed data, an independent sample t-test was used to assess the IL-17 flowcytometric differences between patients and controls. Comparisons of IL-17 and IL-23 genes expression between the control group and TB patient were performed using the non-parametric 2-tailed Mann-Whitney U–test. The Wilcoxon matched pairs t-test was used to analyze the effect of activation with PPD on IL-17 and IL-23 gene expression and compared gene expression before and after PBMCs activation. A Pearson test was used for checking correlations between the result of IL-17 gene expression and the result of IL-17 flowcytometry. The SPSS software package (Windows version 20 SPSS, Chicago) was used for all statistical analyses and a P-value < 0.05 was considered to be significant.
Results
The study population included 28 patients with active TB and 26 latent TB with a history of positive skin test (TST+ or PPD+) as the control group. The mean± SD age of patients was 50.32±21.57, which ranged from 20 to 83 years (64.3% male, 35.7% female). The mean±SD age of the control group was 44.5±10.46, which ranged from 30 to 68 years (65% male and 35% female). No significant differences in age and sex were observed between the two groups (P>0.05).
Reduction of IL-23 gene expression in the patient group and subjects with latent tuberculosis infection after PBMCS stimulation with PPD
To determine IL-23 gene expression, we stimulated PBMCs from subjects with latent TB and the patients group with PPD antigen for 72 hr and measured the expression of IL-23 by real-time PCR, The mean±SD for IL-23 among patients was 21.29± 2.91 and in subjects with latent tuberculosis infection it was 379.58±84.20. The IL-23 gene was significantly lower in the patients than in control groups (P=0.000). The mean expression of IL-23 before stimulation with PPD in latent TB was 244.36±51.70 and in active TB it was 27.24±3.52 (P=0.00) (Figure 1).
Figure 1.
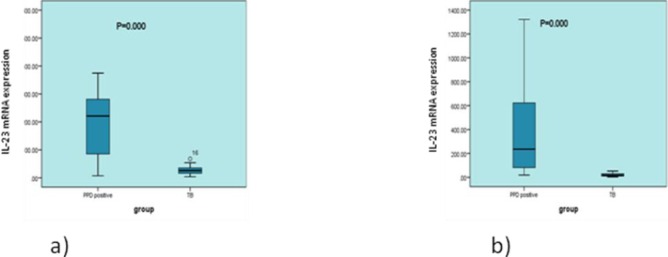
IL-23 mRNA expression before and after cell stimulation with PPD in patients and latent TB. Peripheral blood mononuclear cells from subjects with latent TB (n=26) and patient subjects (n=28) were stimulated with purified protein derivative (PPD) (0.75 TU/ml) for 72 hr, and IL-23 cytokine expression was measured by real-time PCR. IL-23mRNA expression was higher in the control group compared with patients (a) before and (b) after stimulation with PPD antigen (P=0.000 and P=0.000)
Reduction of IL-17 gene Expression in the patient group and subjects with latent tuberculosis infection after stimulation
The mean expression of IL-17 mRNA levels after PBMCs stimulation with PPD in active TB patients was significantly lower (1.45±0.47) compared to the subjects with latent tuberculosis infection (9.70± 2.35) (P=0.000) (Figure 2). When the expression of IL-17 was compared before PBMCs activation between the two groups, the mRNA levels of IL-17 were also lower in patients with active TB (0.03± 0.01) compared to the control group (0.09± 0.01) (P=0.04), respectively (Figure 2).
Figure 2.
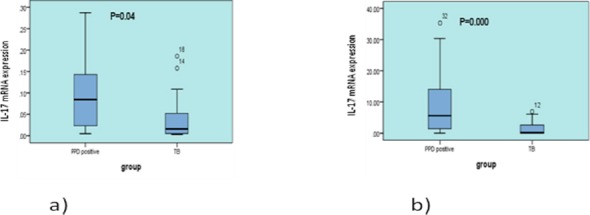
IL-17 mRNA expression before and after cell stimulation with PPD in patients and latent TB. Peripheral blood mononuclear cells from subjects with latent tuberculosis infection (n=26) and patient subjects (n=28) were stimulated with purified protein derivative (PPD) (0.75 Tu/ml) for 72 hr, and IL-17 cytokine expression was measured by real-time PCR. IL-17mRNA expression was higher in the control group compared with patients (a) before and (b) after stimulation with PPD antigen (P=0.04 and P=0.000)
Increased IL-17 expression in PBMCs stimulated with PPD compared to the non-stimulated in cases with active TB and LTB
The mRNA levels of IL-17 were significantly higher in patients after PPD stimulation (1.45±0.47) compared to non-stimulated PBMCs (0.03±0.01) (P=0.00), respectively. Furthermore, significant differences in IL-17 gene expression were observed after and before PPD stimulation in latent TB (9.70± 2.35, 0.09± 0.01, respectively, P=0.00).
No significant differences in IL-23 gene expression before and after PBMCs stimulation were observed in patients with active TB (P=0.1) and in the control group (P=0.2).
Decreased frequency of IL-17 producing CD4+ T cells in patients with active TB
The frequency of IL-17 producing CD4+ T cells was evaluated in patients and PPD+ subjects. The mean± SD for IL-17 production in the CD4+ T cells (Th17 cell subsets) in patients (n=26) and latent TB (n=26) was 1.33±0.77 and 2.52±0.95, respectively. The frequencies of Mtb antigen–specific Th17 response was significantly lower in patients with active TB compared with the controls (P=0.003) (Figure 3). There was a positive correlation between IL-17 gene expression and the frequency of IL-17 producing CD4+ T cells (P=0.030) (data not shown).
Figure 3.
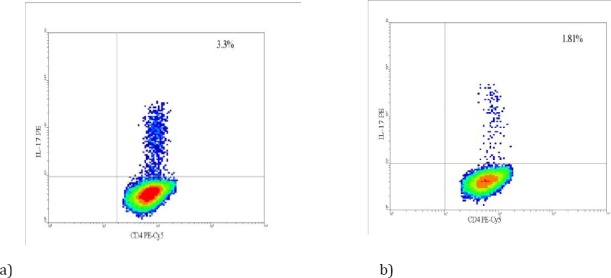
Increased production of IL-17 cytokine in the latent tuberculosis infection controls compared with patients with TB infection. Peripheral blood mononuclear cells from subjects with latent tuberculosis infection (n=26) and patients with TB infection (n=28) were stimulated with 50 ng/ml PMA, 1 μg/ml Ionomycin, 3 μM monensin for 4–5 hr. frequency of IL-17 producing CD4+ T cells was measured by intracellular staining and flowcytometry. Results showing that production of IL-17 cytokine was significantly increased in the (a) latent TB and (b) patients with active TB (P=0.003)
Discussion
The cellular immune response has a key role against tuberculosis infection. IL-17 is a proinfla-mmatory cytokine that is produced predominantly by CD4+ T cells and contributes to immunity against Mtb (5, 24, 25). Induction of chemokines including CXCL9, CXCL10, and CXCL11 by IL-17 leads to the recruitment of IFN-γ-producing T cells to the infection site and inhibits bacterial growth following Mtb infection (26). During the inflammatory process, IL-23 increased the expression of TNF α, IL-1, and IL-6 by activated myeloid cells and also induced the IL-17 producing CD4+ T cells (27). IL-23 is necessary for the establishment of IL-17-producing T cells and acceleration response for early cessation of bacterial growth after Mtb infection (28).
In the present study, we demonstrated that the mRNA expressions of IL-17 and IL-23 are significantly lower in active Mtb patients in stimulated and non-stimulated PBMCs compared to latent TB as the control group. Our results are consistent with previous studies that showed that T cells from Mtb patients produced a reduced amount of IL-17 in response to Mtb antigens compared with T cells from healthy controls with a history of negative tuberculin test (20, 29, 30). Chen et al showed that the frequency of Th17 cells decreased in the blood and pleural fluid of activated TB patients compared to healthy controls and subjects with latent TB infection. The reduced Th17 response was associated with decreased expression of IL-6R on CD4+ T cells (31). In contrast, other studies reported that Th17-cell responses increased in patients with tuberculosis patients and the increased response is associated with disease severity (21, 22). It has been reported that the expressions of IL-23 and IL-17 inPBMCs of subjects with latent TB infection are significantly higher in comparison with negative tuberculin skin test (26). Furthermore, vaccination triggers CD4+ T cells in the lung to respond through an accelerated IFN-γ response, during the subsequent Mtb infection (16). IL-23 was shown to be necessary for the induction and establishment of IL-17-producing CD4+ T cells in the lung (32).
Th17 and Th1 immune responses are decreased in IL-23p19−/− BCG-vaccinated mice and the animals exhibited lower protection following Mtb challenge (18). Studies have shown that during Mtb infection, IL-23 compensates the generation of IFN-γ-producing cells in the absence of IL-12p70, however, this compensatory response could not control the infection(33). Furthermore, it has also been reported that following primary infection with some Mtb strains, IL-17 is required for immunity against (34).
More recently it was reported that percentages of Th17 cells in patients with tuberculosis pleurisy are higher compared with the healthy controls, while expression of T regulatory (Treg) in these patients are lower compared to the healthy group. Furthermore, IL-17 and IL-23 levels were higher than that in the healthy group (35). In contrast, we have previously reported no difference in the frequency of CD4+CD25+FoxP3+ regulatory T cells between active Mtb patients and subjects with latent tuberculosis infection, while the expression of Foxp3 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) was significantly higher in patients than in subjects with latent tuberculosis infection (10). Marin et al showed that the frequency of regulatory T cells increased in patients who suffered from activated TB infection in comparison with latent tuberculosis infection. It seems that Treg cells inhibited a protective response and resulted in bacteria spreading. Down-regulation of Th17 response leads to decreased inflammatory and tissue damage (36). In addition to this, no difference in IL-17 production has been reported among healthy controls, cases with latent tuberculosis infection, and patients with active tuberculosis (23).
These controversial reports might indicate that the results depend on the pathogen and infection stages; with IL-17 showing both harmful and useful effects. In HIV infection, disease progression is associated with a loss of Th17 cells (37), while IL-17-secreting innate lymphoid cells are essential for host defense against certain fungi (38, 39). In contrast, IL-17 aggravates chronic hepatitis B infection (40) and increases pathology in human schistosomiasis (41).
It is not clear why latent and active TB-infected subjects respond differently to Mtb. One of the possible explanations for this issue is that CD4 T producing IL-17 cells from individuals with LTB and active TB recognize different antigens at different phases of the disease (42-45).
It has also been shown that in LTB individuals depletion of regulatory T cells increased the production of IL-17 and IL-23, suggesting regulatory T cells might be involved in the differences of IL-17 and IL23 between active and LTB subjects. Thus, the differences in the expression of IL-17 and IL-23 in different studies in Mtb might be attributed to the stage of disease and the number of regulatory T cells and production of CTLA-4 and FOXp3 (46). The differences in the environmental exposure to Mtb in Iran and developed countries should also be taken into account. Furthermore, BCG vaccination is not routine in some of the developed countries and this could be a cause for the differences in the results of the current study with the results of the published studies in these countries.
Conclusion
Our findings suggest that IL-23 acts as a promoting factor for the promotion of Th17 responses and improving the induction of Th17 induced-protection response against TB. Further studies are needed to clarify the exact mechanisms of IL-23 and IL-17 in the immune response against TB and the role of these cytokines in the pathogenesis of individuals infected with TB.
Acknowledgment
We would like to thank the Vice-Chancellor for Research of Mashhad University of Medical Sciences, Mashhad, Iran for financial support.
References
- 1.Pai-Dhungat JV, Parikh F. Centenary of discovery of Tubercle bacillus. J Assoc Physicians India. 2003;51:668. [PubMed] [Google Scholar]
- 2.Deveci F, Ilhan N. Plasma malondialdehyde and serum trace element concentrations in patients with active pulmonary tuberculosis. Biol Trace Elem Res. 2003;95:29–38. doi: 10.1385/BTER:95:1:29. [DOI] [PubMed] [Google Scholar]
- 3.Sapozhnikova NV, Skvortsova LA, Pavlova MV, Vishnevskii BI, Otten TF, Vasil’eva SN, et al. Pulmonary tuberculosis caused by Mycobacterium tuberculosis of different genotypes. Probl Tuberk Bolezn Legk. 2003:13–15. [PubMed] [Google Scholar]
- 4.Edwards PQ, Edwads LB. Story of the tuberculin test from an epidemiologic viewpoint. Am Rev Respir Dis. 1960;81(Pt 2):1–47. [PubMed] [Google Scholar]
- 5.Dong C. Differentiation and function of pro-inflammatory Th17 cells. Microbes Infect. 2009;11:584–588. doi: 10.1016/j.micinf.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 8.Holscher C, Holscher A, Ruckerl D, Yoshimoto T, Yoshida H, Mak T, et al. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol. 2005;174:3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 9.Lienhardt C, Azzurri A, Amedei A, Fielding K, Sillah J, Sow OY, et al. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur J Immunol. 2002;32:1605–1613. doi: 10.1002/1521-4141(200206)32:6<1605::AID-IMMU1605>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Ghazalsofala R, Rezaee SA, Rafatpanah H, Vakili R, Ghazvini K, Heidarnejad F, et al. Evaluation of CD4+CD25+FoxP3+regulatory T cells and FoxP3 and CTLA-4 gene expression in patients with newly diagnosed tuberculosis in northeast of Iran. Jundishapur J Microbiol. 2015;8:e17726. doi: 10.5812/jjm.8(4)2015.17726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 12.Pitta MG, Romano A, Cabantous S, Henri S, Hammad A, Kouriba B, et al. IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani. J Clin Invest. 2009;119:2379–2387. doi: 10.1172/JCI38813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins 1986. J Immunol. 2005;175:5–14. [PubMed] [Google Scholar]
- 14.Mosmann TR, Coffman RL. TH1 and TH2 cells:different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 16.Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 17.Ekman AK, Sigurdardottir G, Carlstrom M, Kartul N, Jenmalm MC, Enerback C. Systemically elevated Th1-, Th2- and Th17-associated chemokines in psoriasis vulgaris before and after ultraviolet B treatment. Acta Derm Venereol. 2013;93:527–531. doi: 10.2340/00015555-1545. [DOI] [PubMed] [Google Scholar]
- 18.Gopal R, Lin Y, Obermajer N, Slight S, Nuthalapati N, Ahmed M, et al. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol. 2012;42:364–373. doi: 10.1002/eji.201141569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paidipally P, Periasamy S, Barnes PF, Dhiman R, Indramohan M, Griffith DE, et al. NKG2D-dependent IL-17 production by human T cells in response to an intracellular pathogen. J Immunol. 2009;183:1940–1945. doi: 10.4049/jimmunol.0803578. [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Li J, Tian J, Zhu B, Zhang Y, Yang K, et al. IL-17 and IFN-gamma production in peripheral blood following BCG vaccination and Mycobacterium tuberculosis infection in human. Eur Rev Med Pharmacol Sci. 2012;16:2029–2036. [PubMed] [Google Scholar]
- 21.Marin ND, Paris SC, Rojas M, Garcia LF. Reduced frequency of memory T cells and increased Th17 responses in patients with active tuberculosis. Clin Vaccine Immunol. 2012;19:1667–1676. doi: 10.1128/CVI.00390-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jurado JO, Pasquinelli V, Alvarez IB, Pena D, Rovetta AI, Tateosian NL, et al. IL-17 and IFN-gamma expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. J Leukoc Biol. 2012;91:991–1002. doi: 10.1189/jlb.1211619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marin ND, Paris SC, Rojas M, Garcia LF. Functional profile of CD4+and CD8+T cells in latently infected individuals and patients with active TB. Tuberculosis (Edinb) 2013;93:155–166. doi: 10.1016/j.tube.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Radhakrishna S, Frieden TR, Subramani R, Tuberculosis Research C. Association of initial tuberculin sensitivity, age and sex with the incidence of tuberculosis in south India:a 15-year follow-up. Int J Tuberc Lung Dis. 2003;7:1083–1091. [PubMed] [Google Scholar]
- 25.Lin MY, Ottenhoff TH. Not to wake a sleeping giant:new insights into host-pathogen interactions identify new targets for vaccination against latent Mycobacterium tuberculosis infection. Biological Chem. 2008;389:497–511. doi: 10.1515/bc.2008.057. [DOI] [PubMed] [Google Scholar]
- 26.Babu S, Bhat SQ, Kumar NP, Kumaraswami V, Nutman TB. Regulatory T cells modulate Th17 responses in patients with positive tuberculin skin test results. J Infect Dis. 2010;201:20–31. doi: 10.1086/648735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowan J, Pandey S, Filion LG, Angel JB, Kumar A, Cameron DW. Comparison of interferon-gamma-, interleukin (IL)-17- and IL-22-expressing CD4 T cells, IL-22-expressing granulocytes and proinflammatory cytokines during latent and active tuberculosis infection. Clin Exp Immunol. 2012;167:317–329. doi: 10.1111/j.1365-2249.2011.04520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar NP, Anuradha R, Suresh R, Ganesh R, Shankar J, Kumaraswami V, et al. Suppressed type 1, type 2, and type 17 cytokine responses in active tuberculosis in children. Clin Vaccine Immunol. 2011;18:1856–1864. doi: 10.1128/CVI.05366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Zhang M, Liao M, Graner MW, Wu C, Yang Q, et al. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+T Cells. Am J Respir Crit Care Med. 2010;181:734–742. doi: 10.1164/rccm.200909-1463OC. [DOI] [PubMed] [Google Scholar]
- 32.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 33.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 34.Gopal R, Monin L, Slight S, Uche U, Blanchard E, Fallert Junecko BA, et al. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 2014;10:e1004099. doi: 10.1371/journal.ppat.1004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang GQ, Yang CL, Yue DF, Pei LH, Zhong H, Niu JX. The changes and its significance of Th17 and Treg cells and related cytokines in patients with tuberculosis pleurisy. Allergy Asthma Clin Immunol. 2014;10:28. doi: 10.1186/1710-1492-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marin ND, Paris SC, Velez VM, Rojas CA, Rojas M, Garcia LF. Regulatory T cell frequency and modulation of IFN-gamma and IL-17 in active and latent tuberculosis. Tuberculosis. 2010;90:252–261. doi: 10.1016/j.tube.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Singh A, Vajpayee M, Ali SA, Mojumdar K, Chauhan NK, Singh R. HIV-1 diseases progression associated with loss of Th17 cells in subtype ’C’infection. Cytokine. 2012;60:55–63. doi: 10.1016/j.cyto.2012.06.288. [DOI] [PubMed] [Google Scholar]
- 38.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng SC, van de Veerdonk F, Smeekens S, Joosten LA, van der Meer JW, Kullberg BJ, et al. Candida albicans dampens host defense by downregulating IL-17 production. J Immunol. 2010;185:2450–2457. doi: 10.4049/jimmunol.1000756. [DOI] [PubMed] [Google Scholar]
- 40.Yang B, Wang Y, Zhao C, Yan W, Che H, Shen C, et al. Increased Th17 cells and interleukin-17 contribute to immune activation and disease aggravation in patients with chronic hepatitis B virus infection. Immunol Lett. 2013;149:41–49. doi: 10.1016/j.imlet.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Mbow M, Larkin BM, Meurs L, Wammes LJ, de Jong SE, Labuda LA, et al. T-helper 17 cells are associated with pathology in human schistosomiasis. J Infect Dis. 2013;207:186–195. doi: 10.1093/infdis/jis654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park HD, Guinn KM, Harrell MI, Liao R, Voskuil MI, Tompa, et al. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol. 2003;48:833–843. doi: 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schnappinger D, Ehrt S, Voskuil MI, Mangan JA, Monahan IM, et al. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages:insights into the phagosomal environment. J Exp Med. 2003;198:693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherman DR, Voskuil M, Schnappinger D, Liao R, Harrell MI, Schoolnik GK. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc Natl Acad Sci USA. 2001;98:7534–7539. doi: 10.1073/pnas.121172498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voskuil MI, Schnappinger D, Visconti KC, Harrell MI, Dolganvo GM, Sherman DR, et al. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med. 2003;198:705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bandaru A, Devalraju KP, Paidipally P, Dhiman R, Venkatasubramanian S, Barnes PF, et al. Phospho-rylated STAT3 and PD-1 regulate IL-17 production and IL-23 receptor expression in Mycobacterium tuberculosis infection. Eur J Immunol. 2014;44:2013–2024. doi: 10.1002/eji.201343680. [DOI] [PMC free article] [PubMed] [Google Scholar]


