Abstract
Objective(s):
The objective of this study was to investigate the hepatoprotective effect of licochalcone B (LCB) in a mice model of carbon tetrachloride (CCl4)-induced liver toxicity.
Materials and Methods:
Hepatotoxicity was induced in mice by a single subcutaneous injection (SC) of CCl4. The LCB was administered orally once a day for seven days (PO) as pretreatment at three doses of 1, 5, and 25 mg/kg/day. The levels of superoxide dismutase (SOD), malondialdehyde (MDA), glutathione (GSH), glutathione disulfide (GSSG), C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were analyzed by ELISA. The protein expression degrees of p38 mitogen activated protein kinases (p38) and nuclear factor-k-gene binding (NF-κB) were assayed by western blotting.
Results:
CCl4-induced hepatotoxicity was manifested by an increase in the levels of ALT, AST, MDA, IL-6, CRP, and TNF-ɑ, and a decrease in the SOD level and GSH/GSSG ratio in the serum. The histopathological examination of the liver sections revealed necrosis and inflammatory reactions. Pretreatment with LCB decreased the levels of ALT, AST, MDA, GSSG, IL-6, CRP, TNF-ɑ, and the protein expression of p38 and NF-κB, increased the level of SOD and GSH, and normalized the hepatic histo-architecture.
Conclusion:
LCB protected the liver from CCl4-induced injury. Protection may be due to inhibition of p38 and NFκB signaling, which subsequently reduced inflammation in the liver.
Keywords: Antioxidant, Anti-inflammatory, Carbon tetrachloride, Hepatotoxicity, Licochalcone B, NF-κB, P38
Introduction
A vital organ of the human body, the liver is in charge of detoxification of exogenous xenobiotics, drugs, viral infections, and chronic alcoholism. Liver diseases are one of the major causes of mortality and morbidity worldwide, and drug-induced liver toxicity is a major cause of hepatic dysfunction (1). Liver damage is a widespread pathology, which in most cases involves oxidative stress and is characterized by a progressive evolution from steatosis to chronic hepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma (2). In recent years, attention has been focused on biotransformation of chemicals into highly reactive metabolites that initiate cellular toxicity. Carbon tetrachloride -(CCl4-)induced hepatotoxicity in animal models has been widely used to investigate hepatoprotective effect of natural compounds (3, 4).
Hepatotoxicity is a complicated process that involves various mechanisms. Oxidative damage, which is one of the most important mechanisms involved in hepatotoxicity, has an important function in the progression of hepatotoxicity (5, 6). In addition, inflammation is considered to be a mechanism that contributes to the initiation and progression of hepatic damage in a variety of liver disorders (7, 8). Several studies demonstrate that numerous inflammatory cytokines are produced in hepatotoxic liver tissues (9, 10). Therefore, anti-inflammatory drugs and antioxidants obtained from plants represent a logical therapeutic strategy for the treatment of liver diseases.
Flavonoids are the most potent and versatile biologically active compounds in plants and have been known to exhibit outstanding antioxidant, anti- inflammatory, and hepatoprotective effects (11, 12). Licochalcone B (LCB), which belongs to the retrochalcone family, is isolated from the roots of Chinese licorice. Studies on the biological activities of LCB are still in their initial stage. LCB exhibits high antioxidant and free radical-scavenging activities (13). Experimental studies suggest that LCB possesses several other useful pharmacological properties, such as anti-inflammatory activities and cardioprotective effects (14). In this study, we evaluate the hepatoprotective effects of LCB and the mechanisms underlying such effects.
Materials and Methods
Test Compounds, Chemicals, and Reagents
LCB (purity 98%≥) was purchased from Shanghai Li Chen Biotechnology Co., Ltd. (Shanghai, China). CCl4 was obtained from Sigma Chemical Company, USA. The other chemicals and reagents were of analytical grade.
Animals and experimental groups
Kunming mice (20 g to 30 g) were obtained from Jinan Jinfeng Experimental Animal Breeding Co Ltd (license number: SCXK (lu) 2014-0006). All experimental procedures were approved by the Institutional Animal Care and Use Committee of the National Institute Pharmaceutical Education and Research.
The dose of the LCB used in the experiments was determined by the preliminary experiments. For this purpose, 0.1, 1, 5, 25, 50 and 100 mg/kg/day were chosen to conduct this pre-experiment. Through the measurement of biochemical and histopathological parameters, we found that the best treatment effect belongs to the dose of 25 mg/kg/day. In order to establish a dose-response relationship, the doses of 1, 5 and 25 mg/kg/day were chosen to conduct the experiment. The dose of the CCl4 used in the experiments was also determined by the preliminary experiments and previous studies (15). The mice were randomly divided into five groups (n= 12/group): control group, CCl4 group, and LCB + CCl4 groups (1, 5, and 25 mg/kg/day). In the control group, the mice received distilled water for seven days. In the CCl4 group, the mice received distilled water as in the previous group and intraperitoneally administered with 10 ml/kg body weight of CCl4 diluted with corn oil at a ratio of 1:500 once on day 8. In the LCB + CCl4 groups, the LCB was administered intragastrically once daily for seven days (1, 5, and 25 mg/kg/day) followed by a single subcutaneous (SC) dose of CCl4 (10 ml/kg body weight) on day 8.
Determination of biochemical parameters
After 24 hr of CCl4 administration, the animals were anesthetized using ether, and 1 ml of blood was collected through cardiac puncture. Blood was allowed to clot and centrifuged at 4000 g for 10 min. The serum was separated and used for alanine aminotransferase (ALT) and aspartate aminotransferase (AST) assays by standard methods using enzyme assay kits (Nanjing Jiancheng Biological Product, Nanjing, China).
Assay of oxidative stress and inflammation
As previous treatment in determination of biochemical parameters, blood was collected and allowed to clot and then centrifuged at 4000 g for 10 min. The serum was separated and used for oxidative stress assay. Superoxide dismutase (SOD) activity, the levels of malondialdehyde (MDA), glutathione (GSH), glutathione disulfide (GSSG), tumor necrosis factor-α (TNF-α), C-reactive protein (CRP), and interleukin-6 (IL-6) were spectrophotometrically analyzed according to the instructions of ELISA (Tsz Biosciences, Greater Boston, USA).
General histology survey of livers
The mice were killed by cervical dislocation, and the livers were excised, washed in phosphate buffer, and dried using tissue paper. Each mice liver was fixed in 10% formaldehyde and preserved at normal temperature (37 °C). The livers were observed under an optical microscope after hematoxylin and eosin (HE) coloration. A small piece (2 mm × 1 mm × 1 mm) of liver tissue was obtained and fixed in a 0.1 mM phosphate buffer (pH 7.2) containing 3% glutaraldehyde and 1.5% paraformaldehyde at 4 °C. The tissue sample was cut into small 1 mm3 pieces and subsequently fixed in the abovementioned solution for 4 hr. The piece was fixed in 1% osmic acid again at 4 °C for 1.5 hr after being rinsed with phosphate buffer. The tissue was then dehydrated by alcohol and dimethylbenzene and embedded in epoxy resin 618. The tissue was located by semithin sectioning and then sliced into ultrathin sections (60 nm). The sections were dyed with uranium acetate and lead citrate. After staining with HE, the slides were examined under a microscope (Olympus, Japan) at 100× magnification for histopathological changes.
Western blot analysis
p38 and NF-κB protein expression were examined using western blot analysis. The protein extracts from the liver tissue were prepared using a lysis buffer (50 mM Tris-HCl, pH 7.6, 0.5% Triton X-100, and 20% glycerol). The extracts were then subjected to centrifugation (15,000 g, 15 min at 4 °C). The supernatant fractions were assayed for protein concentration using a Bradford reagent (Bio-Rad, Richmond, CA) and were used for the western blot analyses of p38, NF-κB, and β-actin (Cell Signaling, Beverly, MA, USA). Horseradish peroxidase-conjugated IgG (Zymed, South San Francisco, CA, USA) was used as a secondary antibody.
Statistical analysis
The data were presented as means±SD from a minimum of three independent experiments and evaluated using ANOVA followed by Student’s t-test. Statistical significance was considered at P<0.05. Statistical analysis was performed using SPSS software (IBM SPASS, International Business Machines Corporation, Armonk, NY, USA).
Results
Effects of LCB on serum ALT and AST activity in hepatotoxicity
As shown in Table 1, the intoxication of mice with CCl4 resulted in a marked increase in the levels of liver function serum markers, including ALT (224.30 U/l±19.54 U/l) and AST (164.75 U/l±7.50 U/l), in comparison with those in the control group (P<0.01). On the contrary, the levels of these liver function markers decreased nearer to normalcy because of the ameliorative effect of LCB. Significant hepatoprotective activity was noticed in animals treated with LCB at dosages of 5 and 25 mg/kg (P<0.01). The dose-dependent effect of LCB was then observed.
Table 1.
Prophylactic effect of LCB on the restoration of liver function markers in CCl4-intoxicated mice (values are presented as mean±SD, n= 8)
| Groups | ALT (U/l) | AST (U/l) |
|---|---|---|
| Control | 33.10±1.80 | 50.21±9.26 |
| CCl4 | 244.30±19.54## | 164.75±7.50## |
| 1 mg/kg LCB | 255.21±23.26 | 167.71±6.77 |
| 5 mg/kg LCB | 183.53±7.24** | 112.50±12.64** |
| 25 mg/kg LCB | 57.48±7.76** | 98.65±11.63** |
ALT: alanine aminotransferase; AST: aminotransferase
P<0.01 compared with the normal control group,
P<0.01 compared with the CCl4 group
LCB alleviated oxidative stress of hepatotoxicity induced by CCl4
As shown in Table 2, the oxidative stress markers in liver homogenates revealed that the intoxication of mice with CCl4 significantly decreased the activities of oxidative stress marker enzymes in the serum, including SOD (29.82 U/ml±2.86 U/ml) and GSH (385.95 ng/l±24.29 ng/l), in comparison with those in the toxic control group (SOD: 56.67 U/ml ± 5.19 U/ml; GSH: 750.68 ng/l±16.21 ng/l). In addition, a significant increase in the levels of MDA (11.50 nmol/ml±0.30 nmol/ml) and GSSG (11.07 nmol/ml ±0.19 nmol/ml) were observed in CCl4-intoxicated mice in contrast to the control animals.
Table 2.
Effect of LCB pretreatment on the oxidative stress parameters of mice in CCl4-induced hepatotoxicity. (values are presented as means±SD, n = 8)
| Groups | SOD (U/ml) | MDA (nmol/ml) | GSH (ng/l) | GSSG (nmol/ml) |
|---|---|---|---|---|
| control | 56.67 ± 5.19 | 8.16 ± 0.13 | 750.68 ± 16.21 | 8.87 ± 0.29 |
| CCl4 | 29.82 ± 2.8## | 11.50 ± 0.30## | 385.95 ± 24.29## | 11.07 ± 0.19## |
| 1 mg/kg LCB | 32.46 ± 2.53 | 11.02 ± 0.29 | 433.46 ± 25.29 | 10.89 ± 0.87 |
| 5 mg/kg LCB | 37.30 ± 3.99* | 10.26 ± 0.16* | 512.50 ± 73.04* | 10.10 ± 0.83* |
| 25 mg/kg LCB | 48.51 ± 3.41** | 9.30 ± 0.40** | 628.29 ± 26.93** | 9.13 ± 0.53** |
P< 0.01 compared with the normal control group,
P<0.05 and
P<0.01
SOD: superoxide dismutase; MDA: malondialdehyde; GSH: glutathione; GSSG: glutathione disulfide
Compared with the CCl4 group, the LCB-pretreated (5 and 25 mg/kg) groups exhibited significant ameliorative effect by elevating the levels of SOD (37.30±3.99 U/ml and 48.51±3.41 U/ml) and GSH (512.50±73.54 and 628.29 ng/l±26.93 ng/l). Compared with the CCl4 group, the LCB pretreated (5 and 25 mg/kg) groups showed a significant ameliorative effect by reducing the MDA levels (10.26±0.16 and 9.03 nmol/mll±0.04 nmol/ml). Also, compared with CCl4 group, the pretreated group with 25 mg/kg LCB showed significant ameliorative effect by reducing the GSSG levels (9.13 nmol/ml±0.53 nmol/ml).
LCB attenuated inflammation of hepatotoxicity induced by CCl4
Inflammation is an important mechanism under-lying hepatotoxicity. The presence of inflammatory cytokines (IL-6, CRP, and TNF-α) associated with hepatotoxicity was determined in the serum to identify the possible mechanisms underlying hepatoprotective activity of LCB. As shown in Table 3, the content of IL-6 in the LCB-pretreated group (5 and 25 mg/kg) was significantly lower than that in the CCl4 group (110.95 pg/ml± 7.11 pg/ml). The activity of TNF-α in the group pretreated with LCB at 5 (386.63 ng/l±20.50 ng/l) and 25 mg/kg (294.63 ng/l±18.03 ng/l) were significantly lower than those in the CCl4 group (496.83 ng/l±24.93 ng/l). The CRP level significantly decreased in the group pretreated with 25 mg/kg LCB (1583.57 µg/l±92.41 μg/l) compared with that in the CCl4 group (2171.51 µg/l±206.39 µg/l).
Table 3.
Effect of LCB on the levels of IL-6, TNF-α, and CRP in mice subjected to CCl4-induced hepatotoxicity (values are presented as means±SD, n = 8)
| Groups | IL-6 (pg/ml) | TNF-α (ng/l) | CRP (μg/l |
|---|---|---|---|
| Control | 72.52 ± 3.14 | 266.06 ± 18.46 | 1079.07 ± 109.65 |
| CCl4 | 110.95 ± 7.11## | 469.83 ± 24.93## | 2171.51 ± 206.39## |
| 1 mg/kg LCB | 108.80 ± 2.20 | 419.63 ± 41.88 | 1958.57 ± 61.03 |
| 5 mg/kg LCB | 93.55 ± 0.93* | 369.63 ± 26.50 | 1858.57 ± 132.53** |
| 25 mg/kg LCB | 82.60 ± 1.09** | 294.63 ± 18.03** | 1583.57 ± 92.41** |
P<0.01 compared with the normal control group,
P<0.05 and
P<0.01
Determination of histopathological parameters
Histological profile of the liver sections of control animals showed a normal hepatic architecture with well-preserved cytoplasm, prominent nucleus, central vein (CV), and compact arrangement of hepatocytes without fatty lobulation (Figure 1A). Liver sections of the CCl4-treated animals showed hydropic changes in centrilobular hepatocytes with cell necrosis surrounded by neutrophils. Congestion of the CV and sinusoids was observed, along with inflammatory cells infiltrating sinusoids mainly in the central zone (Figure 1B). Liver sections of the mice administered with 1 mg/kg LCB similarly showed hydropic changes in centrilobular hepatocytes with cell necrosis surrounded by neutrophils, and inflammatory cells infiltrating sinusoids mainly in the central zone (Figure 1C). Liver sections of the mice administered with 5 mg/kg LCB showed the absence of necrosis and mild inflammatory cells (Figure 1D). The animals administered with 25 mg/kg LCB exhibited significant liver protection against CCl4-induced liver damage, as evidenced by the presence of hepatic cords and the absence of inflammatory cells and necrosis (Figure 1E).
Figure 1.
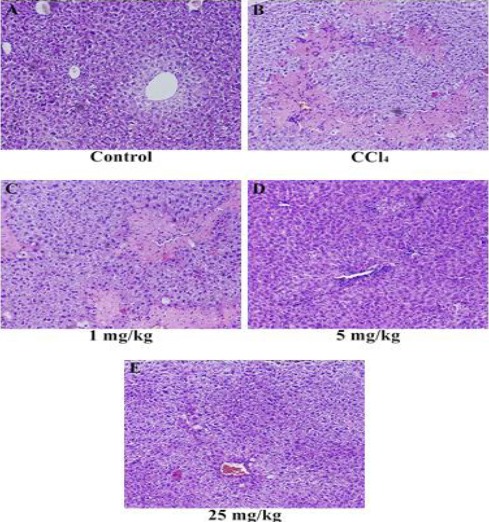
Histopathology of liver tissues. (A) Liver section of normal control mice, showing normal architecture, (B) liver section of CCl4-treated mice, showing massive inflammatory cells and cellular necrosis, (C) liver section of mice treated with CCl4 and 1 mg/kg LCB, showing massive inflammatory cells and cellular necrosis, (D) liver section of mice treated with CCl4 and 5 mg/kg LCB, showing absence of necrosis and mild inflammatory cells, and (E) liver section of mice treated with CCl4 and 25 mg/kg LCB, showing the absence of inflammatory cells and absence of necrosis
The effects of LCB on the protein expression of NF-κB and p38 in liver tissue
The protein expression of NF-κB and p38 in liver tissue was measured by Western blot and ELISA analysis. As shown in Figure 2, compare with control group, the increase in NF-κB protein expression was more significant in the CCl4 group. Compare with CCl4 group, 5 mg/kg and 25 mg/kg of LCB treatment significantly decreased the relative levels of NF-κB. Compare with control group, the p38 protein expression was significantly elevated. However, 5 mg/kg and 25 mg/kg LCB treatment significantly decreased the relative levels of p38.
Figure 2.
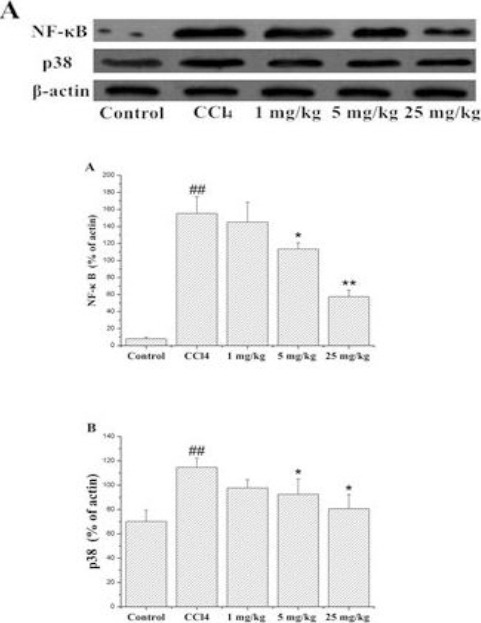
(A) p38 MAPK and NF-κB expression in Western blot. (B) Expression of NF-κB in liver tissue by Western blot analysis. (C) Expression of p38 MAPK in liver tissue by Western blot analysis
##P<0.01 compared with the control group; * P<0.05, ** P<0.01 compared with CCl4 group
Discussion
In this study, LCB could protect the liver in the mice model of CCl4-induced liver toxicity through decrease in MDA, IL-6, CRP, GSSG, and TNF-α levels and increase in the SOD and GSH activity. Thus, the hepatoprotection of LCB may be attributed to its antioxidant and anti-inflammatory activities; in addition, p38 and NF-κB may perform an important function in the hepatoprotective effect of LCB.
CCl4 is a well-known hepatotoxic agent, which is widely used to induce toxic liver injury and to study cellular mechanisms behind oxidative damages in laboratory animals (16). CCl4-induced liver damage has been studied in mice and rats, and findings show a significant elevation of the serum aminotransferase (e.g., AST and ALT) levels (17, 18). CCl4-induced hepatic lesions are characterized by coagulation necrosis and hepatocyte vacuolation, which is mainly situated in the central to middle portion of the hepatic lobules (19). In the present study, a significant elevation in the levels of serum marker enzymes (e.g., AST and ALT) is observed among the animals treated with CCl4. Administration of LCB reduced the toxic effect of CCl4 by restoring the levels of serum marker enzymes to normalcy. The HE staining demonstrated that LCB pretreatment attenuates liver damage in mice upon CCl4 administration. All these results demonstrate the protective effects of LCB on mice liver against CCl4.
A widely accepted assumption is that oxidative damage is the main causes of CCl4-induced acute liver injury. Therefore, anti-oxidative therapy is an effective means of preventing and attenuating oxidative stress-related liver diseases (20). Antioxidant enzymes, such as SOD and GSH, perform important functions in defense mechanisms against the harmful effects of ROS and free radicals in biological systems. In addition, the increased levels of MDA and GSSG in the liver tissue homogenate of mice treated with CCl4 reflect lipid peroxidation and damage to plasma membrane (21, 22) as consequences of oxidative stress. Our previous research demonstrated the anti-oxidative effects of LCB (14). Based on this information, we hypothesize that LCB can attenuate the liver damage induced by CCl4. In the present study, we found that LCB exhibits unique antioxidant properties in vitro. Our results also show that LCB treatment increases SOD and GSH levels and decreases MDA and GSSG levels back to their normal levels.
CCl4 can also indirectly activate the Kupffer cells, which mediate the hepatic inflammation process by producing certain cytotoxic cytokines (e.g., IL-6 and TNF-α) (23, 24). The NF-κB and p38 pathways are all associated with the production of these inflammatory factors in the liver after CCl4 treatment (25, 26). To explore the detailed mechanisms underlying the protective effect of LCB on hepatoprotective activation, we measured the expression of two proteins associated with inflammation (27). In the present study, the results of western blot analysis showed that CCl4 treatment enhances p38 and NF-κB expression. However, LCB treatment decreases p38 and NF-κB expression. Moreover, CCl4-induced liver injury increases CRP, IL-6, and TNF-α production in the serum, but LCB treatment reduces the concentrations of these cytokines. Therefore, LCB possibly exerts its anti-inflammatory effects by inhibiting NF-κB and p38 expression.
Conclusion
In this study, LCB exhibited hepatoprotective activity in CCl4-induced hepatotoxicity in mice. Apart from antioxidative action, LCB may exert an anti-inflammatory effect by mediating the NF-κB and p38 pathways. In the future, LCB may be developed as a drug with antioxidant, antifibrotic, immunomodulatory, antiviral, and regenerative properties for use in human liver diseases.
Acknowledgment
We thank Weihai Municipal Hospital for the support and all the researches who participated in the studies.
Conflict of interest
The authors declare they have no conflict of interests.
References
- 1.Rabinowich L, Shibolet O. Drug Induced Steatohepatitis:An Uncommon Culprit of a Common Disease. Biomed Res Int. 2015;2015:168905. doi: 10.1155/2015/168905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin CS, Chang CS, Yang SS, Yeh HZ, Lin CW. Retrospective evaluation of serum markers APRI and AST/ALT for assessing liver fibrosis and cirrhosis in chronic hepatitis B and C patients with hepatocellular carcinoma. Intern Med. 2008;47:569–575. doi: 10.2169/internalmedicine.47.0595. [DOI] [PubMed] [Google Scholar]
- 3.Krithika R, Jyothilakshmi V, Prashantha K, Verma RJ. Mechanism of protective effect of phyllanthin against carbon tetrachloride-induced hepatotoxicity and experimental liver fibrosis in mice. Toxicol Mech Methods. 2015;4:1–10. doi: 10.3109/15376516.2015.1077361. [DOI] [PubMed] [Google Scholar]
- 4.Tong J, Yao X, Zeng H, Zhou G, Chen Y, Ma B, et al. Hepatoprotective activity of flavonoids from Cichorium glandulosum seeds in vitro and in vivo carbon tetrachloride-induced hepatotoxicity. J Ethnopharmacol. 2015;174:355–263. doi: 10.1016/j.jep.2015.08.045. [DOI] [PubMed] [Google Scholar]
- 5.Moghadam AR, Tutunchi S, Namvaran-Abbas-Abad A, Yazdi M, Bonyadi F, Mohajeri D, et al. Pre-administration of turmeric prevents methotrexate-induced liver toxicity and oxidative stress. BMC Complement Altern Med. 2015;15:246. doi: 10.1186/s12906-015-0773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lv D, Zhu CQ, Liu L. Sesamin ameliorates oxidative liver injury induced by carbon tetrachloride in rat. Int J Clin Exp Pathol. 2015;8:5733–5738. [PMC free article] [PubMed] [Google Scholar]
- 7.Lakshman MR, Reyes-Gordillo K, Varatharajalu R, Arellanes-Robledo J, Leckey LC, Garige M, et al. Novel modulators of hepatosteatosis, inflammation and fibrogenesis. Hepatol Int. 2014;2:413–420. doi: 10.1007/s12072-014-9526-8. [DOI] [PubMed] [Google Scholar]
- 8.Jiang X, Guo H, Shen T, Tang X, Yang Y, Ling W. Cyanidin-3-O-β-glucoside purified from black rice protects mice against hepatic fibrosis induced by carbon tetrachloride via inhibiting hepatic stellate cell activation. J Agric Food Chem. 2015;63:6221–6230. doi: 10.1021/acs.jafc.5b02181. [DOI] [PubMed] [Google Scholar]
- 9.Lin JC, Peng YJ, Wang SY, Young TH, Salter DM, Lee HS. Role of the sympathetic nervous system in carbon tetrachloride-induced hepatotoxicity and systemic inflammation. PLoS One. 2015;10:e0121365. doi: 10.1371/journal.pone.0121365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocha SW, de França ME, Rodrigues GB, Barbosa KP, Nunes AK, Pastor AF, et al. Diethylcarbamazine reduces chronic inflammation and fibrosis in carbon tetrachloride- (CCl₄-) induced liver injury in mice. Mediators Inflamm. 2014;2014:696383. doi: 10.1155/2014/696383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rezende TP, do A Corrêa JO, Aarestrup BJ, Aarestrup FM, de Sousa OV, da Silva Filho AA. Protective effects of Baccharis dracunculifolia leaves extract against carbon tetrachloride- and acetaminophen-induced hepatotoxicity in experimental animals. Molecules. 2014;19:9257–9272. doi: 10.3390/molecules19079257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnappa P, Venkatarangaiah K, Venkatesh Shivamogga Rajanna SK, Kashi Prakash Gupta R. Antioxidant and prophylactic effects of Delonix elata L. stem bark extracts, and flavonoid isolated quercetin against carbon tetrachloride-induced hepatotoxicity in rats. Biomed Res Int. 2014;2014:507851. doi: 10.1155/2014/507851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu Y, Chen J, Li YJ, Zheng YF, Li P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013;141:1063–1071. doi: 10.1016/j.foodchem.2013.03.089. [DOI] [PubMed] [Google Scholar]
- 14.Han J, Wang D, Yu B, Wang Y, Ren H, Zhang B, et al. Cardioprotection against ischemia/reperfusion by LCB in isolated rat hearts. Oxid Med Cell Longev. 2014;2014:134862. doi: 10.1155/2014/134862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu H, Zheng L, Yin L, Xu L, Qi Y, Han X, et al. Protective effects of the total saponins from Dioscorea nipponica Makino against carbon tetrachloride-induced liver injury in mice through suppression of apoptosis and inflammation. Int Immunopharmacol. 2014;19:233–244. doi: 10.1016/j.intimp.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Girish C, Pradhan SC. Hepatoprotective activities of picroliv, curcumin, and ellagic acid compared to silymarin on carbon-tetrachloride-induced liver toxicity in mice. J Pharmacol Pharmacother. 2012;3:149–155. doi: 10.4103/0976-500X.95515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai Z, Lou Q, Wang F, Li E, Sun J, Fang H1, et al. N-acetylcysteine protects against liver injure induced by carbon tetrachloride via activation of the Nrf2/HO-1 pathway. Int J Clin Exp Pathol. 2015;8:8655–8662. [PMC free article] [PubMed] [Google Scholar]
- 18.Yang H, Sung SH, Kim YC. The ethanolic extract of Juglans sinensis leaves and twigs attenuates CCl4-induced hepatic oxidative stress in rats. Pharmacogn Mag. 2015;11:533–539. doi: 10.4103/0973-1296.160463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdel-Moneim AM, Al-Kahtani MA, El-Kersh MA, Al-Omair MA. Free Radical-Scavenging, Anti-Inflammatory/Anti-Fibrotic and Hepatoprotective Actions of Taurine and Silymarin against CCl4 Induced Rat Liver Damage. PLoS One. 2015;10:e0144509. doi: 10.1371/journal.pone.0144509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, He Y, Yu H, Ma F, Wu J, Zhang X. Liquiritigenin Protects rats from carbon tetrachloride induced hepatic injury through PGC-1αpathway. Evid Based Complement Alternat Med. 2015;2015:649568. doi: 10.1155/2015/649568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan KW, Ho WS. Anti-oxidative and hepatoprotective effects of lithospermic acid against carbon tetrachloride-induced liver oxidative damage in vitro and in vivo. Oncol Rep. 2015;34:673–680. doi: 10.3892/or.2015.4068. [DOI] [PubMed] [Google Scholar]
- 22.Sagor AT, Chowdhury MR, Tabassum N, Hossain H, Rahman MM, Alam MA. Supplementation of fresh ucche (Momordica charantia L. var. muricata Willd) prevented oxidative stress, fibrosis and hepatic damage in CCl4 treated rats. BMC Complement Altern Med. 2015;15:115. doi: 10.1186/s12906-015-0636-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatterjee N, Das S, Bose D, Banerjee S, Jha T, Saha KD. Leishmanial lipid affords protection against oxidative stress induced hepatic injury by regulating inflammatory mediators and confining apoptosis progress. Toxicol Lett. 2015;232:499–512. doi: 10.1016/j.toxlet.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Lan T, Kisseleva T, Brenner DA. Deficiency of NOX1 or NOX4 prevents liver inflammation and fibrosis in mice through inhibition of hepatic stellate cell activation. PLoS One. 2015;10:e0129743. doi: 10.1371/journal.pone.0129743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, Liu S, DU T, Chen H, Li Z, Yan J. NF-κB inhibition alleviates carbon tetrachloride-induced liver fibrosis via suppression of activated hepatic stellate cells. Exp Ther Med. 2014;8:95–99. doi: 10.3892/etm.2014.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yue S, Hu B, Wang Z, Yue Z, Wang F, Zhao Y, et al. Salvia miltiorrhiza compounds protect the liver from acute injury by regulation of p38 and NFκB signaling in Kupffer cells. Pharm Biol. 2014;52:1278–1285. doi: 10.3109/13880209.2014.889720. [DOI] [PubMed] [Google Scholar]
- 27.Ma JQ, Ding J, Zhang L, Liu CM. Ursolic acid protects mouse liver against CCl4-induced oxidative stress and inflammation by the MAPK/NF-κB pathway. Environ Toxicol Pharmacol. 2014;37:975–983. doi: 10.1016/j.etap.2014.03.011. [DOI] [PubMed] [Google Scholar]


