Abstract
Objective(s):
Glioblastoma multiforme (GBM) is one of the most lethal forms of human cancer and temozolomide (TMZ) is currently part of the standard treatment for this disease. Combination therapy using natural substances can enhance the anti-cancer activity of TMZ. The purpose of this study was to evaluate the effect of TMZ in combination with thymoquinone (TQ) on human GBM cell line (U87MG).
Materials and Methods:
The cell line was treated with TMZ and/or TQ. Cell viability was assessed using trypan blue and MTT assay. The effect of TMZ and/or TQ on colony-forming ability of the cells was investigated. Apoptosis and autophagy were quantified by fluorescent dye staining. The expression level of two autophagy related genes (ATG) were assessed using RT-PCR. Furthermore, nitric oxide (NO) production was detected by Griess reaction.
Results:
After treatment with TMZ and/or TQ, the cell viability was reduced in a time- and dose-dependent manner, and the cell survival fraction (SF) was significantly decreased (P=0.000). Apoptosis index of U87MG cells was also significantly increased (P=0.000). Autophagy was significantly increased by TMZ (P=0.000) and decreased by TQ (P=0.018). Also TMZ and/or TQ significantly decreased NO production by U87MG cell (P=0.000).
Conclusion:
TQ enhanced the anti-cancer activity of TMZ by inhibition of autophagy at the transcriptional level and decreased the colony-forming ability and NO production of U87MG cell line.
Keywords: Apoptosis, Autophagy, Glioblastoma multiforme, Temozolomide, Thymoquinone
Introduction
The incidence of primary brain tumors has increased over the past 20 years, and this trend is expected to continue. Glioblastoma multiforme (GBM), classified as a grade IV astrocytoma by world health organization (WHO), is the most common malignant primary brain tumor and one of the most aggressive forms of cancer. It accounts for more than 50% of all diagnosed brain tumors (1, 2). Current treatment strategies of GBM are surgery in addition to radiation therapy and alkylating agent-based chemotherapy (3).
Temozolomide (TMZ) (8-carbamoyl-3- methylimi-dazo [5, 1-d] - 1, 2, 3, 5-tetrazin-4 (3H)-one), an alkylating chemotherapeutic prodrug, belonging to imidazotetrazine series (4), is currently part of GBM treatment (5). It is one of a few drugs that is able to cross the blood-brain barrier (6). TMZ is administered orally and undergoes rapid chemical conversion to its active form, MTIC (5-(3- methyltriazen-1-yl) imidazole-4- carboxamide), in a systemic circulation (7). This active form of drug causes DNA damage during cell replication, thereby destroying the proliferating tumor cells (8).
Natural products have been used as useful sources of anti-tumor or cancer prevention agents. Thymoquinone (TQ) (C10H12O2; molecular weight: 164.2), a naturally occurring quinone (2-isopropyl-5-methylbenzo-1, 4-quinone), is the main bioactive component (30%-48%) of the volatile oil of black seed (Nigella sativa, Ranunculaceae family) and has been shown to possess anti-oxidant, anti-inflammatory and anti-neoplastic effects (9).
Previous studies have shown that thymoquinone inhibits the proliferation of different types of cancer cells including breast, colon, ovary, larynx, and lung cancer, as well as myeloblastic leukemia, and osteosarcoma (10).
TQ has been reported to be a potent cytotoxic agent against several multidrug-resistant human tumor cell lines (11).
GBM is one of the most malignant tumors, and despite research efforts, it is still associated with a poor prognosis and a rare long-term survival of the patients (12). Resistance to TMZ is the major therapeutic obstacle to an effective therapy (13), thereby the development of new therapeutic strategies is required to enhance the anti-cancer effect of TMZ in GBM therapy.
Autophagy is an important homeostatic cellular recycling mechanism that has an important role in response to therapeutic stresses. Recent studies have shown that activation of autophagy in response to chemotherapeutic agents acts as a prosurvival mechanism and contributes to anti-cancer drug resistance (14). Most of the genes encoding the key components of autophagy pathway, named autophagy-related genes (ATG), have been characterized. Beclin-1 is a mammalian homolog of yeast autophagy protein, Atg-6, which plays a central role in autophagy pathway. It interacts with several cofactors to induce autophagy (15). ATG-7 is a key autophagy-promoting gene that plays a critical role in regulation of autophagy (16). Inhibition of the autophagic process may reduce cancer cell drug resistance.
Nitric oxide (NO) is a small, easily diffusible gaseous molecule that has shown numerous roles in cellular function. In recent years, NO has been shown to have a ubiquitous role in the physiopathology of human diseases such as malignant gliomas (17).
Studies have indicated that NO is a chemosensitizer and apoptosis inducer in tumor cells at low concentrations. NO can induce or inhibit tumor progression and metastasis depending on the concentration and duration of exposure to glioma (18).
Previous data have demonstrated that natural antioxidants can be used as adjuvant in combination with chemotherapy to lower the side effects and to increase the efficiency of cancer treatments (19). Since TQ can cross the blood-brain barrier and inhibit GBM growth (20), in the present study, we tested the effect of TMZ and TQ alone and in combination with each other on the viability, colony-forming ability, apoptosis, necrosis, autophagy and NO production of human GBM cell line.
Materials and Methods
Cell line and reagents
The human glioblastoma cell line (U87MG) was obtained from the National Cell bank of Iran (NCBI). TMZ, known commercially as Temodal®, TQ, Dulbecco’s modified Eagle’s medium and Ham’s F12 (DMEM/F12), fetal bovine serum (FBS), trypsin, and acridine orange (AO) were purchased from Sigma-Aldrich Chemical Co (St. Louis, MO, USA). RNX-Plus solution was purchased from SinaClon BioScience Co (Tehran, Iran).
Cell culture and treatments
U87MG cells were cultured in DMEM/F12 supplemented with 10% FBS, without antibiotics, at 37 °C and 5% CO2 in a humidified incubator. GBM cells (1×106) were cultured in 25 cm2 flasks, and the culture medium was changed after 48 hr. Sub-confluent cells were detached using trypsin-EDTA solution in calcium-free phosphate buffered saline (PBS) and were counted in hemocytometers. Dose-response study was performed to calculate the IC50 values (the concentration of TMZ and TQ that induced 50% cell inhibition against U87MG cells) of each drug. Cells were treated with 10, 20, 50 and 100 µM TMZ (21) and 10, 20, 50, 100, 150 and 200 µM TQ (22) for trypan blue staining and MTT assay. For other tests, 20 µM concentration of TMZ and/or 50 µM TQ were chosen.
Trypan blue Staining
Cells were cultured in 24-well plates (7×104 per well) and after 24 hr, the culture medium was replaced with a new serum-free medium containing TMZ, TQ or a combined dose of both.
Plates were incubated for 24, 48, 72 and 96 hr. Subsequently, the cells were trypsinized and mixed with an equal volume of 0.4% trypan blue solution. The number of dead cells (stained) versus the total number of cells was calculated as the percentage of viability (23).
MTT assay
MTT assay is a standard colorimetric assay for measuring cell viability, based on tetrazolium dye reduction by mitochondrial dehydrogenase enzymes of viable cells with production of a purple color. U87MG cells (15×103 per well) were plated in 96-well culture dish. After 24 hr incubation, the medium was removed and replaced with a fresh serum-free medium containing TMZ, TQ or a combination of TQ and TMZ and was incubated again at 37 °C. After 24, 48, 72 and 96 hr, the medium was removed and 20 μl MTT solution (5 mg/ml in PBS) was added to each well and incubated for 3 hr at 37 ºC. The formed purple formazan crystals were dissolved in 100 µl dimethyl sulfoxide (DMSO).
The absorbance of each well was measured at 570 nm with reference reading at 630 using a microplate reader. The percentage of viability was calculated as follows: % of cell viability = (absorbance of treated cells / absorbance of control cells) × 100 (24). The IC50 values of TMZ and TQ against U87MG cells were calculated using GraphPad Prism 5 (GraphPad Software Inc, San Diego, USA).
Median effect analysis
The method proposed by Chou (25) was used to determine and quantify the nature of TMZ and TQ interaction (synergistic, additive, or antagonistic). The combination of TMZ and TQ was prepared in constant concentration ratio (1.87:1) based on their corresponding IC50 values (90.63 and 48.50 µM for TMZ and TQ respectively) in serial dilutions above and below the IC50 value of each agent, and then the MTT assay was performed. The combination index (CI) was calculated using CompuSyn software (ComboSyn, Inc., Paramus, NJ, USA). The CI values were interpreted as additive (CI = 1), synergistic (CI < 1) and antagonistic (CI > 1).
Colony formation assay
A colony formation assay was performed to evaluate the effects of TMZ and TQ alone and in combination with each other on the clonogenic capacity of U87MG cells. Colony formation assay is a standard survival assay based on the ability of a single cell to grow into a colony. Single cell suspensions of 500 cells were cultured into 4-well plates and incubated overnight. Subsequently, the cells were treated with TQ and/or TMZ for 72 hr. Then, the culture medium was replaced with a drug-free medium. Plates were incubated for 12-14 days and thereafter the colonies were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet solution. Only the colonies that contained more than 50 cells were selected. The percentage of U87MG seeded cells that formed a colony (the plating efficacy (PE)) was calculated as the percentage of counted colonies/seeded cells × 100. The surviving fractions (SF) of cells were determined as the number of former colonies / number of seeded cells × PE (26).
Terminal transferase dUTP nick end labeling (TUNEL) assay
TUNEL assay was performed to detect apoptosis in U87MG cells using an in situ cell death detection kit, (Roche Diagnostics; Germany) according to the manufacturer’s instructions. Briefly, after 72 hr treatment with 20 μM TMZ and/or 50 μM TQ, the cells were fixed with 4% paraformaldehyde for 1 hr, permeabilized with 0.2% Triton X-100 for 5 min on ice, and incubated with 50 μl of TUNEL mixture solution for 1 hr. For differential staining of the cells, the propidium iodide staining solution was added to each well and incubated for 5 min at room temperature. Finally, the cells were analyzed using a fluorescence microscope. All the described stages were performed in dark condition. The apoptotic index of the cells was calculated as a follow (23):
Apoptotic index (%) = (number of apoptotic cells/total number of cells) ×100
Detection of acidic vesicular organelles (AVOs)
Autophagy is characterized by the formation of AVOs in cells. To analyze autophagy induction, AVOs, which consist predominantly of autophagosomes, were quantified by staining the cells with AO. Autophagy is an evolutionarily conserved process for recycling and maintaining the quality of cells. During this process, cytoplasmic constituents are sequestered in autophagosomes and degraded after fusion with lysosomes and formation of acidic autolysosomes (27). AO accumulates in acidic organelles in a pH-dependent manner in cells. At neutral pH, it emits green fluorescence, but within acidic environments, becomes protonated, and gets trapped within the organelle and then aggregates and emits red fluorescence. The U87MG cells were grown in absence or presence of TMZ, TQ and combination of both for 72 hr. These cells were stained with 1 μg/ml AO for 15 min. A morphological analysis was performed under a fluorescence microscope. At least 1,000 cells in randomly selected microscopic fields were counted. The percentage of autophagic cells was calculated as follows: % of autophagic cells = (the number of cells with AVOs / the total number of stained cells) × 100 (28).
RNA isolation and RT-PCR
The effect of TMZ and/or TQ on the expression level of autophagy marker genes, beclin-1 and ATG-7 was analyzed by RT-PCR. GBM cells were treated with TMZ and/or TQ for 72 hr. Then, total RNA was extracted by RNX-Plus solution according to the manufacturer’s instructions.
The quantity and quality of the purified RNAs were verified by nanodrop spectrophotometer and electrophoresis using 1% agarose gel, respectively. cDNA synthesis was carried out taking 1 μg RNA using cDNA synthesis kit (Vivantis Technologies, Selangor DE, Malaysia) according to the manufacturer’s protocol. RT-PCR was performed taking 1 μl of cDNA as template using RT-PCR kit (Vivantis Technologies, Selangor DE, Malaysia) based on the manufacturer’s protocol. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), a house keeping gene, was served as an internal reference. The PCR products were separated via 2% agarose gel electrophoresis and visualized by ethidium bromide staining. The primer sequences were as follows:
ATG-7 forward: 5’-ATTGCTGCATCAAGAAACCC-3’, reverse: 5’-GATGGAGAGCTCCTCAGCA-3’, beclin-1 forward: 5’-GCCGAAGACTGAAGGTCA,
reverse: 5’-GTCTGGGCATAACGCATC-3’, GAPDH
forward: 5’- CAATGACCCCTTCATTGACC-3’,
reverse: 5’- TTCACACCCATGACGAACAT-3’.
NO measurement
The alteration in NO production following treatment with TMZ and/or TQ was determined by Griess reaction.
This reaction is widely used to determine total nitrate and nitrite concentration as an index of NO production in biological samples (29). U87MG cells were plated in 24-well plates (7×104 cells per well) and incubated overnight. Next, the cells were treated with TMZ, TQ and combination of TMZ and TQ for 72 hr. Subsequently, 400 µl of the culture medium of each sample was collected and 6 mg of zinc sulfate was added to deproteinize it, and centrifuged at 10,000 g for 10 min at 4 ºC. Then, 100 µl of each deproteinized sample was transferred to microplate wells, and 100 μl vanadium (III) chloride (8 mg/ml in HCl 1 M), 50 μl 2% sulfanilamide (in 5% HCl), and 50 µl 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride (in deionized water) were added, respectively. The plates were incubated for 30 min at 37°C, and the absorbance rate was subsequently measured by a microplate reader at 540 and 630 nm. The concentrations of NO were determined from a sodium nitrite standard curve (30).
Statistical analysis
All data were presented as mean ± SD of three independent experiments. Statistical evaluation was performed by SPSS software version 16.0 (SPSS Inc., Chicago, USA) using one-way ANOVA. Differences were considered to be statistically significant when P-value was <0.05.
Results
Cytotoxic assay
The effects of TMZ and TQ on the survival of U87MG cells were evaluated by trypan blue staining and MTT assay after 24, 48, 72 and 96 hr, and presented in Figure 1A and B. The results showed that both TMZ and TQ decreased cell viability in a dose- and time-dependent manner. This toxicity effect was consistent with morphologic changes, including cell rounding and granulation as well as reduction in cell number. IC50 values for each drug were calculated by Graph Pad Prism 5.0 software using the data of MTT assay and presented in Table 1.
Figure 1.
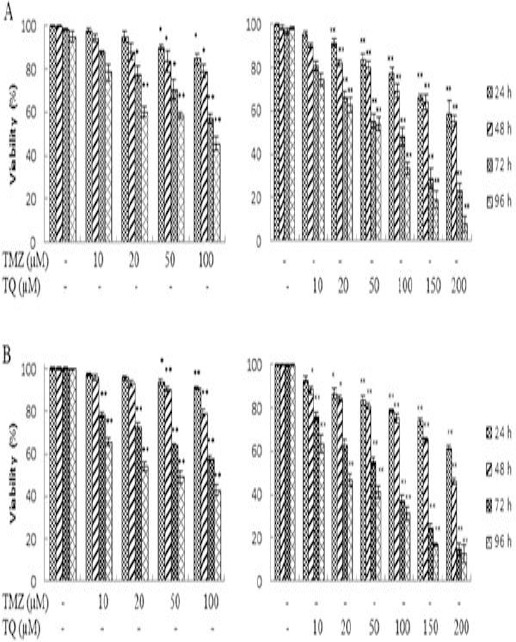
The effect of temozolomide (TMZ) and thymoquinone (TQ) on viability of U87MG cells. Cells were treated with TMZ and TQ for 24, 48, 72 and 96 hr and viability was measured by trypan blue staining (A) and MTT assay (B). Control wells were treated with equivalent amount of medium alone. The results showed the mean±SD from triplicated experiments. (*P < 0.05; ** P < 0.01 compared with control)
U87MG cells were treated with TMZ and TQ in combination for 72 hr. Reduction in the cell viability by TMZ and TQ combination was greater than either TMZ or TQ alone (Figure 2A and B). The CI values were calculated by CompuSyn software using the data of MTT assay and presented in Table 2. The results showed that the CI values in all combinations were smaller than 1, implying a synergistic effect in all combination tests.
Figure 2.
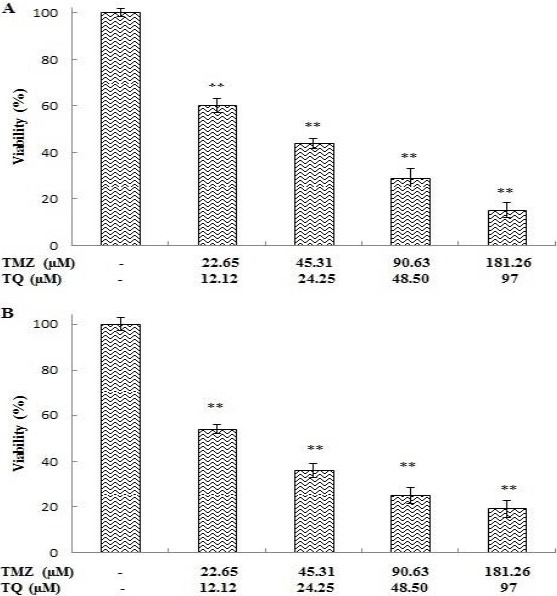
The effect of temozolomide (TMZ) and thymoquinone (TQ) in combination on viability of U87MG cells. Cells were treated with combination of TMZ and TQ for 72 hr and viability was measured by trypan blue staining (A) and MTT assay (B). Control wells were treated with equivalent amount of medium alone. The results showed the mean±SD from triplicated experiments. (*P < 0.05; ** P < 0.01 compared with control)
Table 2.
Combination indexa (CI) values for temozolomide (TMZ) and thymoquinone (TQ) combination
| TMZ (µM) | TQ (µM) | CI | Interpretation |
|---|---|---|---|
| 20 | 10 | 0.51 | Synergism |
| 50 | 20 | 0.37 | Synergism |
| 100 | 50 | 0.35 | Synergism |
| 150 | 100 | 0.33 | Synergism |
Colony formation assay
After 72 hr treatment, TMZ (20 µM) decreased the percentage of SF significantly up to 29% when compared to control (P=0.000). Interestingly, TQ (50 µM) decreased SF significantly up to 46% (P=0.000). However, administration of TQ in combination with TMZ reduced the percentage of SF significantly by approximately 71% (P=0.000) (Figure 3).
Figure 3.
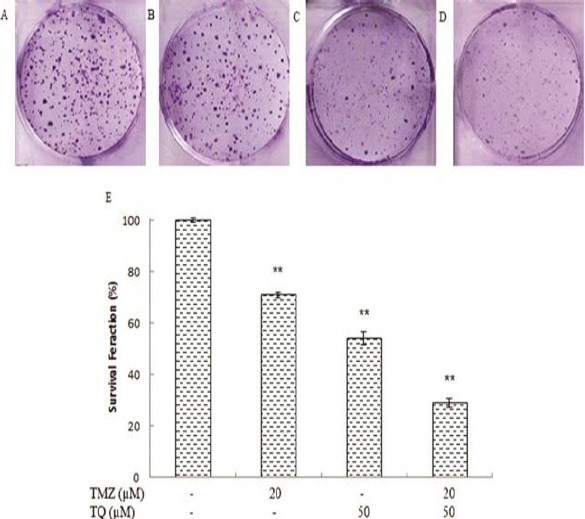
The effect of temozolomide (TMZ) and/or thymoquinone (TQ) on colony formation of U87MG cell line. A) control group; B) in the presence of 20 μM temozolomide; C) in the presence of 50 μM thymoquinone; D) in the presence of combination both; E) Columns mean percentage of colonies from three independent experiments performed in triplicate. (*P < 0.05 compared with control; **P < 0.01 compared with control)
Detection of apoptosis by TUNEL staining
The number of apoptotic cells were quantified and presented as percentage (Figure 4).
Figure 4.
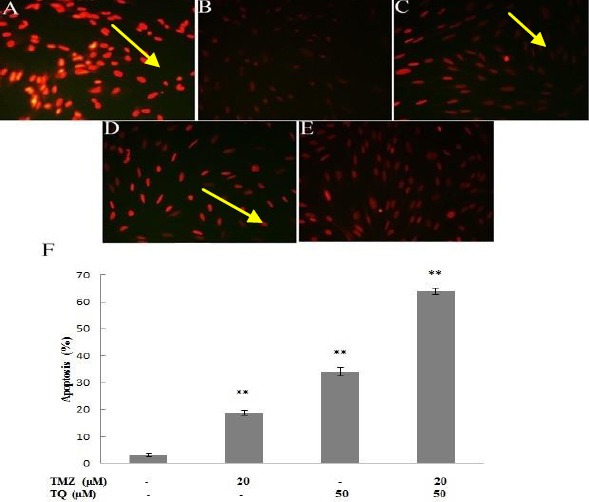
Apoptotic potential of temozolomide (TMZ) and/or thymoquinone (TQ) was monitored by TUNEL (Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling) staining in U87MG cells. A) positive control group; B) negative control group; C) in the presence of 20 μM temozolomide; D) in the presence of 50 μM thymoquinone; E) in the presence of combination both. F) Columns mean percentage of apoptotic and necrotic cells from three independent experiments. Yellow arrows indicate TUNEL-positive cells (*P < 0.05, **P < 0.01 compared with control)
After treatment with TMZ and TQ alone for 72 hr, as much as 19% and 36% of the cells were apoptotic, respectively, whereas 66% of the cells with combination therapy were apoptotic.
Detection of autophagy by AVOs staining
The control cells and cells treated with TQ exhibited a negligible cytoplasmic staining (Figure 5 A and C), and the TMZ-treated tumor cells showed abundant cytoplasmic AVO formation, which is a characteristic of autophagy (Figure 5 B). Comparison of the mean AVO in TMZ and TMZ/TQ treated cells indicated a significant decrease in AVO number in the combination group (P=0.000). So, after incubation with both TMZ and TQ, the acidic vesicular organelle was significantly less than that of TMZ alone (~71%).
Figure 5.
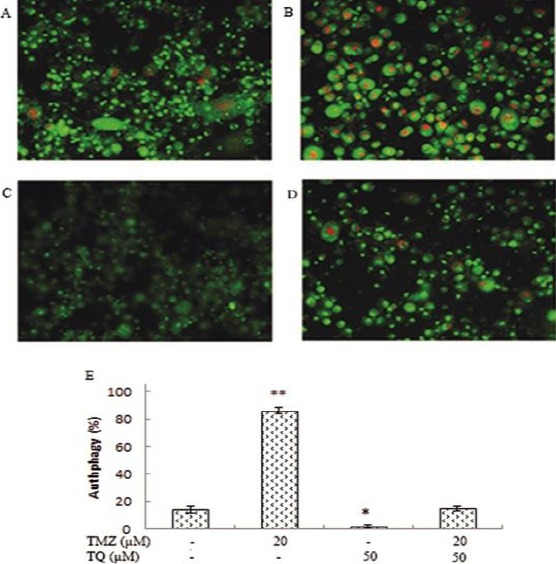
The effect of temozolomide (TMZ) and/or thymoquinone (TQ) on autophagy was monitored by acidic vesicular organelles detection assay in U87MG cells. A) control group; B) in the presence of 20 µM temozolomide; C) in the presence of 50 µM thymoquinone; D) in the presence of combination both. E) Columns mean percentage of autophagic cells from three independent experiments. Red dots indicate autophagic vesicles (*P<0.05, **P<0.01 compared with control)
Analysis of ATG-7 and beclin-1 expression
The results of RT-PCR showed that TMZ increased the beclin-1 and ATG-7 mRNA levels significantly (P=0.000), whereas TQ decreased them significantly (P=0.000) in U87MG cells. However, the combined treatment of TMZ and TQ could prevent TMZ-induced upregulation of beclin-1and ATG-7 mRNA levels significantly (P=0.000) (Figure 6). Thus, TQ inhibited TMZ-induced autophagy at the transcriptional level.
Figure 6.
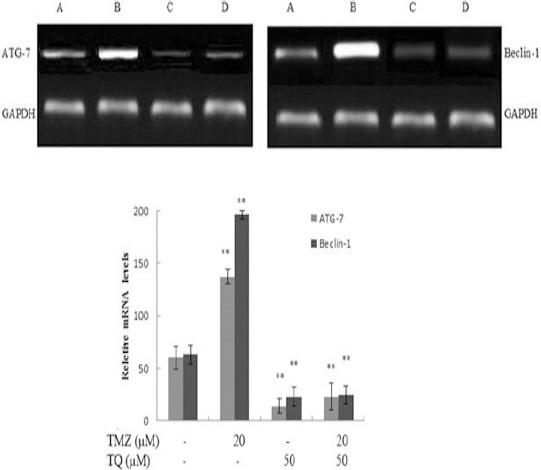
The effect of temozolomide (TMZ) and/or thymoquinone (TQ) on mRNA expression of Beclin-1 and ATG-7 in was monitored by RT-PCR in U87MG cells. A) control group; B) in the presence of 20 µM temozolomide; C) in the presence of 50 µM thymoquinone; D) in the presence of combination both. The results showed the mean±SD from triplicated experiments. (*P<0.05; **P<0.01 compared with control)
NO measurement
Both TMZ and TQ significantly decreased the NO secretion of GBM cells after 72 hr, and combination of both TMZ and TQ had a stronger effect on the reduction of NO production than each of them separately (P=0.000) (Figure 7).
Figure 7.
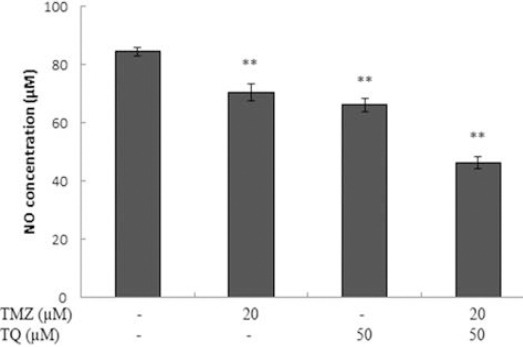
The effect of temozolomide (TMZ) and/or thymoquinone (TQ) on nitric oxide (NO) production in U87MG cell line. Cells were treated with temozolomide, thymoquinone alone and combination for 72 hours and nitric oxide concentration was measured by Griess assay. Control wells were treated with equivalent amount of medium alone. The results showed the mean±SD from triplicated experiments. (*P<0.05; **P<0.01 compared to control)
Discussion
The main purpose of this study was to investigate whether TQ was able to enhance the anti-proliferative effects of TMZ on U87MG cell line. First, the ability of each agent alone was evaluated to promote cell death. Our results showed that both TQ and TMZ decreased the viability of U87MG cells in a time- and dose-dependent manner. We also explored whether TQ could demonstrate a therapeutically beneficial effect when administered in combination with TMZ or not.
Results showed that TQ enhanced the anti-proliferative activity of TMZ and combination of TMZ and TQ exerted synergistic cytotoxic effects on U87MG cell line. In other words, TMZ and TQ acted synergistically to reduce the viability of brain cancer cells. Based on the previous studies, the 20 µM concentration of TMZ was selected because it is the concentration close to the content of TMZ in the brains of patients with glioma. Using microdialysis sampling technique and pharmacokinetic model has shown that the peak concentration of TMZ in brain interstitium was about 0.6 μg/ml (3 μM) (31), and TMZ concentrations in the brain ranged from 1.8 to 3.7 μg/ml (9 μM – 20 μM) (32).
Our data also showed that TMZ and TQ reduced the colony-forming ability, and combination of both exerted a synergistic effect and reduced the number of colonies of GBM cells. The formation of colonies by the original cells requires intensive cell division, so the number of colonies can be a good indicator of the cells growth potential. Further, we found that both drugs induced apoptosis, and enhanced TMZ-induced apoptosis may account for the synergistic cytotoxicity of the combination treatment. We also demonstrated that TQ potentiated the anti-cancer effect of TMZ by increasing apoptotic cell death via suppressing TMZ-induced autophagy, suggesting that TMZ-induced autophagy acts as a prosurvival pathway, which is a reason for chemotherapy resistance.
Despite remarkable improvements in surgical techniques and treatment options, including radiotherapy and chemotherapy with TMZ, GBM is still associated with very poor prognosis, and only 3 to 5% of patients survive more than 3 years owing to inherent chemoresistance (33), so new treatment strategies are needed to be developed for the patients with GBM. Since a large number of different anti-cancer agents have already been recognized for a novel combination treatment, improvement of therapeutic approaches can be one of these new treatment strategies. The current study showed that TQ as the major bioactive component of Nigella sativa Linn seed (also known as black seed) oil has anti-cancer activity against numerous cell lines (10), including glioblastoma (22, 30, 34, 35).
Autophagy is a conserved process that is crucial for development, differentiation, survival and homeostasis, and allows cells to sequester cytoplasmic contents through the formation of vesicles and targets them for degradation with lysosomes. A number of studies have shown that anti-neoplastic agents induce autophagy in human cancer cell lines (36). Whether therapy-induced autophagy causes tumor cell death or resistance to therapy-mediated cell death is still a controversial issue. Increasing evidence supports the key role of autophagy in resistance of cancer cells to anti-neoplastic therapies. Therefore, inhibition of autophagy may be a good strategy to improve the efficacy of cancer therapy or to overcome therapeutic resistance (37).
Our study demonstrated that the number of AVO-containing cells was increased significantly by TMZ, indicating the activation of autophagy, whereas it was significantly decreased by TQ, indicating the inhibition of autophagy. In combination treatment, the number of AVO-containing cells was significantly lower than that of TMZ-treated cells. Also at molecular level, the mRNA expression of two autophagy promoting genes, beclin-1 and ATG-7, were significantly increased by TMZ, whereas they were significantly decreased by TQ in GBM cells. These results are in agreement with the findings of AVO staining. So, induction of autophagy by TMZ may be one of the main reasons of TMZ resistance, and its inhibition in the transcriptional level by TQ can be a good strategy to improve the efficacy of TMZ treatment.
TMZ is an imidazotetrazine derivative of dacarbazine and a novel oral alkylating agent used for the treatment of GBM. Previous studies have demonstrated that TMZ reduces cell viability, induces cell-cycle arrest in the gap 2/mitosis phase, inhibits cell migration (38) and activates apoptosis, autophagy and senescence pathways in glioma cells. It also induces necrosis in low level. These endpoints have a complex interplay and the decision between death and survival depends on the balance of autophagy, senescence and apoptosis. Autophagy serves as a survival mechanism inhibiting apoptosis and stimulating senescence (39). Furthermore, glioblastoma cells are dependent on the autophagic pathway for survival because exposure to autophagy inhibitors reduces glioblastoma cell proliferation (20). Hence, inhibition of autophagy in combination with TMZ treatment might represent a novel strategy for enhancing the killing effect of TMZ on glioma cells and promoting the therapeutic response.
TQ has selective cytotoxicity for glioblastoma cells compared to normal human astrocytes (20). It induces DNA damage, inhibits telomerase activity and causes apoptosis in glioblastoma cells (33).
Nitric oxide mediates many physiological and pathological mechanisms in the brain and has a dual and diverging role in glioma biology. We showed that TMZ and TQ significantly reduced NO production and combination of both had a stronger effect on the reduction of NO level than each one of them separately. NO is a small signaling molecule with complex regulatory roles under physiological and pathological conditions (40). Therefore, it is possible to achieve anti-glioma effects by modulation of NO release. NO has been shown to influence cell proliferation, vascularization, invasion, chemo- and radiotherapy sensitivity and immune reactivity in glioma tumors (41). The role of NO in glioma cell invasion, as its role in glioma cell proliferation, is ambiguous. The effect of NO on the proteolytic enzymes involved in invasion is critical in determining whether invasion is enhanced or inhibited. Thus, NO induces cytotoxicity via a number of different mechanisms.
Conclusion
We showed that combination treatment with TMZ and TQ resulted in synergistic anti-tumor effect in GBM cells. Our study suggested that the mechanism of reducing cell resistance to TMZ by TQ might be attributed to autophagy blockage. Also, both TQ and TMZ reduced NO production in GBM cells. We propose further in vivo studies about TMZ/TQ synergy and future clinical trials to evaluate the efficacy of TMZ and TQ combination therapy among GBM patients.
Acknowledgment
The results described in this paper were part of a PhD dissertation with grant number 93079. The authors would like to thank the Fertility and Infertility Research Center (FIRC) staff, Kermanshah University of Medical Sciences, Kermanshah, Iran for financial support.
Conflict of interest
There is no conflict of interest in this study.
References
- 1.Grossman SA, Batara JF. Current management of glioblastoma multiforme. Semin Oncol. 2004;31:635–644. doi: 10.1053/j.seminoncol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Central Brain Tumor Registry of the United States (CTBRUS) Report. 2010. http://www.cbtrus.org/-factsheet/factsheet.html .
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Marchesi F, Turriziani M, Tortorelli G, Avvisati G, Torino F, De Vecchis L. Triazene compounds:mechanism of action and related DNA repair systems. Pharmacol Res. 2007;56:275–287. doi: 10.1016/j.phrs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 5.FDA Approval for Temozolomide. National Cancer Institute. 2010. http://www.cancer.gov/cancertopics/-treatment/drugs/fda-temozolomide .
- 6.Serwer LP, James CD. Challenges in drug delivery to tumors of the central nervous system:an overview of pharmacological and surgical considerations. Adv Drug Deliv Rev. 2012;64:590–597. doi: 10.1016/j.addr.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Wheelhouse RT, Stevens MFG. Decomposition of the antitumour drug temozolomide in deuteriated phosphate buffer:methyl group transfer is accompanied by deuterium exchange. J Chem Soc Chem Commun. 1993;1993:1177–1178. [Google Scholar]
- 8.Jhaveri N, Cho H, Torres S, Wang W, Schonthal AH, Petasis NA, et al. Noscapine inhibits tumor growth in TMZ-resistant gliomas. Cancer Lett. 2011;312:245–252. doi: 10.1016/j.canlet.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Gali-Muhtasib H, Roessner A, Schneider-Stock R. Thymoquinone:A promising anticancer drug from natural sources. Int J Biochem Cell Biol. 2006;38:1249–1253. doi: 10.1016/j.biocel.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee S, Padhye S, Azmi A, Wang Z, Philip PA, Kucuk O, et al. Review on molecular and therapeutic potential of thymoquinone in cancer. Nut Cancer. 2010;62:938–946. doi: 10.1080/01635581.2010.509832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worthen DR, Ghosheh OA, Crooks PA. The in vitro antitumor activity of some crude and purified components of blackseed, Nigella sativa L. Anticancer Res. 1998;18:1527–1532. [PubMed] [Google Scholar]
- 12.Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 13.Lena A, Rechichi M, Salvetti A, Bartoli B, Vecchio D, Scarcelli V, et al. Drugs targeting the mitochondrial pore act as cytotoxic and cytostatic agents in temozolomide-resistant glioma cells. J Transl Med. 2009;5:7–13. doi: 10.1186/1479-5876-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, et al. Autophagy and chemotherapy resistance:a promising therapeutic target for cancer treatment. Cell Death Dis. 2013 Oct 10;4:e838. doi: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weidberg H, Shvets E, Elazar Z. Biogenesis and cargo selectivity of autophagosomes. Annu Rev Biochem. 2011;80:125–156. doi: 10.1146/annurev-biochem-052709-094552. [DOI] [PubMed] [Google Scholar]
- 17.Miller MR, Megson IL. Recent developments in nitric oxide donor drugs. Br J Pharmacol. 2007;151:305–321. doi: 10.1038/sj.bjp.0707224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 19.Ladas EJ, Jacobson JS, Kennedy DD, Teel K, Fleischauer A, Kelly KM. Antioxidants and cancer therapy:a systematic review. J Clin Oncol. 2004;22:517–528. doi: 10.1200/JCO.2004.03.086. [DOI] [PubMed] [Google Scholar]
- 20.Racoma IO, Meisen WH, Wang QE, Kaur B, Wani AA. Thymoquinone inhibits autophagy and induces cathepsin-mediated, caspase-independent cell death in glioblastoma cells. PLoS One. 2013;8:8e72882. doi: 10.1371/journal.pone.0072882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renata MŻ, Maria HB, Anna F, Sylwia KN, Diana S, Halina C. Propolis changes the anticancer activity of temozolomide in U87MG human glioblastoma cell line. BMC Complement Altern Med. 2013;13:50. doi: 10.1186/1472-6882-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valentina C, Luana Q, Alessia DB, Laura B, Massimo B, Giulio L, et al. Effects of thymoquinone on isolated and cellular proteasomes. FEBS Journal. 2010;277:2128–2141. doi: 10.1111/j.1742-4658.2010.07629.x. [DOI] [PubMed] [Google Scholar]
- 23.Rezakhani L, Rashidi Z, Mirzapur P, Khazaei M. Antiproliferatory effects of crab shell extract on breast cancer cell line (MCF7) J Breast Cancer. 2014;17:219–225. doi: 10.4048/jbc.2014.17.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirzapur P, Rashidi Z, Rezakhani L, Khazaei M. In vitro inhibitory effect of crab shell extract on human umbilical vein endothelial cell. In Vitro Cell Dev Biol Anim. 2015;51:36–41. doi: 10.1007/s11626-014-9810-x. [DOI] [PubMed] [Google Scholar]
- 25.Chou TC. Drug combination studies and their synergy quantificationusing the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 26.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 27.Hale AN, Ledbetter DJ, Gawriluk TR, Rucker EB., 3rd Autophagy:regulation and role in development. Autophagy. 2013;9:951–972. doi: 10.4161/auto.24273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bądziul D, Jakubowicz-Gil J, Paduch R, Głowniak K, Gawron A. Combined treatment with quercetin and imperatorin as a potent strategy for killing HeLa and Hep-2 cells. Mol Cell Biochem. 2014;392:213–227. doi: 10.1007/s11010-014-2032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J, Zhang X, Broderick M, Fein H. Measurement of nitric oxide production in biological systems by using Griess reaction assay. Sensors. 2003;3:276–284. [Google Scholar]
- 30.Khazaei M, Roshankhah S, Ghorbani R, Chobsaz F. Sildenafil effect on nitric oxide secretion by normal human endometrial epithelial cells cultured In vitro. IJFS. 2011;5:142–147. [PMC free article] [PubMed] [Google Scholar]
- 31.Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors:potential implications for the current approach to chemoradiation. Clin Cancer Res. 2009;15:7092–7098. doi: 10.1158/1078-0432.CCR-09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosso L, Brock CS, Gallo JM, Saleem A, Price PM, Turkheimer FE, et al. A new model for prediction of drug distribution in tumor and normal tissues:pharmacokinetics of temozolomide in glioma patients. Cancer Res. 2009;69:120–127. doi: 10.1158/0008-5472.CAN-08-2356. [DOI] [PubMed] [Google Scholar]
- 33.Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, et al. Longterm survival with glioblastoma multiforme. Brain. 2007;130:2596–2606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 34.Gurung RL, Lim SN, Khaw AK, Soon JF, Shenoy K, Mohamed Ali S, et al. Thymoquinone induces telomere shortening, DNA damage and apoptosis in human glioblastoma cells. PLoSOne. 2010;5:e12124. doi: 10.1371/journal.pone.0012124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolli-Bouhafs K, Boukhari A, Abusnina A, Velot E, Gies JP, Lugnier C, et al. Thymoquinone reduces migration and invasion of human glioblastoma cells associated with FAK MMP-2 and MMP-9 down-regulation. Invest New Drugs. 2012;30:2121–2131. doi: 10.1007/s10637-011-9777-3. [DOI] [PubMed] [Google Scholar]
- 36.Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Han W, Sui X, Fang Y, Pan H. Autophagy:A novel therapeutic target for hepatocarcinoma (Review) Oncol Lett. 2014;7:1345–1351. doi: 10.3892/ol.2014.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen W, Hu JA, Zheng JS. Mechanism of temozolomide-induced antitumour effects on glioma cells. J Int Med Res. 2014;42:164–172. doi: 10.1177/0300060513501753. [DOI] [PubMed] [Google Scholar]
- 39.Knizhnik AV, Roos WP, Nikolova T, Quiros S, Tomaszowski KH, Christmann M, et al. Survival and death strategies in glioma cells:autophagy, senescence and apoptosis triggered by a single type of temozolomide-induced DNA damage. PLoS One. 2013;8:e55665. doi: 10.1371/journal.pone.0055665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung HT, Pae HO, Choi BM, Billiar TR, Kim YM. Nitric oxide as a bioregulator of apoptosis. Biochem Biophys Res Commun. 2001;282:1075–1079. doi: 10.1006/bbrc.2001.4670. [DOI] [PubMed] [Google Scholar]
- 41.Badn W, Siesjö P. The dual role of nitric oxide in glioma. Curr Pharm Des. 2010;16:428–430. doi: 10.2174/138161210790232158. [DOI] [PubMed] [Google Scholar]


