Abstract
Objective(s):
Although low-dose radiotherapy (RT) that involves low collateral damage is more suitable for hepatocellular carcinoma (HCC) than traditional high-dose RT, but to achieve satisfactory therapeutic effect with low-dose RT, it is necessary to sensitize HCC cells to irradiation. This study was aimed to determine whether radiosensitivity of HCC cells can be enhanced using miR-26b by targeting erythropoietin producing human hepatocelluar A2 (EphA2).
Materials and Methods:
The levels of miR-26b and EphA2 expression in multiple HCC cell lines were assessed by qPCR and western blotting, respectively, and compared with those in a hepatic cell line. HCC 97H cells were transfected with miR-26b mimics, EphA2-ShRNA or EphA2 over-expression vector before exposure to low-dose irradiation.
Results:
Different degrees of miR-26b down-regulation and EphA2 up-regulation were observed in all HCC cell lines, among which the HCC 97H cell line expressed the lowest level of miR-26b and highest level of EphA2. EphA2 was verified as the target of miR-26b by dual luciferase reporter assay. HCC 97H cells transfected with miR-26b mimics or EphA2-ShRNA reduced the expression of EphA2 protein, with significantly lower cell proliferation rate and cell invasion ability and higher apoptosis rate in response to low-dose irradiation than those in the non-transfected cells. These results were reversed after EphA2 was overexpressed by transfection with the EphA2 overexpression vector. Co-transfection with miR-26b mimics and EphA2 overexpression vector barely altered EphA2 expression level and cell response to low-dose irradiation.
Conclusion:
These data suggest that miR-26b enhances radiosensitivity of HCC 97H cells by targeting EphA2 protein.
Keywords: EphA2, HCC 97H cells, Hepatocellular carcinoma, miR-26, Radiosensitivity
Introduction
Hepatocellular carcinoma (HCC) currently ranks as the sixth most common and third most deadly cancer worldwide, posing a huge threat to human life (1). HCC usually occurs in the context of a cirrhotic liver disease with poor hepatic reserves, which makes it difficult to undertake conventional high-dose radiotherapy (RT), as high-dose irradiation inflicts serious damage not only to HCC but also to hepatic tissues (1, 2). Low-dose RT, a recent advance in the field of RT, is characterized by little collateral damage to neighboring organs and tissues, but it needs that cancer cells (e.g., HCC) be sensitized to low-dose irradiation in order to achieve satisfactory therapeutic effect. To date, the precise molecular mechanism involved in modulating the radiosensitivity of HCC cells is poorly defined, which hinders extensive clinical application of low-dose RT in HCC.
miRNAs, a class of small endogenous non-coding single-stranded RNA, drew great attention after many of them were found to be aberrantly expressed in diverse kinds of cancers (3-5). Ongoing research has identified miRNAs that tightly correlate with multiple hallmarks of cancer cells including radiosensitivity, proliferation, migration, and inva-sion. With regard to the radiosensitivity of cancer cells, miR-181, for example, sensitizes human gliomas to irradiation by targeting Bcl-2 (6). miR-221 or miR-222 increases the susceptibility of gastric carcinoma cells to irradiation by targeting PTEN (7). miR-26b has been observed to be down-regulated in many cancers and is commonly associated with cancer development and worst outcome after cancer therapy (8). However, to date, the role of miR-26b in the radiosensitivity of cancer cells, especially HCC, is barely understood.
Erythropoietin producing human hepatocelluar A2 (EphA2), acting as an important member of the Eph receptor family, is highly expressed in many cancers such as breast cancer, prostate cancer, lung cancer, malignant glioma, and gastric cancer (9). A previous study found that up-regulated EphA2 expression is associated with cancer lymphogenous metastasis and poor prognosis, suggesting that EphA2 contributes to cancer progression (9). Moreover, EphA2 provides a protective mechanism by which tumors can attenuate irradiation-induced antivascular effect, so that the tumors can easily migrate and escape radiation (10). A previous literature represents EphA2 as an important target of miR-26b in gliomas (11). We, therefore, speculated that down-regulated miR-26b expression in many cancers is an important reason for the up-regulation of EphA2 expression. If true, overexpressing miR-26b to block EphA2 expression is expected to be a feasible strategy to inhibit EphA2-mediated irradiation-protective effect and to sensitize cancer cells to irradiation.
In the present study, the expression of miR-26b gene and EphA2 protein in multiple HCC cell lines were evaluated by qPCR and western blotting, respectively, to gather basic understanding of the expression profiles of miR-26b and EphA2 in HCC cells and their potential interrelationship. Dual luciferase reporter assay was performed to validate EphA2 as an important target of miR-26b in HCC cells. Subsequently, HCC 97H cells were separately transfected with miR-26b mimics, EphA2 overexpression vector, and EphA2-ShRNA, and co-transfected with miR-26b mimics and EphA2 overexpression vector to investigate the changes in cell response to low-dose irradiation in terms of proli-feration rate, apoptosis, and invasion ability. This study aimed to determine whether the radiosensitivity of HCC cells can be enhanced by miR-26b by targeting EphA2, which is further expected to provide a theoretical foundation for modulation of the radiosensitivity of HCC cells during low-dose RT.
Materials and Methods
Cell culture
Human HCC cell lines, including HepG2, SMMC7721, Huh7, Bel-7402, GGY7703, 97L, and 97H, as well as the hepatic cell line L02 were purchased from the American Type Culture Collection (Manassas, VA, USA) and grown in Dulbecco’s Minimal Essential Medium (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, Utah, USA) and 1% penicillin/streptomycin at 37 °C in a humidified incubator containing a mixture of 95% air and 5% CO2.
Quantitative polymerase chain reaction (qPCR)
Total RNA from the cells was obtained using Trizol reagent (Invitrogen Life Technologies). Complementary DNA (cDNA) was synthesized using a First-Strand cDNA Synthesis kit (Fermentas, Vilnius, Lithuania) and using 1 µg of RNA template. qPCR was performed in a 7300 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using a SYBR® Green PCR kit (Applied Biosystems). The primers of miR-26b and the housekeeping gene (U6) were as follows: miR-26b forward primer 5’-GGGACCCAGTTCAAGTAATTCAGG-3’, reverse primer 5’-TTTGGCACTAGCACATT-3’; U6 forward primer 5’-CTCGCTTCGGCAGCACA-3’, reverse primer 5’-AACG-CTTCACGAATTTGCGT-3’. The relative expression level of miR-26b was calculated using comparative computerized tomography methods, with U6 as an internal control.
Western blot analysis
Cells were solubilized with the lysis buffer (1% Non-ionic detergent-40, 0.1% Sodium dodecyl sulfate, 50 mM Dithiothreitol, 2 μg/ml Aprotinin, 2 μg/ml Leupeptin, and 1 mM Phenylmethanesulfonyl fluoride) and the solution was boiled for 5 – 10 min, and an equal amount of protein from different groups was separated by SDS-PAGE. After electrophoresis, the proteins were transferred onto a nitrocellulose membrane. The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline and then incubated with primary antibody directed against EphA2 and β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 1 hr at room temperature. The blots were developed using peroxidase-conjugated anti-mouse IgG secondary antibody (Santa Cruz Biotechnology, Inc.), and the proteins were visualized by enhanced chemilu-minescence (Amersham Bio-sciences, NJ, USA). β-actin was used as a loading control.
Dual luciferase reporter assay
The predicted miR-26b-binding site sequence (miRNA response element, MRE) on the EphA2 3’UTR were inserted (subcloned) into pmirGLO (Promega, WI) to construct a miR-26b MRE luciferase reporter. This reporter was transfected into HCC 97H cells by using Lipofectamine 2000 (Invitrogen Life Technologies) in the presence or absence of miR-26b mimics that was used to up-regulate intracellular miR-26b level. The reporter vector without the miR-26b MRE on the EphA2 3’UTR (empty reporter vector) as well as the reporter vector inserted with the mutated miR-26b MRE on the EphA2 3’UTR (mutated reporter vector) were used as two negative control. The luciferase activity was measured at 48 hr after transfection by using GloMax 20/20n luminous detector (Promega, WI, USA).
Transfection
To knockout or overexpress EphA2, the cells were grown in complete medium for 24 hr and then transfected with EphA2-ShRNA (GenePharma, Co., Ltd, Shanghai, China) and EphA2 overexpression vector (pEGFP-C1; Invitrogen Life Technologies), respectively, by using Lipofectamine™ 2000 (Invitrogen Life Technologies), following the procedure recommended by the manufacturer. The miR-26b mimics were purchased from GenePharma, Co., Ltd., and transfected into 97H cells with artificially overexpressed EphA2 or non-silenced EphA2. Through modulating the time of the transfection, we made sure a similar transfection efficiency shared by the miR-26b mimics, EphA2-ShRNA, and EphA2 overexpression vector transfection.
Irradiation
The 97H cells were plated in 3.5-cm dishes and incubated in the culture medium until 70 – 80% confluency was attained. Cells were next cultured in the medium without FBS and exposed to irradiation at a dose of 1 Gy, a dose determined based on a preliminary experiment where the cells were subjected to 1, 2, 4, and 6 Gy. Seventy-two hours after irradiation, the rates of cell proliferation and apoptosis were measured by CCK-8 assay and Annexin V-FITC / Propidium Iodide (PI) double-staining test.
Cell proliferation assay
The cells were seeded at a density of 1 × 105 cells per well into 96-well plates containing 100 μl / well of the culture medium for 24 hr. One hour before the incubation period ended, 10 μl of CCK-8 reagent was added to each well. The optical density at 490 nm (OD490nm) of each well was determined by an enzyme immunoassay analyzer.
Apoptosis rate assay
Cells were dual-stained with Alexa Fluor 488-Annexin V and propidium iodide (PI) by using an Annexin V-fluorescein isothiocyanate/PI apoptosis kit (Kaiji Biological Inc., Nanjing, China), according to the manufacturer’s instructions. The rate of apoptosis was analyzed using a dual laser flow cytometer (Becton Dickinson, San Jose, CA, USA) and estimated using the ModFit LT software (Verity Software House, Topsham, ME, USA).
Cell invasion assay
Transwell chamber (Corning, New York, USA) was used to test cancer cell invasion, with 8 μm membrane filter inserts coated with Matrigel (BD Biosciences, California, USA). Briefly, the cells were trypsinized and suspended in serum-free medium. Next, 1 × 104/ml cells were added to the upper chamber, and the lower chamber was filled with the medium containing 10% FBS. After 36 hr of incubation, the cells that had invaded the lower chamber were fixed with 95% ethyl alcohol for 15–20 min and stained with hematoxylin for 10 min. Then, the cells were enumerated microscopically.
Statistical analysis
The results are presented as the mean±SD of three independent experiments. One-way analysis of variance (ANOVA) with post-hoc testing was used for multiple comparisons between each group (SPSS13.0 software, IBM, USA). Significant differences were established at P<0.05.
Results
Down-regulated miR-26b and up-regulated EphA2 expression in different HCC cell lines
The levels of miR-26b gene expression and EphA2 protein expression in different HCC cell lines are presented in Figures 1A and 1B, respectively. miR-26b gene expression showed different degrees of down-regulation in all tested HCC cell lines, compared to normal liver cells, but EphA2 protein expression level was up-regulated in the HCC cell lines. It is interesting that lower miR-26b gene expression in HCC cell lines corresponds to higher EphA2 protein expression. Most obviously, HCC 97H cells with the lowest level of miR-26b gene expression expressed highest level of EphA2 expression, implying a potential interrelationship between miR-26b and EphA2 in HCC cells. HCC 97H cells, considered the ideal cell line of our study, was used in the following trials.
Figure 1.
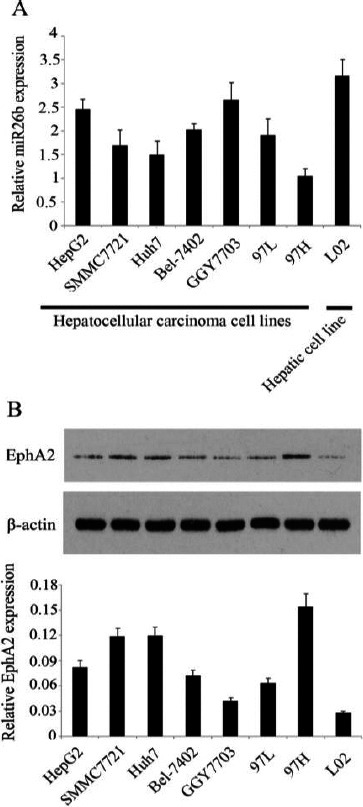
Down-regulated miR-26b and up-regulated erythropoietin producing human hepatocelluar A2 (EphA2) in different hepatocellular carcinoma (HCC) cell lines
Human HCC cell lines, including HepG2, SMMC7721, Huh7, Bel-7402, GGY7703, 97L, and 97H, and the hepatic cell line L02 were used to detect miR-26b gene and EphA2 protein expression by PCR and western blotting, respectively. (A) miR-26b gene shows reduced expression in all HCC cell lines, compared to the hepatic cell line. (B) EphA2 protein shows increased expression in all HCC cell lines compared to the normal hepatic cell line. Each bar represents the mean of three independent experiments
The predicted miR-26b-binding site sequence (miRNA response element, MRE) on the EphA2 3’UTR were inserted (subcloned) into pmirGLO (Promega, WI) to construct a miR-26b MRE luciferase reporter. This reporter was transfected into HCC 97H cells by using Lipofectamine 2000 (Invitrogen Life Technologies) in the presence or absence of miR-26b mimics that was used to up-regulate intracellular miR-26b level. The reporter vector without the miR-26b MRE on the EphA2 3’UTR (empty reporter vector) and the reporter vector inserted with the mutated miR-26b MRE on the EphA2 3’UTR (mutated reporter vector) were used as two negative control. *P< 0.05, **P<0.01
EphA2 as an important target of miR-26b in HCC 97H cells
To determine whether EphA2 is an important target of miR-26b in HCC cells, we performed dual luciferase reporter assay. The predicted miR-26b-binding site sequence on the EphA2 3’UTR cloned downstream to a luciferase reporter gene rendered almost 52% loss of luciferase activity (P<0.05, Figure 2), compared to both the empty reporter vector and mutated reporter vector. The up-regulation of intracellular miR-26b with miR-26b mimics trans-fection resulted in a greater loss of the luciferase activity that was 83% lower than those of two loading control (P<0.01).
Figure 2.
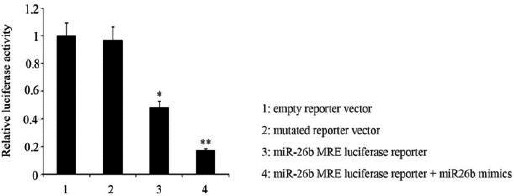
Erythropoietin producing human hepatocelluar A2 (EphA2) is an important target of miR-26b in HCC 97H cells
Optimum irradiation dose is 1 Gy
To obtain the optimum low dose of irradiation for use in our experiments, HCC 97H cells were exposed to different doses of irradiation from 1 to 6 Gy. Compared to the control, irradiation with 1 Gy caused minor damage to HCC 97H cells; only marginal alterations in the proliferation and apoptosis rate, as well as invasion ability were observed after irradiation (Figures 3A, 3B, and 3C), but with increasing dose of irradiation, the damage inflicted by irradiation evidently increased. Irradiation with 2 Gy significantly decreased the rate of cell proliferation and invasion and increased the rate of apoptosis (P<0.05). Irradiation with 1 Gy was determined as the optimum low dose of irradiation to generate cells for further studies because enhancing HCC 97H cell radiosensitivity is necessary in case of exposure to low-dose irradiation.
Figure 3.
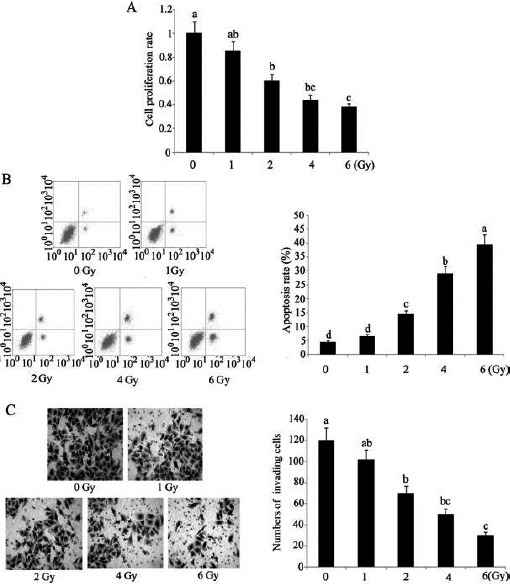
HCC 97H cells were subjected to different doses of irradiation
HCC 97H cells were subjected to different doses of irradiation before testing (A) cell proliferation rate, (B) apoptosis rate, and (C) cell invasion ability by using CCK-8 kits, Annexin V-fluorescein isothiocyanate/PI apoptosis kit and flow cytometer, and Transwell chambers. Each bar represents the mean of three independent experiments. Bars not sharing a common letter differ (P<0.05)
EphA2 expression level in HCC 97H cells following different treatments
We first evaluated the effects of non-targeting miRNA mimic, non-targeting siRNA, and empty overexpression vector transfection on EphA2 expression level in HCC 97H cells, and found no noticeable alteration in EphA2 expression level compared to the non-transfected cells (Figure 4A), thereby excluding the possibility that the intro-duction of non-targeting miRNA and siRNA and empty vector causes the change in EphA2 expre-ssion. Figure 4B shows the photomicrographs and corresponding fluorescent images of cells that were transfected with miR-26b mimics, EphA2-ShRNA or EphA2 overexpression vector. As can be seen from these pictures, the efficiency of these transfections was similar. Transfection with miR-26b mimics was used to up-regulate miR-26b level in HCC 97H cells. Both miR-26b mimics and EphA2-ShRNA transfec-tion resulted in significant reduction of EphA2 expression (P<0.05, Figure 4C) in compare-son with the non-transfected cells. Transfection with EphA2 overexpression vector up-regulated EphA2 expre-ssion (P<0.05). In contrast, co-transfection with miR-26b mimics and EphA2 overexpression vector barely altered EphA2 expression. We further investigated the EphA2 expression level in the non-transfected and transfected cells upon low-dose irradiation. As shown in Figure 4D, exposure to 1 Gy irradiation alone only caused slight reduction in EphA2 expression in HCC 97H cells. Transfection with miR-26b mimics and following exposure to low-dose irradiation significantly inhibited EphA2 expression (P<0.05). EphA2-ShRNA transfected cells upon low-dose irradiation showed the same inhibition rate of EphA2 expression (P<0.05). Up-regulated EphA2 expression was still observed in the cells transfected with EphA2 overexpression vector in despite of the exposure to low-dose irradiation. Co-transfection with miR-26b mimics and EphA2 overexpression vector before irradiation has no effect on EphA2 expression.
Figure 4.
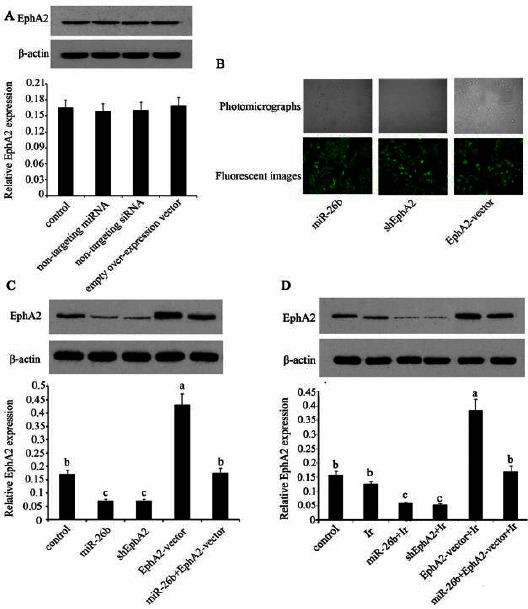
EphA2 expression level in HCC 97H cells following different treatments
(A) The erythropoietin producing human hepatocelluar A2 (EphA2) expression level in HCC 97H cells that were transfected with non-targeting miRNA mimic, non-targeting siRNA, or empty over-expression vector. (B) The photomicrographs and corres-ponding fluorescent images of cells that were transfected with miR-26b mimics, EphA2-ShRNA or EphA2 overexpression vector. These pictures were used for the identification of the transfection and to make sure similar transfection efficiency among these transfections. Both the miR-26b mimics and EphA2-ShRNA were attached with a FAM green fluorescent tag. EphA2 over-expression vector was inserted with the sequence of green fluorescent protein (GFP). (C) The EphA2 expression level in HCC 97H cells that were separately transfected with miR-26b mimics, EphA2 overexpression vector, and EphA2-small hairpin RNA, as well as cells that were co-transfected with miR-26b mimics and EphA2 overexpression vector. (D) HCC 97H cells were separately transfected with miR-26b mimics, EphA2 overexpression vector, and EphA2-small hairpin RNA, in addition to cells co-transfected with miR-26b mimics and EphA2 overexpression vector. EphA2 expression level was assessed after these cells were exposed to 1 Gy irradiation. Each bar represents the mean of three independent experiments. Bars not sharing a common letter differ (P<0.05). Ir: irradiation; miR-26b: miR-26b mimics; EphA2-vector: EphA2 over-expression vector; shEphA2: EphA2-small hairpin RNA
Changes in the radiosensitivity of HCC 97H cells after different treatments
The radiosensitivity of HCC 97H cells after different treatments was studied by evaluating the changes in cell proliferation and apoptosis rate, as well as invasion ability. Both miR-26b mimics and EphA2-ShRNA transfection caused ~ 45% decrease in the cell proliferation rate after treatment with low-dose irradiation, compared to the control (both P<0.05; Figure 5). Transfection with EphA2 overexpression vector enhanced the rate of cell proliferation, relative to the control (P<0.05). Co-transfection with miR-26b mimics and EphA2 overexpression vector had no significant effect on cell proliferation. The post-irradiation apoptosis rate showed a dramatic increase after transfection with miR-26b mimics and EphA2-ShRNA transfection (both P<0.01; Figure 6). Transfection with EphA2 overexpression vector lowered the rate of apoptosis to 35% of that of the control (P<0.05). Co-transfection with miR-26b mimics and EphA2 overexpression vector did not significantly alter post-irradiation apoptosis rate. Post-irradiation cell invasion ability was remarkably attenuated in HCC 97H cells that were transfected with miR-26b mimics or EphA2-ShRNA, as can be determined by the decrease in the number of cells passing through the Transwell (~ 40% compared to the control; P<0.05; Figure 7, and 30% compared to the cells exposed to low-dose irradiation only; P<0.05). Transfection with EphA2 overexpression vector conferred significant increase in the cell invasion ability after treatment with low-dose irradiation (P<0.05). No significant changes were observed in the invasion ability of HCC 97H cells that were co-transfected with miR-26b mimics and EphA2 overexpression vector.
Figure 5.
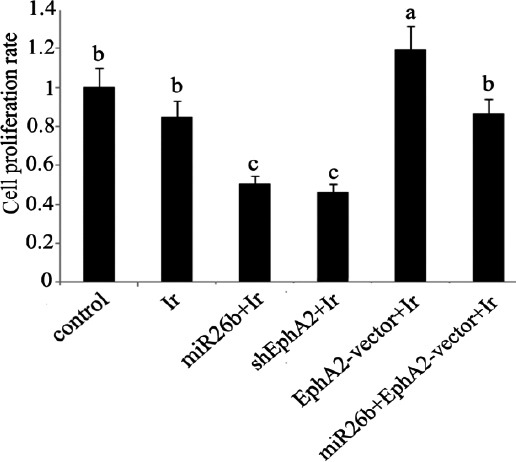
Cell proliferation rates of HCC 97H cells following different treatments.
Before exposure to irradiation at dose of 1 Gy, HCC 97H cells were separately transfected with miR-26b mimics, EphA2 overexpre-ssion vector and EphA2-small hairpin RNA in addition to co-transfection with miR-26b mimics and EphA2 overexpression vector. Cell proliferation rate was tested by using CCK-8 kits. Each bar represents the mean of three independent experiments. Bars not sharing a common letter differ (P<0.05). Ir: irradiation; miR-26b: miR-26b mimics; EphA2-vector: EphA2 expression vector; shEphA2: EphA2-small hairpin RNA
Figure 6.
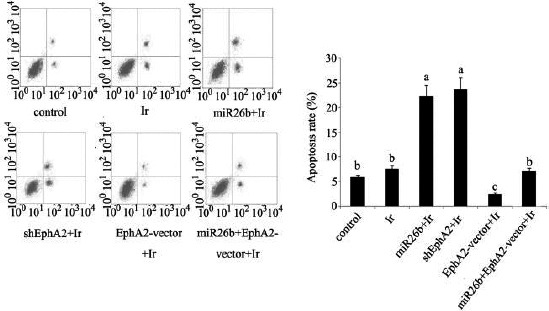
Apoptosis rates of HCC 97H cells following different treatments
Before exposure to irradiation at dose of 1 Gy, HCC 97H cells were separately transfected with miR-26b mimics, EphA2 over-expression vector and EphA2-small hairpin RNA and cells that co-transfected with miR-26b mimics and EphA2 overexpression vector. Apoptosis rate was tested by using Annexin V-fluorescein isothiocyanate / PI apoptosis kit and flow cytometer. The horizontal axes of the flow cytometer images represent Annexin V FITC, and the vertical axes represent PI. Each bar represents the mean of three independent experiments. Bars not sharing a common letter differ (P < 0.05). Ir: irradiation; miR-26b: miR-26b mimics; EphA2-vector: EphA2 over-expression vector; shEphA2: EphA2-small hairpin RNA
Figure 7.
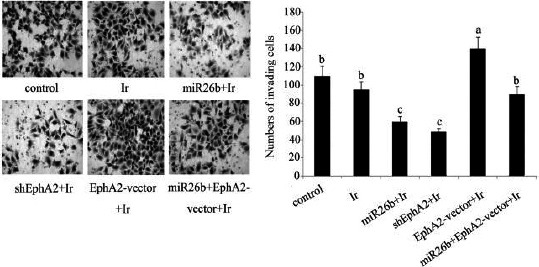
Cell invasion ability of HCC 97H cells following different treatments. Before exposure to irradiation at dose of 1 Gy, HCC 97H cells were separately transfected with miR-26b mimics, EphA2 overexpression vector and EphA2-small hairpin RNA and also were co-transfected with miR-26b mimics and EphA2 overexpression vector. Cell invasion ability was tested by using transwell chamber. Each bar represents the mean of three independent experiments. Bars not sharing a common letter differ (P<0.05). Ir: irradiation; miR-26b: miR-26b mimics; EphA2-vector: EphA2 over-expression vector; shEphA2: EphA2-small hairpin RNA
Discussion
In the present study, miR-26b showed different levels of down-regulation in multiple HCC cell lines compared to the normal hepatic cells. The level of down-regulated miR-26b is inversely correlated with the grade of HCC and its prognosis after therapy. Grade I and II liver tumors maintain relatively high levels of miR-26b expression, but grade III and IV tumors have markedly lower levels of miR-26b expression (8). In HCC, low miR-26b expression often indicated poor survival (12). Related research showed that down-regulated miR-26b contributes to epithelialmesen-chymal transition and chemo-resistance in HCC, which may be responsible for the relatively rapid malignant transformation and poor chemotherapeutic effect in HCC (8, 13). In our study, the effect of different degrees of miR-26b down-regulation on HCC radiosensitivity was not assessed, but artificially elevated miR-26b expression in HCC 97H cells with lowest miR-26b expression among tested HCC cells, and the change of radiosensitivity were evaluated. Up-regulated miR-26b effectively diminished post-irradiation cell proliferation rate and promoted irradiation-induced apoptosis, indicating that miR-26b enhances HCC radiosensitivity.
Furthermore, HCC 97H cells with up-regulated miR-26b expression significantly decreased cell invasion ability under treatment with low-dose irradiation. According to clinical experience, cancer cells migrating to and invading other organs and tissues not only make it difficult to undertake RT, but also increase the possibility of cancer cells bypassing irradiation attack (14). Thus, miR-26b up-regulation can, in fact, enhance the effect of RT by inhibiting the invasion of HCC 97H cells. The fact that miR-26b decreases E-cadherin expression and increases vimentin expression may explain miR-26b-induced attenuation of the invasion and migration abilities of HCC 97H cells (8).
As observed with miR-26b down-regulation in all tested HCC cell lines, EphA2 was up-regulated in these cell lines, implying a potential interrelationship between miR-26b and EphA2. Our dual luciferase reporter assay, along with previous findings, verified that EphA2 is the target of miR-26b (11). Further analysis using western blotting indicated that miR-26b exerted inhibitory effect on endogenous EphA2 protein level in HCC 97H cells. It has been well established that miRNAs form a class of small noncoding RNAs that can negatively regulate gene expression by interacting with the 3’-UTR of protein-coding genes, further leading to translational inhibition (8). With regard to miR-26b, there is evidence that miR-26b silences USP9X, TAK1, TAB3, TNKS1BP1, CPSF7, and COL12A1 proteins, which could be the underlying reason for the increase in chemosensitivity and the inhibition of cell proliferation and migration (8, 13, 15).
To determine whether EphA2 expression influences the enhancing effect of miR-26b on the radiosensitivity of HCC, EphA2 was down-regulated and overexpressed by transfecting with EphA2-ShRNA and EphA2 over-expression vector, respectively. Our results showed that the radiosensitivity of HCC is inversely correlated with EphA2 expression level, which indicates that EphA2 itself can influence radiosensitivity. Our data showed that transfection with miR-26b mimics and EphA2-ShRNA, both inhibit EphA2 expression cooperatively, resulting decrease in cell proliferation rate and cell invasion ability and increase in apoptosis rate after exposure of cells to low-dose irradiation. This suggests that miR-26b enhances the radiosensitivity of HCC 97H cells mainly by targeting EphA2, because if there are other targets that mediate the role of miR-26b in enhancing radiosensitivity, they would not be able to show similar levels of radiosensitivity at a similar rate of inhibition of EphA2 expression. Moreover, co-transfection with miR-26b mimics and EphA2 overexpression vector that maintains EphA2 expression constant, did not cause any significant difference in the radiosensitivity of HCC 97H cells, which further strengthened our point. A previous study showed that EphA2 induces pro-oncogenic effects including increased tumorigenesis, migration and invasion of tumor cells, angiogenesis, and metastasis by many molecular mechanisms (16), but only some of them are involved in the modulation of radiosensitivity. Our studies are dedicated toward investigating the precise mechanism underlying EphA2-mediated regulation of radiosensitivity.
Conclusion
In summary, EphA2 was identified as a target of miR-26b by the dual luciferase reporter assay and confirmed to be inversely correlated with the radiosensitivity of HCC cells. In addition, our finding showed that EphA2 overexpression and down-regulation decreased and increased the radiosensitivity of HCC 97H cells, respectively. Both transfection with miR-26b mimics and EphA2-ShRNA, can inhibit EphA2 expression, and exert similar effects on increasing radiosensitivity. Co-transfection with miR-26b mimics and EphA2 expression vector that maintains EphA2 expression constant failed to change the radio-sensitivity of HCC 97H cells. This study indicated that miR-26b enhances the radiosensitivity of HCC by targeting EphA2 protein. EphA2 and miR-26b are supposed to be new therapeutic targets to improve the therapeutic effect of HCC low-dose RT.
Acknowledgement
Sincerely thank Prof. Pei Guo Cao for his valuable suggestions and critical reading of the manuscript. This research is funded by all authors. The authors declare that they have no competing interests.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hung CH, Chiu YC, Chen CH, Hu TH. MicroRNAs in hepatocellular crcinoma:carcinogenesis, progression, and therapeutic target. Biomed Res Int. 2014;2014:486–407. doi: 10.1155/2014/486407. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Ursino S, Greco C, Cartei F, Colosimo C, Stefanelli A, Cacopardo B, et al. Radiotherapy and hepatocellular carcinoma:update and review of the literature. Eur Rev Med Pharmacol Sci. 2012;16:1599–1604. [PubMed] [Google Scholar]
- 3.Wang S, Bian C, Yang Z, Bo Y, Li J, Zeng L, et al. miR-145 inhibits breast cancer cell growth through RTKN. Int J Oncol. 2009;34:1461–1466. [PubMed] [Google Scholar]
- 4.Ostenfeld MS, Bramsen JB, Lamy P, Villadsen SB, Fristrup N, Sørensen KD, et al. miR-145 induces caspase-dependent and-independent cell death in urothelial cancer cell lines with targeting of an expression signature present in Ta bladder tumors. Oncogene. 2010;29:1073–1084. doi: 10.1038/onc.2009.395. [DOI] [PubMed] [Google Scholar]
- 5.Salim H, Akbar NS, Zong D, Vaculova AH, Lewensohn R, Moshfegh A, et al. miRNA-214 modulates radiotherapy response of non-small cell lung cancer cells through regulation of p38MAPK, apoptosis and senescence. Br J Cancer. 2012;107:1361–1373. doi: 10.1038/bjc.2012.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen G, Zhu W, Shi D, Lv L, Zhang C, Liu P, et al. MicroRNA-181a sensitizes human malignant glioma U87MG cells to radiation by targeting Bcl-2. Oncol Rep. 2010;23:997–1003. doi: 10.3892/or_00000725. [DOI] [PubMed] [Google Scholar]
- 7.Chun-Zhi Z, Lei H, An-Ling Z, Yan-Chao F, Xiao Y, Guang-Xiu W, et al. MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN.BMC Cancer. 2010;10:367. doi: 10.1186/1471-2407-10-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen G, Lin Y, Yang X, Zhang J, Xu Z, Jia H. MicroRNA-26b inhibits epithelial-mesenchymal transition in hepatocellular carcinoma by targeting USP9X. BMC Cancer. 2014;14:393. doi: 10.1186/1471-2407-14-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosch B, Pietzsch D, Pietzsch J. Irradiation affects cellular properties and Eph receptor expression in human melanoma cells. Cell Adh Migr. 2012;6:113–125. doi: 10.4161/cam.20655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fokas E, Kamlah F, Hänze J, Engenhart-Cabillic R, Rose F, An HX. EphA2 blockade enhances the anti-endothelial effect of radiation and inhibits irradiated tumor cell-induced migration of endothelial cells. Thorac Cancer. 2010;1:153–162. doi: 10.1111/j.1759-7714.2010.00029.x. [DOI] [PubMed] [Google Scholar]
- 11.Wu N, Zhao X, Liu M, Liu H, Yao W, Zhang Y, et al. Role of microRNA-26b in glioma development and its mediated regulation on EphA2. PLoS One. 2011;6:16264. doi: 10.1371/journal.pone.0016264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji J, Shi J, Budhu A, Yu Z, Forgues M, Roessler S, et al. MicroRNA Expression, Survival, and Response to Interferon in Liver Cancer. N Engl J Med. 2009;361:1437–1447. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verghese ET, Drury R, Green CA, Holliday DL, Lu X, Nash C, et al. MiR-26b is down-regulated in carcinoma-associated fibroblasts from ER-positive breast cancers leading to enhanced cell migration and invasion. J Pathol. 2013;231:388–399. doi: 10.1002/path.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu HG, Chen C, Yang H, Pan YF, Zhang XH. Cancer stem cell subsets and their relationships. J Transl Med. 2011;9:50. doi: 10.1186/1479-5876-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao N, Wang R, Zhou L, Zhu Y, Gong J, Zhuang SM. MicroRNA-26b suppresses the NF-κB signaling and enhances the chemosensitivity of hepatocellular carcinoma cells by targeting TAK1 and TAB3. Mol Cancer. 2014;13:35. doi: 10.1186/1476-4598-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Annamalai B, Liu XG, Gopal U, Isaacs JS. Hsp90 is an essential regulator of epha2 receptor stability and signaling:implications for cancer cell migration and metastasis. Mol Cancer Res. 2009;7:1021–1032. doi: 10.1158/1541-7786.MCR-08-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]


