Abstract
Objective(s):
Hyperthermia is one of the most common environmental stressors that affect multi-biological systems in the body including the central nervous system as well as the hematopoietic organs. The objective of the present study was to investigate the protective role of ethanolic extract of propolis (EEP) on some selective stress markers, hematological, biochemical, and histopathological changes in rats subjected to hyperthermia (40 °C/12 hr).
Materials and Methods:
The experimental groups (10 rats each) were classified as follows; Group A; control, (C), was kept at a controlled room temperature (25±5 °C). Group B; ethanolic extract of propolis, (EEP), was fed a basal diet supplemented with 3 g EEP/kg diet for 10 days. Group C; heat stress, (HS), was fed a basal diet for 10 days, and then exposed to high temperature (40±1 °C) for 12 hr. Group D; co-exposed, (EEP+HS) was fed a basal diet supplemented with 3 g EEP/kg diet for 10 days, and then subjected to high temperature (40±1 °C) for 12 hr. At the end of the experimental period, animals were decapitated; blood and tissue samples (brain and spleen) were collected for hematological, biochemical, and histopathological examination.
Results:
EEP at a dose of 3 g/kg diet has a potent protective effect against hematotoxicity and brain damage as well as oxidative stress induced by heat stress in rats.
Conclusion:
The present study indicates that pre-treatment with EEP protects from hematotoxicity and neurological damage induced by high environmental temperature.
Keywords: Caspase-3, GABA, Heat stress, Malondialdehyde, Neurotransmitters, Propolis
Introduction
Recently, many deaths due to hyperthermia were recorded, and all over the world heat-related illnesses in the human population produce serious medical problems (1). Heatstroke is defined as a form of excessive hyperthermia associated with a systemic inflammatory response that results in multiple organ dysfunctions in which central nervous system disorders such as delirium, convulsions, and coma are predominant (2). HS-induced deaths are increasing with global warming and with a worldwide increase in the frequency and intensity of heat waves (3). Nowadays, oxidative stress attracts the attention of many researchers. Heat stress is one of the environmental phenomena that lead to reactive oxygen species (ROS) activation (4). Animal studies suggested that exposure to high temperature (more than 42°C) leads to brain damage through increased free radical production, causing an elevation of lipid peroxidation and limitation of the antioxidant enzymes activity in the brain tissue (5). Erythrocytes are constantly used for oxidative stress evaluation, due to the presence of polyunsaturated fatty acids in their membranes which are the main target for free radical reaction and are very sensitive to lipid peroxidation that results in membrane fluid loss and cellular lysis inducing hemolysis (6, 7).
Propolis is a natural plant product derived from numerous plant resins collected by honey bees. It has been used as a folk medicine for centuries. Propolis has been recorded to exhibit many properties including anticancer (8), antimicrobial, antioxidant, anti-inflammatory (9), antibacterial, antifungal (10), antiviral (11), hepatoprotective (12), and nephropro-tective (13) properties. It has been used as a protective agent against lead-induced neurotoxicity in an animal model (14). Propolis has a protective role in chlorpyrifos-induced changes in the hematological parameters and the oxidative/antioxidative status of Cyprinus carpio carpio (15). Propolis can be effective in the prevention of cypermethrin (CYP)-induced toxicity in rainbow trout, especially hematopoiesis (16). EEP at the supplemented dose of 3 g/kg diet might be considered to prevent oxidative stress in the broilers exposed to heat stress (17). From all of the above, we aspire to focus on the harmful effects resulting from exposure to heat, which lead to hematopoietic disorders and brain damage as a result of oxidative stress.
Materials and Methods
Tested substances and chemicals
Ethanolic extract of propolis 70% (EEP) (Dosic Imp and Exp.Co. Ltd, China) was used in the present experiment. All reagents and chemicals were used of analytical grade purchased from Sigma-Aldrich Co St Louis, MO, USA.
Ethical circumstance
All animals were treated in accordance with the guide for the care and use of laboratory animals (National Institute of Health Publication) and were approved by the Research and Ethics Committee of Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt.
Heat stress (HS) exposure procedure
Heat stressed rats were placed in an insulated wooden box warmed by a thermostatically controlled infra-red electric lamp (E27, R125 HAOBANG, China) for 12 hr. The box temperature was kept at 40 °C (18). During this time, up to 40 °C rectal temperature was recorded by thermometer.
Animal grouping and experimental design
Forty adult male albino rats (3 months old, weighing 220±10 g) were purchased from the Laboratory Animal Breeding Unit, Faculty of Veterinary Medicine, Zagazig University. Rats were acclimatized for one week prior to the beginning of the experimental study. The animals were housed in an insulated wooden box in a temperature-controlled room (25±5°C) with relative humidity (50±10) and with 12 hr light/dark cycle. Rats were allowed a standard commercial chow diet.
This study was carried out on 40 adult male albino rats, divided into four main groups (n=10);
Group A (C) was kept at a controlled room temperature (25±5°C).
Group B (EEP) was fed a basal diet supplemented with 3 g EEP/kg diet for 10 days (17).
Group C (HS) was fed a basal diet for 10 days and then exposed to high temperature (40±1°C) for 12 hr.
Group D (EEP+HS) was fed a basal diet supplemented with 3 g EEP/kg diet for 10 days and then subjected to high temperature (40±1°C) for 12 hr.
Sampling
Animals were fasted overnight, decapitated, and sacrificed for obtaining the blood and tissue samples. Whole blood was used for hematological analysis, serum was collected for serum iron, total, direct, and indirect bilirubin meanwhile, the collected plasma was used for determination of malondialdehyde (MDA), noradrenaline (NA), adrenaline (A), corticosterone (Cs), and glucose levels while the sedimented erythrocytes were washed three times with saline 0.9 and lysed with distilled water (1:3, v/v) at 0°C for 30 min then extracted from the lysate and stored at -80°C until used for measuring the reduced glutathione (GSH) level. The brain was removed from the skull and rinsed with sterile physiological saline (0.9%, NaCl) and cut into two equal parts; the first part was homogenized in 5 ml of cold phosphate buffer saline (pH 7.4) by Universal Laboratory Aid Homogenizer (MPW-309, Mechanika Precyzyjna, Warsaw, Poland). Homogenates were centrifuged using BOECO centrifuge (Germany) at 3000 rpm for 15 min at 4°C. The supernatants were collected and frozen at −20°C until used for assaying of caspase-3, Bcl-2, dopamine, serotonin, gamma-aminobutyric acid (GABA), MDA, and GSH, meanwhile, the second part of the brain and additional tissue fragments from spleen were fixed with neutral buffered formalin 10% for histopathological examination.
Hematological evaluation
Total erythrocytes counts (TEC), hemoglobin (Hb), hematocrit (Hct), mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration (MCHC) were evaluated by using an automated hematology analyzer (Hospitex Hema Screen 18 analyzer, Italy) (19). Reticulocytes (retics) were counted on blood smear stained with new methylene blue stain (20). Retics number was expressed in percentage (number of reticulocytes per total number of RBC x 100%).
Estimation of hemolysis markers
Serum iron was analyzed colorimetrically using SGM kits according to the methods of Ruutu (21). Spleen iron was measured colorimetrically after acid digestion of tissues (22). The serum bilirubin levels (total and direct) were assayed by using a commercial kit obtained from Diamond Diagnostics, Egypt accor-ding to Jendrassik (23). Indirect bilirubin was calculated by subtracting the obtained direct bilirubin level from the total bilirubin level.
Estimation of stress biomarkers and neurotrans-mitters
Plasma noradrenaline (NA) and adrenaline (A) concentrations were measured by using commercial radioimmunoassay (RIA) kits (IBL, Germany) following the instructions of the manufacturer. Corticosterone level was determined by the method of Vazquez-Palacios (24) using ELISA kit (DRG instruments GmbH, Germany). Moreover, glucose level was estimated by glucose oxidase–peroxidase method of Trinder (25) using a colorimetric kit of Biodiagnostics, Egypt. Sero-tonin, dopamine, and GABA levels were measured according to the methods described by Hou; Ciarlone, and Seiler, respectively (26-28).
Parameters related to DNA damage and apoptosis assaying
Brain caspase-3 was measured according to Fernandes-Alnemri et al (29). The Bcl-2 protein level was determined by ELISA kits of Uscn Life Science Inc., the procedure was carried out through the enclosed pamphlet method.
Oxidative stress indices
Total proteins in brain homogenate were measured colorimetrically according to Lowry et al (30). Brain homogenate MDA and GSH were determined according to the methods described previously by Ohkawa et al and Beutler et al, respectively (31, 32). Meanwhile, in blood, malondialdehyde, and reduced glutathione levels were estimated using the methods of Ohkawa et al and Beutler, respectively (31, 33).
Histopathological investigation
Brain and spleen specimens were obtained, fixed in neutral buffered formalin 10% for 7 days. The formalin-fixed samples were continuously transferred to freshly prepared fixative. The preserved samples were briefly dehydrated in a graded series of ethanol, cleared in 3 changes of xylene and embedded in paraffin wax. Paraffin blocks were sectioned into 4–5 μm thick sections. Finally, the paraffin sections were subjected to hematoxylin and eosin stain (H&E) according to Bancroft and Gamble (34). Histological sections were viewed and representative photomicrographs were taken using Olympus BX41 research optical photomicroscope equipped with an Olympus DP25 digital camera, courtesy Cytology and Histology Department, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt. Histopathological scoring was microscopically done and scored blindly in a table.
Statistical analysis
Data were analyzed by means of one-way (ANOVA) using SPSS statistical software (ver. 16.00, USA). Data are expressed as the mean±SE, and the results were statistically significant at P<0.05 (35).
Results
Effect of heat stress and EEP co-exposure on erythrogram
Basic statistics for the erythrogram of different experimental groups were shown in Table 1. Hemolytic anemia could be easily observed in the heat stressed animals showing macrocytosis and hypochromasia with reticulocytosis while pretreatment with EEP improves the erythrogram picture.
Table 1.
Erythrogram variables in different experimental groups
| Treatments | ||||
|---|---|---|---|---|
| Parameters | C | EEP | HS | EEP + HS |
| TEC (106/mm3) | 6.22± 0.32a | 6.07± 0.54a | 4.09±0.32b | 5.13± 0.19ab |
| Hb (gm/dl) | 16.00± 0.23a | 15.11± 1.06a | 10.60± 0.30b | 12.60± 0.61b |
| Ht (%) | 39.60± 0.83a | 38.60± 1.80ab | 31.06± 0.40c | 35.00±1.73bc |
| MCV (fl) | 63.89± 2.22b | 64.07± 3.07b | 76.60± 4.96a | 68.09±1.31ab |
| MCHC (%) | 40.41± 0.32a | 39.06± 0.98a | 34.10± 0.61b | 36.01± 0.57b |
| Retics (%) | 2.56± 0.23c | 2.46± 0.24c | 5.63± 0.34a | 4.03± 0.14b |
C: Control; EEP: ethanolic extract of propolis; HS: heat stress; TEC: Total erythrocytes counts; Hb: Hemoglobin; Ht: Hematocrit; MCV: Mean corpuscular volume; MCHC: Mean corpuscular hemoglobin concentration
Data are expressed as the mean±SE. Means within the same row carrying different superscripts are significantly different (one-way ANOVA followed by Duncan’s multiple range test, P<0.05, n=5 /group)
HS and/or EEP exposure induced oxidative hemolytic changes
Concerning the erythrocytes oxidative stress and hemolysis features, Table 2 showed a significant (P< 0.05) increase in MDA level, serum and spleen iron as well as total and indirect bilirubin with a significant (P<0.05) decrease in erythrocyte GSH contents in animals exposed to high temperature (40 °C/12 hr) compared to the control rats. Meanwhile, all these parameters were improved in animals pre-treated with ethanolic extract of propolis at a dose of 3 g EEP/kg diet for 10 days prior to heat exposure but were not returned to the control level.
Table 2.
Effect of heat stress exposure and/or ethanolic extract of propolis on blood oxidative and hemolysis indices in rats
| Treatments | ||||
|---|---|---|---|---|
| Parameters | C | EEP | HS | EEP + HS |
| GSH (nmol/ml) | 1147.07±7.66a | 1155.50±6.64a | 1023.49±11.33b | 1103.87±6.61c |
| MDA (nmol/ml) | 3.27±0.17c | 3.13±0.14c | 12.31±0.36a | 6.49±0.39b |
| Serum Fe (µmol/l) | 15.82±0.15c | 15.99±0.35c | 36.06±0.38a | 23.82±0.81b |
| Spleen Fe (µg Fe/g) | 45.78±0.50c | 45.65±0.97c | 65.63±0.29a | 54.20±0.48b |
| T.bilirubin (mg%) | 1.61±0.11c | 1.64±0.18c | 4.49±0.22a | 2.89±0.08b |
| D.bilirubin (mg%) | 0.40±0.03a | 0.37±0.03a | 0.36±0.02a | 0.36±0.02a |
C: Control; EEP: ethanolic extract of propolis; HS: heat stress; GSH: Reduced glutathione; MDA: Malondialdehyde; Fe:Iron; T.bilirubin: Total bilirubin; D.bilirubin: Direct bilirubin.
Data are expressed as the mean±SE. Means within same row carrying different superscripts are significantly different (one-way ANOVA followed by Duncan’s mcultiple range test, P<0.05, n= 5 /group)
Stress biomarkers alterations following HS and/or EEP exposure
Regarding the biochemical analysis of selective stress biomarkers including, corticosterone, adrenaline, noradrenaline, and glucose showed a significant increase in animals acutely exposed to high temperature compared with control ones. Addition of EEP to diet prior to high-temperature exposure resulted in marked (P<0.05) reduction in the above-mentioned stress markers compared with the HS group but not return to the normal control values as shown in Table 3.
Table 3.
Effect of heat stress exposure and/or ethanolic extract of propolis on stress markers level in rats
| Treatments | ||||
|---|---|---|---|---|
| Parameters | C | EEP | HS | EEP+HS |
| Corticosteroe (µg/dl) | 64.88±1.28c | 63.04±1.16c | 117.23±3.29a | 92.59±1.36b |
| Adrenaline (pg/ml) | 425.59±2.20c | 428.87±0.70c | 833.1±25.51a | 600.7±5.34b |
| Noradrenalie (pg/ml) | 1141.85±17.66c | 1138.0±12.4c | 1667.6±23.7a | 1321.1±21.4b |
| Glucose | 103.22±2.24c | 104.15±1.70c | 272.76±2.27a | 179.2±1.56b |
| (mg/dl) | ||||
C: Control; EEP: ethanolic extract of propolis; HS: heat stress
Data are expressed as the mean±SE. Means within same row carrying different superscripts are significantly different (one-way ANOVA followed by Duncan’s multiple range test, P<0.05, n=5/group
Oxidative, apoptotic brain injury following HS and/or EEP exposure
As shown in Table 4, a significant increase in brain dopamine, serotonin, and caspase-3 contents was found in addition to a marked decrease in brain GABA; BCL-2 also was noticed in heat stressed rats compared to the control group. On the other hand, pre-treatment with propolis ameliorates the level of these parameters near to the control group compared with the HS group.
Table 4.
Effect of heat stress and/or EEP on neurotransmitters, apoptotic, and oxidative stress indices in rats
| Treatments | ||||
|---|---|---|---|---|
| Parameters | C | EEP | HS | EEP+HS |
| Neurotransmitters | ||||
| Dopamine (µg/g tissue) | 17.26± 0.33c | 17.21±0 .78c | 29.26 ±1.11a | 21.81 ±1.38b |
| Serotonin (µg/g tissue) | 6.36±0.33c | 6.19± 0.40c | 16.36 ±0.33a | 11.64± 0.69b |
| GABA (µg/g tissue) | 99.83± 0.54a | 100.27± .65a | 71.02 ±2.07c | 92.77 ± 1.13b |
| Apoptosis markers | ||||
| Caspase-3 (ng/100mg) | 119.14 ±0.60c | 118.60±0.74c | 164.74±7.20a | 135.81±2.90b |
| BCL-2 (ng/mgtissue) | 74.82± 5.46a | 72.06± 2.04a | 42.98± 1.12c | 60.04± 3.33b |
| Oxidative stress markers | ||||
| GSH (nmol/mg protein) | 30.35±0.25a | 30.85±0.12a | 15.96±0.21c | 29.12±0.13b |
| MDA(nmol/mg protein) | 0.71±0.02c | 0.69±0.04c | 1.38±0.01a | 0.94±0.02b |
Data are expressed as the mean±SE. Means within same row carrying different superscripts are significantly different (one-way
ANOVA followed by Duncan’s multiple range test, P<0.05, n=5/group); C: Control; EEP: ethanolic extract of propolis; HS: heat stress
Also, a significant (P< 0.05) increase in MDA level with a decrease in GSH content was found in brain homogenate of animals exposed to high temperature compared to the control rats. Observable reduction in MDA level and elevation in GSH content close to the control level were noticed in animals supplemented with ethanolic extract of propolis at a dose of 3 g EEP/kg diet for 10 days prior to acute heat exposure compared to the heat exposed animals.
Histopathological findings following HS and/or EEP exposure
The severity of alterations in the brain and spleen specimens of different experimental groups was blindly scored microscopically, and the scores are presented in Table 5.
Table 5.
Scoring of the histopathological alterations in brain and spleen sections of rats of different treated groups
| Lesions | Treatments | |||
|---|---|---|---|---|
| C | EEP | HS | EEP +HS | |
| Brain tissue | ||||
| Meningeal cellular proliferation | -- | -- | +++ | + |
| Neuronal degeneration | -- | -- | ++ | + |
| Focal gliosis | -- | -- | ++ | -- |
| Congested blood vessels | -- | -- | ++ | -- |
| Submeningeal hemorrhage | -- | -- | + | -- |
| Spleen tissue | ||||
| Activated lymphoid follicles | -- | + | -- | -- |
| Congested blood vessels | -- | -- | ++ | + |
| Lymphocytic depletion | -- | -- | ++ | -- |
| Hemosiderosis | -- | -- | +++ | + |
C: Control; EEP: ethanolic extract of propolis; HS: heat stress
+++ : Severe histopathological alteration
++ : Moderate histopathological alteration
+ : Mild histopathological alteration
-- : Nil histopathological alteration
Microscopical examination of H&E stained brain sections of control animals showing normal cerebral architecture with different sized intact basophilic neurons and randomly oriented axonal fibers, normally distributed neuroglia cells and intact meningeal cell layers (Figure 1 a). High magnification of the previous figure focused on the intact basophilic neurons (Figure 1 b). The propolis-treated group showed normal histological cerebral architecture (Figure 1 c). Meanwhile, the brain of heat stressed animals’ revealed meningeal cellular proliferation with congested blood vessels and mild submeningeal hemorrhage (Figure 1 d).
Figure 1.
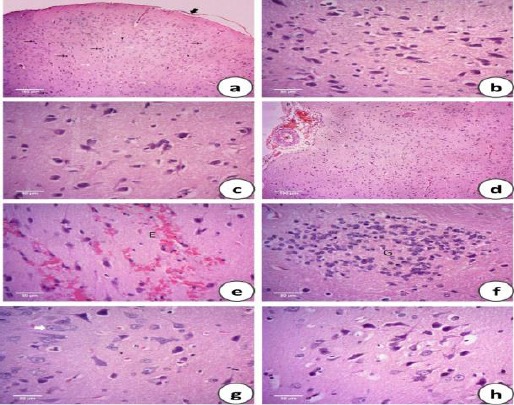
Photomicrographs of the H&E stained brain sections of the control group (C) showing normal cerebral architecture with different sized intact basophilic neurons (black arrows) and randomly oriented axonal fibers (white arrows), normally distributed neuroglia cells (head arrows) and intact meningeal cell layers (thick arrow) (a). High magnification of the previous figure focused on the intact basophilic neurons (b). Propolis administered group (EEP) showing normal histological cerebral architecture (c). Heat stressed group (HS) showing meningeal cellular proliferation (P) with congested blood vessels (C) and mild submeningeal hemorrhage (H) (d), additional extravasated blood cells (E) were also observed between the neuronal tissues (e) with evidence of focal gliosis (G) detected by occasional aggregation of small rounded deeply basophilic neuroglia cells (f). Neuronal degeneration was observed and characterized by swollen rounded vacuolated neurons with pyknotic nuclei (white arrow) (g). The heat stressed group treated with propolis (EEP + HS) showing normal intact meninges and absence of both hemorrhage and gliosis with few to moderate numbers of neurons showing mild degenerative changes with occasional swelling and vacuolation (h)
Extravasated blood cells were also observed between the neuronal tissues (Figure 1 e). Evidence of focal gliosis was detected by occasional aggregation of small rounded deeply basophilic neuroglia cells (Figure 1 f). Neuronal degeneration was observed and characterized by swollen rounded vacuolated neurons with pyknotic nuclei (Figure 1 g). Regarding heat stressed groups treated with propolis, the neuronal tissues showed normal intact meninges and absence of both hemorrhage and gliosis.
Few to moderate numbers of neurons showed mild degenerative changes with occasional swelling and vacuolation (Figure 1 h).
Spleen of the control group showed a normal splenic structure with normal white and red pulp integrity and absence of hemosiderosis (Figure 2 a). The propolis administered group (EEP) showing normal histological splenic architecture as control one with active lymphoid follicles (Figure 2 b). Heat stressed group showing congested blood vessels and sinusoids among the red pulp (Figure 2 c) along with hemosiderosis was observed as yellowish brown pigment inside the phagocytic cells (Figure 2 d). Moderate to marked lymphocytic depletion was represented by smaller lymphatic nodules with occasional degeneration and necrosis of lymphocytes especially at the rim of lymphoid follicles (Figure 2 e). The co-exposed group showing significant improvement of the splenic architecture with mildly congested blood vessels and slight yellowish-brown pigmentation (Figure 2 f).
Figure 2.
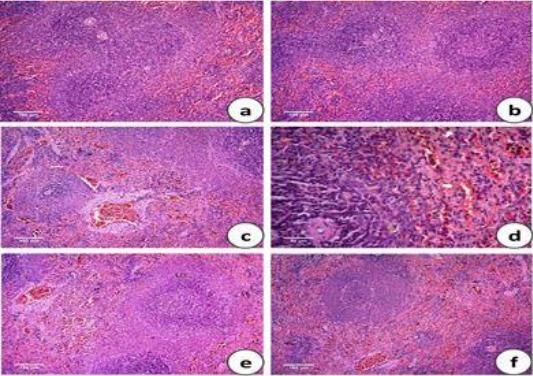
Photomicrographs of the H&E stained spleen of the control group (C) showing a normal splenic structure with normal white (W) and red pulp (R) integrity and absence of hemosiderosis (a). Propolis administered group (EEP) showing normal histological splenic architecture as control one with active lymphoid (L) follicles (b). Heat stressed group (HS) showing congested (C) blood vessels and sinusoids among the red pulp (c), along with hemosiderosis was observed as yellowish brown pigment inside the phagocytic cells (white arrows) (d). Moderate to marked lymphocytic depletion (LD) was represented by smaller lymphatic nodules with occasional degeneration and necrosis of lymphocytes especially at the rim of lymphoid follicles (e). The co-exposed group showing significant improvement of the splenic architecture with mildly congested blood vessels and slight yellowish-brown pigmentation (f)
Discussion
Studies on the heat stress induced alterations are still increasing, as temperature is one of the most encountered stressful factors in different biological systems. Heat stress (HS) is considered a model of thermal injury to the central nervous system (CNS) (36).
Hyperthermia as a result of high summer heat exposure was found to be one of the most common environmental stressors that induce excess ROS production (37), causing oxidative damage to many vital organs, especially the brain, leading to neurodegenerative disorder (38) and consequently decline in the animal’s performance (39).
Upon exposure to higher temperatures in the present work, an increase in oxidative damage markers such as MDA combined with a reduction in GSH in brain homogenate resulted in brain damage was recorded. The presented data in this study agreed with researchers (40) who found that heat acclimation (HA) on acute exhaustive exercise-rats model induced oxidative stress (OxS) and inflammatory reactions. These results were confirmed by the histopathological findings which revealed meningeal cellular proliferation with congested blood vessels, mild submeningeal hemorrhage, extravasated blood cells between the neuronal tissues, focal gliosis and neuronal degeneration in the heat stressed group. These findings were previously obtained by Boulant (41) who reported that rats with hyperpyrexia showed local hemorrhages and parenchymatous degeneration of brain cells. Research (42) found that brains of heat exposed rats (40°C /12 hr) show hyperemic capillaries and blood vessels and focal hemorrhage in cerebral tissue.
Heat stroke influences excitatory and inhibitory amino acid neurotransmitters in the central nervous system. Our results showed a significant increase in adrenaline, noradrenaline, corticosterone, glucose, dopamine, and serotonin as stress markers. Increased levels of these neurotransmitters could be attributed to their great role in temperature regulation. Noradrenaline and adrenaline are chemical transmitters at most sympathetic postganglionic endings, stored in the synaptic knobs of adrenergic neurons. Corticosterone is the terminal product of the HPA axis and one of the adrenal cortical synthetic glucocorticoids (43). Dopamine is the noradrenaline precursor found in the dopaminergic neurons of brain hypothalamus. Serotonin is a synaptic mediator formed from tryptophan hydroxylation and decarboxylation. Our results appeared to be parallel with literature (42, 44, 45). GABA is a non-protein amino acid acting as a signaling molecule that helps in the regulation of many stress responses through controlling the carbon/nitrogen balance, osmotic potential, free radical scavenging, and pH regulation (46, 47). In the present study, the level of GABA significantly decreased under heat stress, this was observed by (1) who found that experimental rats exposed to high temperature (38 °C/4 hr) showed a marked decrease in GABA.
Heat-stress induced apoptosis and necrosis as shown in our work by increasing brain caspase-3 and decreasing Bcl-2 content. Caspase-3 is an essential apoptosis marker; its stimulation is a feature of cellular apoptosis (48), and Bcl2 is one of the antiapoptotic proteins (49). The changes in both can be attributed to oxidative damage induced by heat stress in brain cells leading to its death. Our results agreed with the previous results, which found an elevation of caspase-3 and declining of Bcl-2 in cardiomyocyte (50) and liver cells (51) of heat-stressed animals.
Regarding hematological alterations resulting from exposure to high ambient temperature; we found that heat exposed rats showed hemolytic anemia, which was confirmed by reticulocytosis, increased serum and spleen iron with hyperbilirubinemia and splenic hemosiderosis. These results could be linked to the increased generation of highly free radicals in mitochondria or due to the elevation of erythrocyte osmotic fragility in hot-dry seasons (52). Our results agreed with researchers (53) who reported that heat-stress induced hemolysis in rats during hot-dry seasons with decreased erythrocyte count, hemoglobin concentration, and packed cell volume.
Propolis, a natural therapeutic agent, shows antioxidant activities (54). Propolis extract contains many flavonoids and phenolic compounds which are powerful antioxidants, free radical scavengers, and lipid peroxidation inhibitors. Several studies suggested that propolis and its components protect the brain from ischemia-reperfusion damage through their antioxidant (55) and anti-inflammatory activities (56). In the present work, we found that EEP protects the brain and erythrocyte from oxidative damage induced by heat stress and this was confirmed by normal intact meninges of neuronal tissues with absence of both hemorrhage and gliosis and presence of few to moderate numbers of degenerative neurons.
Conclusion
From the above-mentioned details, we found that ethanolic extract of propolis protects from oxidative hematological alterations and neurological damage induced by high environmental temperature.
Acknowledgment
The authors wish to thank Dr Mohamed Afifi for his sincere advice in the statistical analysis of data.
References
- 1.Sharma HS. Hyperthermia induced brain edema:Current status & future perspectives. Indian J Med Res. 2006;123:629–652. [PubMed] [Google Scholar]
- 2.Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 3.Patz JA, Campbell-Lendrum D, Holloway T, Foley JA. Impact of regional climate change on human health. Nature. 2005;438:310–317. doi: 10.1038/nature04188. [DOI] [PubMed] [Google Scholar]
- 4.Imen BS, Taha N, Abdeljelil G, Hajer D, Moncef BM, Manef A. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage;A review. Int J Hyperthermia. 2014;30:513–523. doi: 10.3109/02656736.2014.971446. [DOI] [PubMed] [Google Scholar]
- 5.Chang CK, Chang CP, Liu SY, Lin MT. Oxidative stress and ischemic injuries in heat stroke. Prog Brain Res. 2007;162:525–546. doi: 10.1016/S0079-6123(06)62025-6. [DOI] [PubMed] [Google Scholar]
- 6.Brzezinska-Slebodzinska E. Species differences in the susceptibility of erythrocytes exposed to free radicals in vivo. Vet Res Commun. 2003;27:211–217. doi: 10.1023/a:1023344607691. [DOI] [PubMed] [Google Scholar]
- 7.Adenkola AY, Ayo JO. Effect of eight hours road transportation stress on erythrocyte osmotic fragility of pigs administered ascorbic acid during the harmattan season. J Cell Anim Biol. 2009b;3:004–008. [Google Scholar]
- 8.Amini-Sarteshnizi N, Mobini-Dehkordi M, Khosravi-Farsani S, Teimori H. Anticancer activity of ethanolic extract of propolis on AGS cell line. J HerbMed Pharmacol. 2015;4:29–34. [Google Scholar]
- 9.Jaqueline FC, Uilson PS, Paola SR, Marcio JD, José BPB, Claudia ALC, et al. Antimicrobial, antioxidant, anti-Inflammatory, and cytotoxic activities of propolis from the stingless Bee Tetragonisca fiebrigi (Jataí) Evid Based Complement Alternat Med. 2015:1–11. doi: 10.1155/2015/296186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Séverine B, Anne-Marie LR, Anne L, Marie K, Viviane C, Catherine F, et al. Antifungal and antibacterial metabolites from a french poplar type propolis. Evid Based Complement Alternat Med. 2015:1–10. doi: 10.1155/2015/319240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilherme RC, Ronaldo ZM, Karina SV, Cristina AF, Juliana CB, Noemi T, et al. Antiviral action of hydromethanolic extract of geopropolis from Scaptotrigona postica against antiherpes simplex virus (HSV-1) Evid Based Complement Alternat Med. 2015:1–10. doi: 10.1155/2015/296086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abeer ME, Amal AS, Faiza AM. The potential protective effect of propolis on experimentally induced hepatitis in adult male albino rats. Histological and immunohistochemical study. J Histol Histopathol. 2015;2:1–9. [Google Scholar]
- 13.Murat B, Sibel S, Mehtap Ö, Osman G, Nuri E, Mehmet B. In vivo nephroprotective efficacy of propolis against contrast-induced nephropathy. Diagn Interv Radiol. 2015;21:317–321. doi: 10.5152/dir.2015.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thanaa AE, Ashraf ME, Nagla AE. Possible protective effect of propolis against lead induced neurotoxicity in animal model. J Evol Biol Res. 2011;3:4–11. [Google Scholar]
- 15.Enis YM, Yonar SM, Ural Mş, Silici S, Düşükcan M. Protective role of propolis in chlorpyrifos-induced changes in the hematological parameters and the oxidative/antioxidative status of Cyprinus carpio carpio. Food Chem Toxicol. 2012;50:2703–2708. doi: 10.1016/j.fct.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Orun I, Selamoglu Z, Gulhan MF, Erdogan K. Role of propolis on biochemical and hematological parameters of Oncorhynchus mykiss exposed to cypermethrin. J Survey Fisheries Sci. 2014;1:21–35. [Google Scholar]
- 17.TatlıSeven P, Seval Y, Ismail S, Ibrahim HC, Mehmet AA, Mehmet Y. Effects of propolis on selected blood indicators and antioxidant enzyme activities in broilers under heat stress. Acta Vet Brno. 2009;78:75–83. [Google Scholar]
- 18.Joseph IM, Suthanthirarajan N, Namasivayam A. Effect of acute heat stress on certain immunological parameters in albino rat. Indian J Physiol Phannacol. 1991;35:269–271. [PubMed] [Google Scholar]
- 19.Feldman BR, Zinkl JG, Jain NC. Shalm’s veterinary hematology. 5th ed. Baltimore, Maryland: Lippincott, Williams & Wilkins; 2000. p. l344. [Google Scholar]
- 20.NCCLS. Methods for reticulocyte counting (Automated blood cell counters, flow cytometry, and supravital dyes);Approved guideline. 2nd ed. Wayne, Pennsylvania, USA: NCCLS; 2004. [Google Scholar]
- 21.Ruutu R. Determination of iron and unsaturated iron-binding capacity in serum with ferrozine. Clin Chem Acta. 1975;61:229–232. doi: 10.1016/0009-8981(75)90319-8. [DOI] [PubMed] [Google Scholar]
- 22.Torrance JD, Bothwell TH. Tissue iron stores. In: Cook J D, editor. Methods in hematology:Iron. New York NY: Churchill Livingstone; 1980. pp. 90–115. [Google Scholar]
- 23.Jendrassik G. Determination of serum bilirubin. Biochem. 1938;297:81. [Google Scholar]
- 24.Vazquez-Palacios GP. Biochem Behavior. 2001;70:305–310. doi: 10.1016/s0091-3057(01)00620-7. [DOI] [PubMed] [Google Scholar]
- 25.Trinder P. Enzymatic method for glucose determination. Ann Clin Biochem. 1969;6:24–28. [Google Scholar]
- 26.Hou C, Jia F, Liu Y, Li L. CSF serotonin, 5-hydroxyindolacetic acid and neuropeptide Y levels in severe major depressive disorder. Brain Res. 2006;1095:154–158. doi: 10.1016/j.brainres.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 27.Ciarlone AE. Determination of catecholamines spectrophotofluro-metrically. Am J Physiol. 1978;125:731–737. [Google Scholar]
- 28.Seiler N. Glick D, editor. In methods of biochemical analysis. 1970;18:259–337. doi: 10.1002/9780470110362.ch5. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994;269:30761–30764. [PubMed] [Google Scholar]
- 30.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol. 1951;193:265–275. [PubMed] [Google Scholar]
- 31.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 32.Beutler E, Duron O, Kelly MB. Improved method for determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 33.Beutler E. Reduced glutathione (GSH) In: Beutler E, editor. Red cell metabolism. New York: A manual of biochemical methods. Grune and Straton; 1975. [Google Scholar]
- 34.Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th ed. London: Churchill Livingstone; 2008. [Google Scholar]
- 35.SPSS. Statistical package for social science, computer software, Ver 16. London, UK: SPSS Company; 2008. [Google Scholar]
- 36.Ahmed RG. Heat stress induced histopathology and pathophysiology of the central nervous system. Int J Dev Neurosci. 2005;23:549–557. doi: 10.1016/j.ijdevneu.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Larkindale J, Knight MR. Protection against heat stress-induced oxidative damage in arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002;128:682–695. doi: 10.1104/pp.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein JA, Ackerman SL. Oxidative stress, cell cycle, and neurodegeneration. J Clin Invest. 2003;111:785–793. doi: 10.1172/JCI18182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mujahid A, Akiba Y, Toyomizu M. Acute heat stress induces oxidative stress and decreases adaptation in young white leghorn cockerels by downregulation of avian uncoupling protein. Poult Sci. 2007;86:364–371. doi: 10.1093/ps/86.2.364. [DOI] [PubMed] [Google Scholar]
- 40.Triin K, Jaak K, Vahur Ö, Mihkel Z, Kersti Z, Jaan E, et al. Effects of heat acclimation on changes in oxidative stress and inflammation caused by endurance capacity test in the heat. Oxid Med Cell Longev. 2014;2014:1–8. doi: 10.1155/2014/107137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boulant J. Mechanisms sensitivity to temperature. Ann N Y Acad Sci. 2004;856:108. doi: 10.1111/j.1749-6632.1998.tb08319.x. [DOI] [PubMed] [Google Scholar]
- 42.Faisal AB. Hazardous effects of hyperthermia on brain and testicular responses in rats. Life Sci J. 2012;9:992–996. [Google Scholar]
- 43.Beleen C, Martinez Fuentes AJ, Gracia Navarro F. Role of SST, COR and ghrelin and its receptors at the endocrine pancreas. Front Endocrinol. 2012;3:114. doi: 10.3389/fendo.2012.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y, Xing C, Wang L. Effects of heat stress on DA mediated PI signal transduction system in rat straitum. Space Med Eng. 2001;14:116–119. [PubMed] [Google Scholar]
- 45.Blatteis C, Li S, Li Z. Signaling the brain in systemic inflammation. The role of complement. Front Biosci. 2004;9:915. doi: 10.2741/1297. [DOI] [PubMed] [Google Scholar]
- 46.Kinnersley AM, Turano FJ. Gamma aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci. 2000;19:479–509. [Google Scholar]
- 47.Bouche N, Fromm H. GABA in plants. Just a metabolite? Trends Plant Sci. 2004;9:110–115. doi: 10.1016/j.tplants.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Fesik SW, Shi Y. Structural biology;Controlling the caspases. Science. 2001;294:1477–1478. doi: 10.1126/science.1062236. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Zheng Q, Wu H. The effects on expression ratio of Bax/Bcl-2 and the expression of activated caspase 3 in different types of tumor cells. Tumor. 2013;33:138–145. [Google Scholar]
- 50.Qian L, Xueli S, Huirong R, Jingbo G, Suqi C. Mitochondrial mechanism of heat stress-induced injury in rat cardiomyocyte. Cell Stress Chaperones. 2004;9:281–293. doi: 10.1379/CSC-20R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoue H, Sameshima N, Ishida T, Tsuji A, Kudo K, Ikeda N. Vulnerability of experimentally induced fatty liver to heat stress in rats. J Gastroenterol. 2006;41:55–61. doi: 10.1007/s00535-005-1722-9. [DOI] [PubMed] [Google Scholar]
- 52.Oladele SB, Ayo JO, Ogundipe SO, Esievo KAN. Seasonal and species variations in erythrocytes osmotic fragility of indigenous poultry species in Zaria, Northern Guinea Savannah zone of Nigeria. Bull Anim Health Product Afr. 2003;51:204–214. [Google Scholar]
- 53.Alhassan AW, Mohamed AM, Joseph OA, Ambali FS, Shittu M, Adenkola AY, Salawu EO. Effects of co-administration of antioxidants on erythrocyte osmotic fragility of Wistar rats during the hot-dry season. Eur J Sci Res. 2010;46:073–079. [Google Scholar]
- 54.Talas ZS, Dundar SP, Gulhan MF, Orun I, Kakoolaki S. Effects of propolis on some blood parameters and enzymes in carp exposed to arsenic. Iran J Fish Sci. 2012;11:405–414. [Google Scholar]
- 55.Shapour K, Zeliha ST, Oguz C, Osman C, Ilknur O. Role of propolis on oxidative stress in fish brain. Basic Clin Neurosci. 2013;4:153–158. [PMC free article] [PubMed] [Google Scholar]
- 56.Kasai M, Fukumitsu H, Soumiya H, Furukawa S. Ethanol extract of chinese propolis facilitates functional recovery of locomotor activity after spinal cord injury. Evid Based Complement Alternat Med. 2011;2011 doi: 10.1155/2011/749627. [DOI] [PMC free article] [PubMed] [Google Scholar]


