Abstract
Effective pulp-capping materials must have antibacterial properties and induce dentin bridge formation; however, many current materials do not satisfy clinical requirements. Accordingly, the effects of an experiment pulp-capping material (Exp) composed of an antibacterial resin monomer (MAE-DB) and Portland cement (PC) on the viability, adhesion, migration, and differentiation of human dental pulp stem cells (hDPSCs) were examined. Based on a Cell Counting Kit-8 assay, hDPSCs exposed to Exp extracts showed limited viability at 24 and 48 h, but displayed comparable viability to the control at 72 h. hDPSC treatment with Exp extracts enhanced cellular adhesion and migration according to in vitro scratch wound healing and Transwell migration assays. Exp significantly upregulated the expression of osteogenesis-related genes. The hDPSCs cultured with Exp exhibited higher ALP activity and calcium deposition in vitro compared with the control group. The novel material showed comparable cytocompatibility to control cells and promoted the adhesion, migration, and osteogenic differentiation of hDPSCs, indicating excellent biocompatibility. This new direct pulp-capping material containing MAE-DB and PC shows promise as a potential alternative to conventional materials for direct pulp capping.
Dental pulp is occasionally exposed by dental caries, trauma, or iatrogenic injury in clinical practice. Untreated pulp exposure can cause pulp necrosis in the case of bacterial infections, and can undermine the integrity of the tooth structure. Additionally, dental tissues under stress are associated with a high risk of tooth fracture. Pulp capping, including direct and indirect pulp capping, in which biocompatible and bioregenerative dental materials are used to seal the pulpal wound, facilitates the formation of a dentin bridge and maximises pulp preservation, while offering an alternative to root canal therapy1,2. A number of pulp-capping materials have been introduced. Historically, calcium hydroxide was the standard direct pulp-capping material because it facilitates the formation of a dentinal bridge over the pulp wound area3. However, it has many known disadvantages, such as a high solubility, a lack of adhesive qualities, degradation after acid etching, and the presence of tunnels in the dentinal bridge, which allow the pulp to become infected or necrotic over time; thus, it cannot prevent the pulp from continued bacterial penetration owing to its poor sealing ability4,5.
Calcium silicate-based cements, such as mineral trioxide aggregate (MTA), Biodentine, and Portland cement (PC), have been studied for applications to direct pulp capping2,6,7. MTA is an alternative to Ca(OH)2 that achieves the same results in clinical procedures and can induce a significantly greater frequency of dentinal bridge formation, bridges with greater mean thicknesses and less pulp inflammation8,9,10. However, MTA has some disadvantages, such as a prolonged setting time, high cost, potential for discolouration, poor handling characteristics, and inadequate antibacterial effects11,12. Many studies have focused on PC, which is compositionally similar to MTA, but less expensive. Several studies have suggested that PC is biocompatible and can be used as a safe pulp-capping material, and many PC-based materials have been investigated to meet clinical requirements13,14.
Although contemporary pulp-capping materials have demonstrated various benefits, microorganisms are one of the main reasons for failure15. Accordingly, it is necessary to develop a material that can effectively limit bacterial infection and induce dentin bridge formation11. In our previous study, we synthesised a resin-based direct pulp-capping material that contained the quaternary ammonium salt monomer MAE-DB and PC16. This novel material showed constant contact inhibition against Streptococcus mutans in vitro, even after 6 months in water16. We speculated that the Ca and Si from PC might confer favourable properties and bioactivity for pulp healing, similar to MTA17,18,19.
The cell viability, adhesion, migration, and differentiation of human dental pulp stem cells (hDPSCs) have been implicated in the healing response to pulp injury process20. Thus, in the present study, we investigated the effect of the experimental direct pulp-capping material containing MAE-DB and PC (Exp) on hDPSCs in terms of cell viability, migration, adhesion, and osteogenic differentiation.
Methods
Reagents
The following reagents were used in the study: 2-hydroxyethylmethacrylate (HEMA, Sigma–Aldrich, St. Louis, MO, USA), bisphenol glycerolate dimethacrylate (BisGMA; Esstech, Essington, PA, USA), triethylene glycol dimethacrylate (TEGDMA, Esstech), photoinitiator campho9rquinone (CQ, Sigma-Aldrich), ethyl 4-(dimethylamino)benzoate (EDMAB, Sigma-Aldrich), and white PC (P. W. Grade 52.5) provided by the Aalborg Portland Group (Anqing, China). The QAS antibacterial monomer (MAE-DB) was developed by our team.
Sample preparation
The new resin-based composite containing MAE-DB and PC was fabricated according to the methods described in our previous study16. Briefly, a synthesised resin matrix containing HEMA-BisGMA-TEGDMA (mass ratio, 4:3:1) with MAE-DB at 5 wt% was mixed with white PC (P. W. 52.5, Aalborg) at a PC: HEMA-BisGMA-TEGDMA resin mass ratio of 2:1. The resins were photo-cured for 60 s on each side with a light activation unit (QHL75; Dentsply, Tulsa, OK, USA) in a 24-well plate. A cured resin matrix without PC was fabricated for the cell viability assay. White ProRoot MTA (Dentsply) (hereafter referred to as MTA) was used as a commercially available material for comparison; it was prepared according to manufacturer’s instructions on the bottom of a 24-well plate and left to set for 48 h at 37 °C in a humidified 5% CO2, 95% air atmosphere. All of the prepared disks were sterilised with ethylene oxide before the subsequent experiments.
The sample elutes were used for the cell adhesion assay and cell migration assay. The ratio between the surface of the samples and the volume of the medium (α-MEM containing 5% foetal bovine serum [FBS]) was 0.5 cm2/mL, according to ISO standards (10993-5). After incubation at 37 °C for 24 h, the resulting supernatant was sterilised using a 0.22-μm filter before use with the cell cultures and was changed every three days.
Isolation and culture of hDPSCs
Human third molars or premolars extracted from young orthodontic patients (18–19 years old) after obtaining informed consent were collected for the further study. All the experimental protocols were reviewed and approved by the Institutional Review Board of Stomatological Hospital of Fourth Military Medical University (approval number: IRB-REV-2015036; Supplementary information). Methods were carried out in accordance with relevant guidelines and regulations. Briefly, freshly extracted teeth were washed with phosphate-buffered saline (PBS) supplemented with antibiotics. Dental pulp tissues were separated and minced into 1 mm2 fragments, followed by digestion with a solution of 3 mg/mL type I collagenase with 4 mg/mL dispase (Sigma) for 45–60 min at 37 °C. Single-cell suspensions were obtained by passing the cells through a 70-μm cell strainer followed by incubation in α-MEM supplemented with 20% FBS (HyClone, Logan, UT, USA), 100 units/mL penicillin G, 100 mg/mL streptomycin, and 50 mg/mL ascorbic acid (Sigma) at 37 °C in 5% CO2. Flow cytometry was used to characterise hDPSCs. The cells between the third and fifth passages were used in the subsequent analysis.
Flow cytometry assay
The cultured cells were identified based on the surface antigens of hDPSCs using a flow cytometry method. Cells were trypsinised and incubated in PBS containing 0.1% FBS for 45 min with fluorescein-conjugated monoclonal antibodies against CD29, CD34, CD45, CD90, and CD105 (BD Biosciences, San Jose, CA, USA). The flow cytometry test was performed using a flow cytometer (FACSCalibur; BD Biosciences).
Cell viability assay
Cell viability was measured using the Cell Counting Kit-8 (7Sea Biotech, Shanghai, China) according to the manufacturer’s protocol. Briefly, hDPSCs were plated at a density of 1 × 104 cells per well and a volume of 100 μL in 96-well plates. The cells were starved for 24 h with serum-free medium at 37 °C in a humidified 5% CO2 atmosphere before the test. Then the serum-free medium was replaced with different material elutes. Cells cultured in α-MEM medium containing 10% FBS served as a blank control. At each time point (24, 48, and 72 h after seeding cells), 10 μL of CCK8 assay solution was added to each well and incubated for 4 h at 37 °C. Cell number and viability were calculated by measuring absorbance at a wavelength of 450 nm on a multi-plate reader (BIO-TEK, Winooski, VT, USA). The experiments were performed in triplicate.
Adhesion assay
Type I collagen (40 mg/L in PBS; Corning, Discovery Labware, Inc., Bedford, MA, USA) was added to 24-well plates and placed at 4 °C overnight. Subsequently, unbound collagen was removed using PBS and the nonspecific binding sites were blocked with 1% bovine serum albumin (BSA) at 37 °C in a 5% CO2 atmosphere for 1 h. hDPSCs (1 × 104 cells/mL) were resuspended in 100 μL of elute prepared with serum-free α-MEM and were seeded on the wells. After incubation at 37 °C in a 5% CO2 atmosphere for 1 h, non-adherent cells were rinsed with PBS and adherent cells were fixed in 4% formaldehyde for 10 min and stained with 0.5% toluidine blue for 10 min. An inverted phase contrast microscope (Olympus, Tokyo, Japan) was used to obtain images and the wells were then washed with PBS and supplemented with 33% acetic acid (v/v). The optical density (OD) was determined using a microplate reader (BIO-TEK) at an absorbance of 595 nm.
Migration assay
Transwell migration assay
Cell migration was assessed using a two-chamber Transwell system (8 mm pore size and 6.5 mm diameter). hDPSCs (1 × 104 cells/mL) suspended in 100 μL of serum free α-MEM were planted in the top chamber of the Transwell, and 500 μL of the eluate prepared with serum-free α-MEM was added to the lower chambers. After incubation at 37 °C in 5% CO2 for 24 h, filters were removed with sterile tweezers and cells that did not migrate through the filter were gently wiped with a cotton swab. Migrating cells beneath the filter were fixed with 4% paraformaldehyde for 15 min and stained with crystal violet for 20 min. The filters were observed using an inverted microscope (Olympus). The numbers of the migrating cells in each well were counted in 6 random microscopic fields per filter at 200× magnification. The experiments were performed in triplicate independently.
Wound healing assay
Cell migration was also assessed using an in vitro scratch wound healing assay. hDPSCs were cultured with α-MEM supplement with 10% FBS until 100% confluence in 6-well plates. Then, the cells were starved overnight with serum-free α-MEM and a scratch was made using a sterile 1-mL pipette tip. The cell debris was removed with PBS and cells were cultured with elutes for 24 h. An inverted microscope was used to observe cell migration.
In vitro osteogenic differentiation assay
hDPSCs were co-cultured with various material extracts and serum-free α-MEM. Normal culture medium containing α-MEM supplemented with 10% FBS was used as blank control medium (Control/N). Cells cultured with osteogenic differentiation medium were used as a positive control (Control/M). Control/M consisted of the aforementioned normal culture medium supplemented with 50 mg/mL ascorbic acid, 10 mmol/L beta-glycerophosphate, and 10 nmol/L dexamethasone (all from Sigma). The effects of MTA and PC on hDPSC osteogenic differentiation were evaluated.
Alizarin Red staining and quantification
Approximately 1 × 105 hDPSCs were cultured with the elutes supplemented with serum-free α-MEM in 6-well plates for 2 weeks. The cells were washed with PBS and fixed with dehydrated ethanol for 20 min. Cells were stained using the Alizarin Red S Kit (Leagene Biotechnology, Beijing, China) according to the manufacturer’s instructions for 20 min and then washed with H2O 5 times. To quantify Alizarin Red staining, the stained cells were incubated in 10% acetic acid for 30 min and absorbance was measured at 405 nm with a microplate reader.
Alkaline phosphatase staining and quantification
For alkaline phosphatase (ALP) staining, approximately 1 × 105 hDPSCs were seeded in 6-well plates. After reaching 80% confluence, the cells were divided into five groups (MTA, PC, Exp, Control/N, and Control/M). After 9 days of cultivation, wells were washed twice with PBS and fixed with 4% polyoxymethylene for 30 min at room temperature. An ALP Staining Kit (Jiancheng Bioengineering Institute, Jiangsu, China) was used according to the manufacturer’s instructions. After staining for 30 min at room temperature, cells were observed under a light microscope. To quantify ALP activity, hDPSCs were plated in 96-well plates at a density of 1 × 104 hDPSCs/well and incubated overnight in 5% CO2 at 37 °C. After incubation in media without FBS for 24 h, the samples were then divided into the 5 groups described above; each group included 5 wells and media was changed every 3 days. On days 3, 5, 7, and 9, the ALP activity of each well was assayed with an ALP Kit (Jiancheng Bioengineering Institute) according to the manufacturer’s instructions. Absorbance was measured at 520 nm with a microplate reader.
Quantitative real-time polymerase chain reaction
Osteogenic potential was examined using the bone-associated markers ALP, osteonectin (ON), osteocalcin (OCN), dentin matrix protein 1 (DMP-1), and dentin sialophosphoprotein (DSPP) using qRT-PCR. hDPSCs (1 × 105) were seeded in 6-well plates and divided into five groups. After incubation for 2 weeks, the total RNA was extracted and reverse transcribed into complementary DNA with reverse transcriptase using the TaKaRa MiniBEST Universal RNA Extraction Kit and PrimeScript™ RT Master Mix (Perfect Real Time, Takara, Otsu, Japan) according to the manufacturers’ protocols. Subsequently, PCR amplification was performed with SYBR® Premix Ex Taq™ (Tli RNaseH Plus) (Takara) using the ABI 7500 Thermal Cycler (Applied Biosystems, Foster City, CA, USA). The PCR primers are shown in Table 1. The gene expression levels were normalised to the β-actin mRNA level and averaged from triplicate samples.
Table 1. Primer sequences used for qRT-PCR analysis.
| Gene | Code | Forward primer sequences | Reverse primer sequences |
|---|---|---|---|
| ALP | NM 000478 | CATGCTGAGTGACACAGACAAGAA | ACAGCAGACTGCGCCTGGTA |
| ON | MM 182120 | GAAGGCCAAAATCAAGAGTGAGA | AAAAGGGGAGGGTGAAGAAAAG |
| OCN | NM 199173 | GACGAGTTGGCTGACCACA | CAAGGGGAAGAGGAAAGAAGG |
| DMP-1 | NM 004407 | ACTGTGGAGTGACACCAGAACACA | AGCTGCAAAGTTATCATGCAGATCC |
| DSPP | NM 014208 | GCATTTGGGCAGTAGCATGG | CTGACACATTTGATCTTGCTAGGAG |
| β-actin | NM 001101.3 | CATGGATGATGATATCGCCGCG | ACATGATCTGGGTCATCTTCTCG |
Statistical analysis
The data are expressed as means ± standard deviations of three independent experiments performed in triplicate. We preformed normal distribution test and homogeneity test for error variance and the results showed that the distribution of the dates in this study were all alone to normal distribution after test of normality. The p values of homogeneity test are all greater than 0.05. Thus One-way ANOVA and LSD post-hoc tests were used to determine differences among groups using SPSS 19.0. Statistically significant differences were accepted at P < 0.05.
Results
Characterisation of hDPSCs
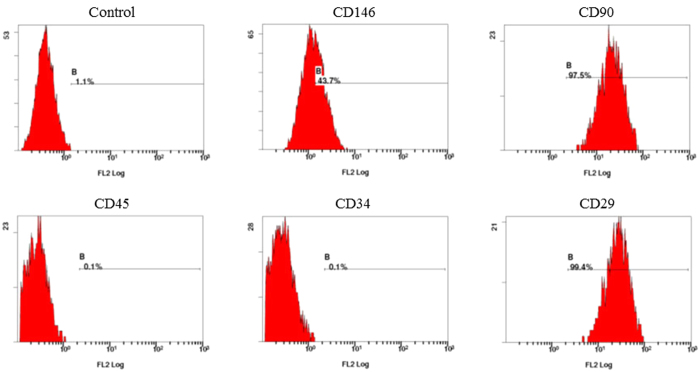
Based on flow cytometry, cells exhibited high expression of mesenchymal surface molecular markers (CD29, 99.4%; CD146, 43.7%; CD90, 97.5%) and low expression of surface makers for hematopoietic system-derived cells (CD34, 0.1%; CD45, 0.1%) (Fig. 1).
Figure 1. Flow cytometry of mesenchymal-related antigens in human dental pulp stem cells (hDPSCs).
Representative diagrams are given for the negative control, CD146, CD90, CD45, CD34, and CD29 expression.
Cell viability
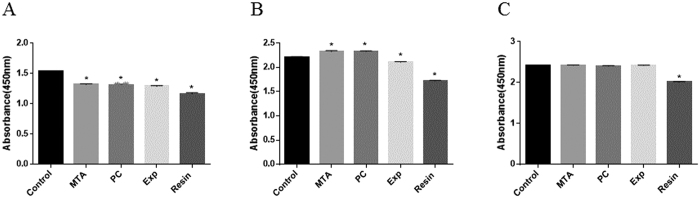
Using the CCK-8 assay, the viability of hDPSCs exposed to various material extracts was examined at 24 h, 48 h, and 72 h. As shown in Fig. 2, the viabilities of cells exposed to MTA, PC, Exp, and Resin were significantly lower than that of the negative control group (P < 0.001) at 24 h, and no significant differences were found among the MTA, PC, and Exp groups (P > 0.05). After 48 h of incubation, cells exposed to Exp showed lower viability than the control group (P < 0.001), while viability was higher in the MTA and PC groups than the control (P < 0.001). The Resin group showed significantly lower cell viability than all other groups. (P < 0.001). After 72 h, there were no significant differences among the MTA, PC, Exp, and control groups (P > 0.05), all of which had higher viabilities than that of the Resin group (P < 0.001).
Figure 2.
Results of cell viability assays after hDPSCs were exposed to various material extracts at 24 h (A), 48 h (B), and 72 h (C). The viability of cells under treatments with different material extracts was investigated using the Cell Counting Kit-8. Data are shown as means ± standard deviation of three independent experiments. *P < 0.05 represents a significant change compared with the control.
Cell Adhesion
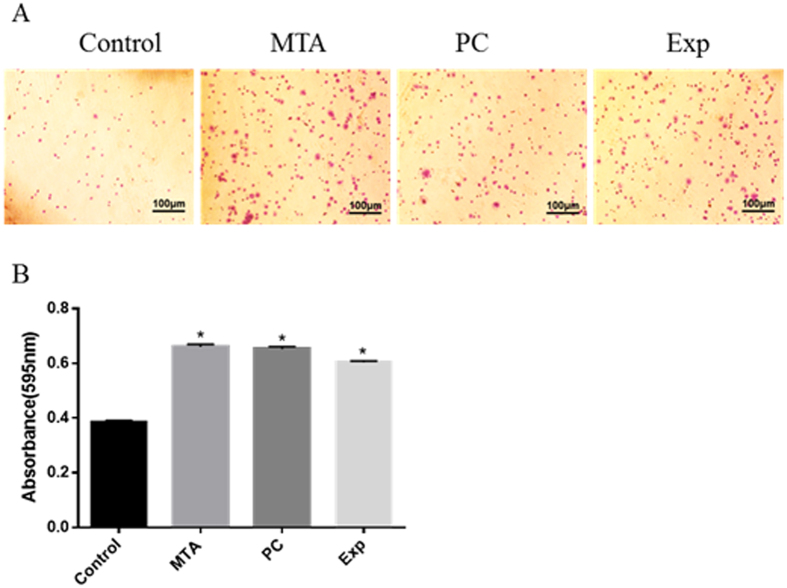
As shown in Fig. 3, considerably more adherent cells were detected for the Exp group than the control. There were no significant differences among the MTA, PC, and Exp groups (P > 0.05).
Figure 3. The effect of material extracts on adhesion ability in hDPSCs.
Cells were incubated with various material extracts for 1 h. Adherent cells were fixed and stained. The coloured solution was quantified at 595 nm on a microplate reader. (A) Representative diagrams for stained cells. (B) Quantification of the adherent cells. Data are presented as means ± standard deviation and measurements were performed in triplicate, with results summarised as the mean for each experiment. *P < 0.05 represents a significant change compared with the control.
Cell Migration
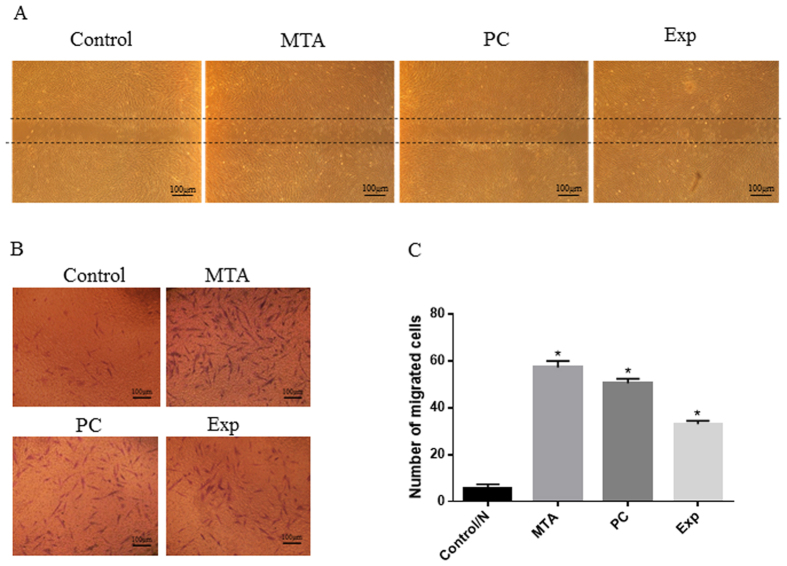
In order to determine the effect of the extracts on the motility of hDPSCs, a wound-healing assay and a two-chamber Transwell assay were performed. As shown in Fig. 4A, Exp shortened the migration distance compared with the control group. Based on the Transwell assay, the numbers of migrated cells in the MTA, Exp, and PC groups were significantly higher than that of the control (P < 0.001), while no detectable differences were observed among the MTA, PC, and Exp groups (P > 0.05) (Fig. 4B,C). These results indicated that Exp had a positive effect on the migration ability of hDPSCs.
Figure 4. Effects of material extracts on migration in hDPSCs.
(A) Wound-healing assay. Cells were incubated with different material extracts for 24 h. Microphotographs of the scratches were obtained at 24-h post-wounding. (B) Cell migration assays were performed using a two-chamber Transwell system. Cells were treated with different material extracts for 24 h, and the migrated cells were fixed and stained. Representative photos of migrated hDPSCs were observed under a phase-contrast microscope. (C) Quantification of migrated cells. Data are presented as means ± standard deviation of three independent experiments. *P < 0.05 represents a significant change compared with the control.
In vitro osteogenic differentiation assay
Alizarin Red staining and quantification
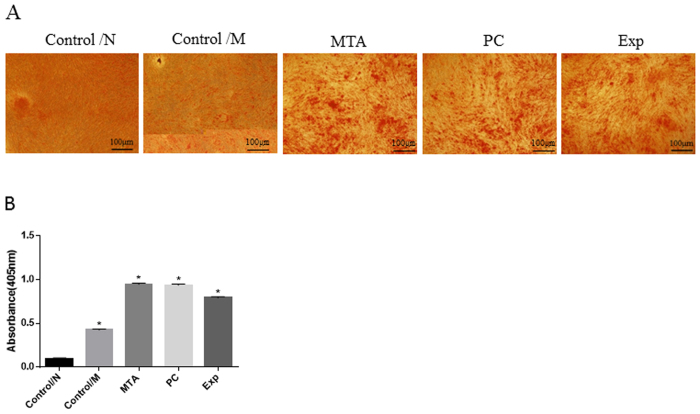
Mineral nodule formation is often used to assay the differentiation of hDPSCs and Alizarin Red staining is an indicator of mineralized-like nodules. Figure 5 shows representative images of calcium deposits produced by hDPSCs in each group. The least calcium deposition was observed for hDPSCs in the Control/N group (P < 0.001). Compared with the Control/M group, Exp significantly promoted calcium deposition in hDPSCs (P < 0.001), and more calcium deposition was detected for the MTA and PC groups compared with the other groups (P < 0.001).
Figure 5. Alizarin Red staining and quantification.
hDPSCs were co-cultured with different material extracts supplemented with serum-free α-MEM for 2 weeks. Normal culture medium containing α-MEM supplemented with 10% foetal bovine serum was used as blank control medium (Control/N). Cells cultured with osteogenic differentiation medium were used as positive controls (Control/M). (A) Mineralised nodules formed by differentiated cells after incubation were stained with Alizarin Red S. (B) Quantitative measurements of Alizarin Red S staining of hDPSCs. The results are expressed as means ± standard deviation and *P < 0.05 represents a significant difference compared with the control/N group.
Alkaline phosphatase staining and quantification
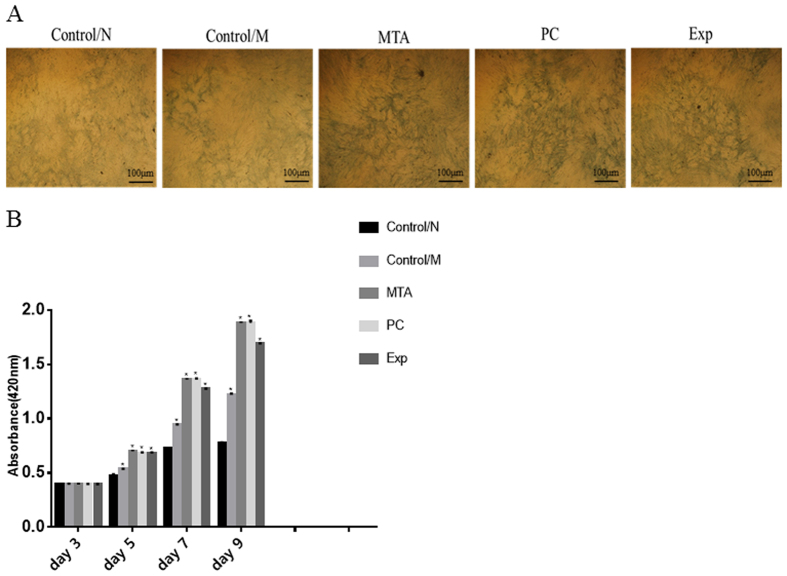
Results of ALP staining are shown in Fig. 6. After 2 weeks of mineralization induction, compared with the control/M group and the control/N group, Exp, MTA, and PC induced positive ALP staining (Fig. 6A). The ALP activity of hDPSCs on days 3, 5, 7, and 9 is indicated in Fig. 6B. The ALP activity of all groups increased until day 9. Compared with the control/M and control/N groups, treatment with Exp markedly increased ALP activity (P < 0.001), and treatment with MTA and PC had the greatest effect on ALP activity at day 9 (P < 0.001).
Figure 6. Alkaline phosphatase (ALP) staining and quantification.
Cells were co-cultured with different material extracts supplemented with serum-free α-MEM. Normal culture medium containing α-MEM supplemented with 10% FBS was used as blank control medium (Control/N). Cells cultured with osteogenic differentiation medium were used as positive controls (Control/M). ALP staining after 9 days of cultivation was performed using an ALP Staining Kit (A). ALP activity was evaluated at 3 days, 5 days, 7 days, and 9 days using an ALP Kit (B). The results are expressed as means ± standard deviation and *P < 0.05 represents a significant difference compared with the control/N group.
Quantitative real-time polymerase chain reaction
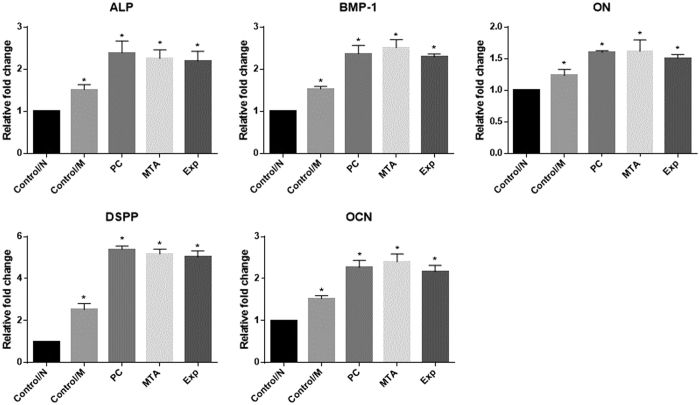
Figure 7 summarises the expression levels of osteogenic differentiation markers in hDPSCs for each group, which were normalised to β-actin. After exposure to the five treatments for 14 days, the hDPSCs exposed to Exp exhibited significantly higher expression levels of OCN, DSPP, DMP1, ON, and ALP mRNA than the control/N and Control/M groups (P < 0.05), while no detectable differences were observed among the MTA, PC, and Exp groups (P > 0.05).
Figure 7. The effects of material extracts on the expression of ALP, BMP-1, ON, DSPP, and OCN in hDPSCs.
Cells were co-cultured with different material extracts supplemented with serum-free α-MEM for 2 weeks. Normal culture medium containing α-MEM supplemented with 10% FBS was used as blank control medium (Control/N). Cells cultured with osteogenic differentiation medium were used as positive controls (Control/M). ALP, BMP-1, ON, DSPP, and OCN mRNA expression levels in hDPSCs were determined by qRT-PCR. The results are expressed as means ± standard deviation and *P < 0.05 represents a significant difference compared with the control/N group.
Discussion
Direct pulp capping is defined as the capping of exposed vital pulp with a bioactive material to maintain dental pulp viability and facilitate the formation of reparative dentin. It offers an alternative conservative treatment approach to root canal therapy2. hDPSCs in dental pulp tissue show the capacity for self-renewal and multilineage differentiation, and are a source of pulp healing21,22,23. When exposed pulp is capped by biocompatible materials, hDPSCs proliferate, migrate to the injured site, and differentiate into odontoblast-like cells, which subsequently deposit reparative dentine22,24. In addition to appropriate physical properties, nontoxicity to pulp tissue, acceptable sealing ability, a short setting time, and excellent handling and antibacterial properties, an ideal pulp-capping material should also demonstrate the ability to stimulate and modulate the healing process induced by hDPSCs25. Although numerous materials have been developed, none fulfils these clinical demands. Exp was created in an attempt to combine the antibacterial properties of MAE-DB with the biological properties of PC. In the present study, its effects on the proliferation, adhesion, migration, and odontogenic differentiation of the hDPSCs were evaluated.
The influence of cytotoxic compounds from pulp-capping materials on cell viability and apoptosis is of great concern26,27. The preservation of pulp vitality following restorative intervention depends on the degree to which pulpal cell populations can survive28. Pulp-capping materials should either promote cell survival and proliferation or be biologically neutral29. In our study, cell viability was significantly reduced when cells were cultured with MTA, PC, and Exp compared with the control group for 24 h, but there were no significant differences among the MTA, PC, Exp, and control group at 72 h, indicating a comparatively good cytocompatibility. The cured resin without PC exhibited significant cytotoxicity at each time point. Resin monomers can induce cell death via an ROS pathway30,31, while MTA can promote cell proliferation at certain concentrations32. Thus, we speculated that the PC constituent in Exp may offset the negative effect induced by resin monomers leached from the material. This phenomenon may also be explained by the increase in resin components in the Resin than the Exp group.
The adhesion and migration behaviours of hDPSCs are fundamental during pulp healing. Accordingly, it is vital to clarify the changes in hDPSCs migration and adhesion with respect to dentin–pulp regeneration when different pulp-capping materials are used33,34. As shown in Figs 3 and 4, Exp promoted cellular migration and adhesion. Our findings are in agreement with previous studies showing that MTA could accelerate the migration and adhesion of dental pulp-derived cells35,36. A migration assay also provided the first in vitro evidence of a similar migration effect of PC on hDPSCs, but Vincenzo et al.37 found that MTA enhances HMSC migration significantly more than PC. Interestingly, in our study, the number of migrated cells in the Exp group was significantly higher than that of the control group, but less than those of the MTA and PC groups. We speculated that the reduced biocompatible could be attributed to the HEMA(2-hydroxyethyl methacrylate) in Exp, which, according to Williams et al.38, inhibits the migration of DPSCs.
Bioactive materials used for pulp tissue healing should enhance the dentinogenic potential of pulp stem cells. In the present study, odontoblastic differentiation-related makers (ALP, DMP-1, DSPP, OCN, and ON) were tracked by qRT-PCR, and intracellular ALP enzyme activity and matrix mineralization were detected using ALP and Alizarin Red staining. ALP is a marker of early odontoblast differentiation and plays a key role in reparative dentin mineralization39. DSPP, composed of two odontoblast-specific proteins, dentin sialoprotein and dentin phosphoprotein, is related to dentinogenesis40. DMP1 regulates collagen matrix organization and promotes dentin matrix mineralization, similar to DSPP41,42. OCN and ON are also thought to be terminal indicators of dentin regeneration43,44,45. The increased and earlier upregulation of these genes suggest that Exp could promote hDPSCs to odontogenic differentiation. These findings are consistent with the increase in calcified nodules in Exp detected by Alizarin Red staining compared with the control group. Thus, we can conclude that Exp enhances the odontogenic potential of hDPSCs, similar to MTA and PC46,47.
Taken together, the results of this study indicate that Exp supports tissue regeneration via the promotion of human pulp stem cell adhesion, migration, and differentiation. These result reflect the similarity in the compositions of Exp and MTA based on PC, which is biocompatible in cultures of several cell lines48,49,50,51. Calcium silicate-based materials, include MTA, PC, and Biodentine, were believed to release Ca ions, which can trigger the recruitment and proliferation of undifferentiated cells from the pulp52 and activate stem cells53. However, these materials lack effective antimicrobial properties54. Torabinejad et al.55 reported that Loma Linda MTA does not exert an inhibitory effect against Enterococcus faecalis, Staphylococcus aureus, or Fusobacterium nucleatum. In a study of GMTA mixed with sterile water, no anti-bacterial effect against E. faecalis was observed55. Another study reported only bacteriostatic effects of MTA against E. faecalis using a direct contact testing protocol56. However, this activity of MTA is limited against some facultative bacteria and has no effect on strictly anaerobic bacteria55.
Recontamination by the restoration of microleakages and residual bacteria in dentin tubes may have adverse effects on pulp healing. The presence of bacteria and their byproducts stimulate pulpal inflammatory activity and reduce the area of dentin bridge formation, irrespective of the material used for pulp capping, and subsequently lead to the failure of pulp capping57,58, even when a dentine bridge forms59. Cox et al.60 have shown that pulp healing is more highly dependent on the capacity of the capping material to prevent bacterial microleakage than on the specific properties of the material itself. In our previous experiments, Exp released comparable amounts of Ca ions as MTA and exhibited effective antibacterial activity throughout the experimental period, which promotes pulp healing.
In summary, the experimental direct pulp-capping material containing MAE-DB and PC showed comparable cytocompatibility on hDPSCs and induced adhesion, migration, and osteogenic differentiation in vitro. Based on its favourable bioactivities and effective antibacterial characteristics, this new direct pulp-capping material shows promise as used for vital pulp therapy. In vivo examinations of its effect on dentine bridge formation are in progress.
Additional Information
How to cite this article: Yu, F. et al. Effect of an Experimental Direct Pulp-capping Material on the Properties and Osteogenic Differentiation of Human Dental Pulp Stem Cells. Sci. Rep. 6, 34713; doi: 10.1038/srep34713 (2016).
Acknowledgments
Supported by grant 81130078, 81470773, 81371187 from National Natural Science Foundation of China, grant 2016JM8086, 2016JQ8008 from Natural Science Foundation of Shaanxi Province and Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13051).
Footnotes
Author Contributions F.Y., Y.D. and Y.-W.Y. performed the experiments and analytical part of the study and wrote the manuscript. X.-F.S. contributed to the flow cytometry test. H.-H.Y., P.-T.L. and H.Z. contributed to the specimen preparation. L.H. and X.S. advised on the experimental design and edited the manuscript. J.-H.C. supervised the project and edited the manuscript. All authors reviewed the manuscript.
References
- Cho S. Y. et al. Prognostic factors for clinical outcomes according to time after direct pulp capping. Journal of endodontics 39, 327–331, doi: 10.1016/j.joen.2012.11.034 (2013). [DOI] [PubMed] [Google Scholar]
- Komabayashi T., Zhu Q., Eberhart R. & Imai Y. Current status of direct pulp-capping materials for permanent teeth. Dental materials journal 35, 1–12, doi: 10.4012/dmj.2015-013 (2016). [DOI] [PubMed] [Google Scholar]
- Farhad A. & Mohammadi Z. Calcium hydroxide: a review. International dental journal 55, 293–301 (2005). [DOI] [PubMed] [Google Scholar]
- Cox C. F., Subay R. K., Ostro E., Suzuki S. & Suzuki S. H. Tunnel defects in dentin bridges: their formation following direct pulp capping. Operative dentistry 21, 4–11 (1996). [PubMed] [Google Scholar]
- Schuurs A. H., Gruythuysen R. J. & Wesselink P. R. Pulp capping with adhesive resin-based composite vs. calcium hydroxide: a review. Endodontics & dental traumatology 16, 240–250 (2000). [DOI] [PubMed] [Google Scholar]
- Kim J. et al. Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry. Restorative dentistry & endodontics 41, 29–36, doi: 10.5395/rde.2016.41.1.29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies S. R. Comparative Performance of Mineral Trioxide Aggregate Versus Calcium Hydroxide as a Direct Pulp Capping Agent. Journal of esthetic and restorative dentistry : official publication of the American Academy of Esthetic Dentistry … [et al.], doi: 10.1111/jerd.12202 (2016). [DOI] [PubMed] [Google Scholar]
- Accorinte Mde L. et al. Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. Journal of endodontics 34, 1–6, doi: 10.1016/j.joen.2007.09.012 (2008). [DOI] [PubMed] [Google Scholar]
- Oguntebi B. R., Heaven T., Clark A. E. & Pink F. E. Quantitative assessment of dentin bridge formation following pulp-capping in miniature swine. Journal of endodontics 21, 79–82, doi: 10.1016/S0099-2399(06)81100-3 (1995). [DOI] [PubMed] [Google Scholar]
- Chacko V. & Kurikose S. Human pulpal response to mineral trioxide aggregate (MTA): a histologic study. The Journal of clinical pediatric dentistry 30, 203–209 (2006). [DOI] [PubMed] [Google Scholar]
- Poggio C. et al. In vitro antibacterial activity of different pulp capping materials. Journal of clinical and experimental dentistry 7, e584–588, doi: 10.4317/jced.52401 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler B. & Rossi-Fedele G. A Review of Tooth Discoloration after Regenerative Endodontic Therapy. Journal of endodontics 42, 563–569, doi: 10.1016/j.joen.2015.12.022 (2016). [DOI] [PubMed] [Google Scholar]
- Yildirim C., Basak F., Akgun O. M., Polat G. G. & Altun C. Clinical and Radiographic Evaluation of the Effectiveness of Formocresol, Mineral Trioxide Aggregate, Portland Cement, and Enamel Matrix Derivative in Primary Teeth Pulpotomies: A Two Year Follow-Up. The Journal of clinical pediatric dentistry 40, 14–20, doi: 10.17796/1053-4628-40.1.14 (2016). [DOI] [PubMed] [Google Scholar]
- Portella F. F. et al. Glycerol Salicylate-based Pulp-Capping Material Containing Portland Cement. Brazilian dental journal 26, 357–362, doi: 10.1590/0103-6440201300218 (2015). [DOI] [PubMed] [Google Scholar]
- Sundqvist G. Ecology of the root canal flora. Journal of endodontics 18, 427–430, doi: 10.1016/S0099-2399(06)80842-3 (1992). [DOI] [PubMed] [Google Scholar]
- Yang Y. et al. In vitro antibacterial activity of a novel resin-based pulp capping material containing the quaternary ammonium salt MAE-DB and Portland cement. PloS one 9, e112549, doi: 10.1371/journal.pone.0112549 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayegan A., Petein M. & Vanden Abbeele A. The use of beta-tricalcium phosphate, white MTA, white Portland cement and calcium hydroxide for direct pulp capping of primary pig teeth. Dental traumatology : official publication of International Association for Dental Traumatology 25, 413–419, doi: 10.1111/j.1600-9657.2009.00799.x (2009). [DOI] [PubMed] [Google Scholar]
- Borges A. H. et al. Comparative study of physico-chemical properties of MTA-based and Portland cements. Acta odontologica latinoamericana : AOL 23, 175–181 (2010). [PubMed] [Google Scholar]
- Min K. S. et al. Human pulp cells response to Portland cement in vitro. Journal of endodontics 33, 163–166, doi: 10.1016/j.joen.2006.07.022 (2007). [DOI] [PubMed] [Google Scholar]
- Schroder U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. Journal of dental research 64 Spec No, 541–548 (1985). [DOI] [PubMed] [Google Scholar]
- Kawashima N. Characterisation of dental pulp stem cells: a new horizon for tissue regeneration? Archives of oral biology 57, 1439–1458, doi: 10.1016/j.archoralbio.2012.08.010 (2012). [DOI] [PubMed] [Google Scholar]
- Gronthos S., Mankani M., Brahim J., Robey P. G. & Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America 97, 13625–13630, doi: 10.1073/pnas.240309797 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S. et al. Stem cell properties of human dental pulp stem cells. Journal of dental research 81, 531–535 (2002). [DOI] [PubMed] [Google Scholar]
- Olsson H., Petersson K. & Rohlin M. Formation of a hard tissue barrier after pulp cappings in humans. A systematic review. International endodontic journal 39, 429–442, doi: 10.1111/j.1365-2591.2006.01116.x (2006). [DOI] [PubMed] [Google Scholar]
- Hilton T. J. Keys to clinical success with pulp capping: a review of the literature. Operative dentistry 34, 615–625, doi: 10.2341/09-132-0 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindeler A., McDonald M. M., Bokko P. & Little D. G. Bone remodeling during fracture repair: The cellular picture. Seminars in cell & developmental biology 19, 459–466, doi: 10.1016/j.semcdb.2008.07.004 (2008). [DOI] [PubMed] [Google Scholar]
- Nikitakis N. G., Sauk J. J. & Papanicolaou S. I. The role of apoptosis in oral disease: mechanisms; aberrations in neoplastic, autoimmune, infectious, hematologic, and developmental diseases; and therapeutic opportunities. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics 97, 476–490, doi: 10.1016/S1079210404000290 (2004). [DOI] [PubMed] [Google Scholar]
- Murray P. E. et al. Human odontoblast cell numbers after dental injury. Journal of dentistry 28, 277–285 (2000). [DOI] [PubMed] [Google Scholar]
- Modena K. C. et al. Cytotoxicity and biocompatibility of direct and indirect pulp capping materials. Journal of applied oral science : revista FOB 17, 544–554 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- RV D. E. C. et al. The influence of concentration of HEMA on degree of conversion and cytotoxicity of a dental bonding resin. Minerva stomatologica 65, 65–71 (2016). [PubMed] [Google Scholar]
- Jiao Y. et al. Epigallocatechin-3-Gallate Reduces Cytotoxic Effects Caused by Dental Monomers: A Hypothesis. Medical science monitor : international medical journal of experimental and clinical research 21, 3197–3202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I., Salhab I., Setzer F. C., Kim S. & Nah H. D. A New Calcium Silicate-based Bioceramic Material Promotes Human Osteo- and Odontogenic Stem Cell Proliferation and Survival via the Extracellular Signal-regulated Kinase Signaling Pathway. Journal of endodontics 42, 480–486, doi: 10.1016/j.joen.2015.11.013 (2016). [DOI] [PubMed] [Google Scholar]
- Sloan A. J. & Smith A. J. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral diseases 13, 151–157, doi: 10.1111/j.1601-0825.2006.01346.x (2007). [DOI] [PubMed] [Google Scholar]
- Lin L. M. & Rosenberg P. A. Repair and regeneration in endodontics. International endodontic journal 44, 889–906, doi: 10.1111/j.1365-2591.2011.01915.x (2011). [DOI] [PubMed] [Google Scholar]
- Lymperi S. et al. Dental Stem Cell Migration on Pulp Ceiling Cavities Filled with MTA, Dentin Chips, or Bio-Oss. BioMed research international 2015, 189872, doi: 10.1155/2015/189872 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Yang J., Zhang J. & Peng B. A comparative study of BioAggregate and ProRoot MTA on adhesion, migration, and attachment of human dental pulp cells. Journal of endodontics 40, 1118–1123, doi: 10.1016/j.joen.2013.12.028 (2014). [DOI] [PubMed] [Google Scholar]
- D’Anto V. et al. Effect of mineral trioxide aggregate on mesenchymal stem cells. Journal of endodontics 36, 1839–1843, doi: 10.1016/j.joen.2010.08.010 (2010). [DOI] [PubMed] [Google Scholar]
- Williams D. W. et al. 2-Hydroxyethyl methacrylate inhibits migration of dental pulp stem cells. Journal of endodontics 39, 1156–1160, doi: 10.1016/j.joen.2013.06.004 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garimella R., Bi X., Anderson H. C. & Camacho N. P. Nature of phosphate substrate as a major determinant of mineral type formed in matrix vesicle-mediated in vitro mineralization: An FTIR imaging study. Bone 38, 811–817, doi: 10.1016/j.bone.2005.11.027 (2006). [DOI] [PubMed] [Google Scholar]
- Feng J. Q. et al. Genomic organization, chromosomal mapping, and promoter analysis of the mouse dentin sialophosphoprotein (Dspp) gene, which codes for both dentin sialoprotein and dentin phosphoprotein. The Journal of biological chemistry 273, 9457–9464 (1998). [DOI] [PubMed] [Google Scholar]
- Prescott R. S. et al. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. Journal of endodontics 34, 421–426, doi: 10.1016/j.joen.2008.02.005 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almushayt A., Narayanan K., Zaki A. E. & George A. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene therapy 13, 611–620, doi: 10.1038/sj.gt.3302687 (2006). [DOI] [PubMed] [Google Scholar]
- Peng W. et al. Effect of tricalcium silicate on the proliferation and odontogenic differentiation of human dental pulp cells. Journal of endodontics 37, 1240–1246, doi: 10.1016/j.joen.2011.05.035 (2011). [DOI] [PubMed] [Google Scholar]
- Eghbali-Fatourechi G. Z. et al. Characterization of circulating osteoblast lineage cells in humans. Bone 40, 1370–1377, doi: 10.1016/j.bone.2006.12.064 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. et al. Demineralized Dentin Matrix Induces Odontoblastic Differentiation of Dental Pulp Stem Cells. Cells, tissues, organs 201, 65–76, doi: 10.1159/000440952 (2016). [DOI] [PubMed] [Google Scholar]
- Ajlan S. A., Ashri N. Y., Aldahmash A. M. & Alnbaheen M. S. Osteogenic differentiation of dental pulp stem cells under the influence of three different materials. BMC oral health 15, 132, doi: 10.1186/s12903-015-0113-8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoluzzi E. A. et al. Cytotoxicity and osteogenic potential of silicate calcium cements as potential protective materials for pulpal revascularization. Dental materials : official publication of the Academy of Dental Materials 31, 1510–1522, doi: 10.1016/j.dental.2015.09.020 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda Y., Ogawa M., Arakawa T., Kadowaki T. & Saito T. The effect of mineral trioxide aggregate on the mineralization ability of rat dental pulp cells: an in vitro study. Journal of endodontics 34, 1057–1060, doi: 10.1016/j.joen.2008.06.007 (2008). [DOI] [PubMed] [Google Scholar]
- Ribeiro D. A., Duarte M. A., Matsumoto M. A., Marques M. E. & Salvadori D. M. Biocompatibility in vitro tests of mineral trioxide aggregate and regular and white Portland cements. Journal of endodontics 31, 605–607 (2005). [DOI] [PubMed] [Google Scholar]
- Camilleri J., Montesin F. E., Di Silvio L. & Pitt Ford T. R. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. International endodontic journal 38, 834–842, doi: 10.1111/j.1365-2591.2005.01028.x (2005). [DOI] [PubMed] [Google Scholar]
- De Deus G., Ximenes R., Gurgel-Filho E. D., Plotkowski M. C. & Coutinho-Filho T. Cytotoxicity of MTA and Portland cement on human ECV 304 endothelial cells. International endodontic journal 38, 604–609, doi: 10.1111/j.1365-2591.2005.00987.x (2005). [DOI] [PubMed] [Google Scholar]
- Goldberg M. & Smith A. J. Cells And Extracellular Matrices Of Dentin And Pulp: A Biological Basis for Repair And Tissue Engineering. Critical reviews in oral biology and medicine: an official publication of the American Association of Oral Biologists 15, 13–27 (2004). [DOI] [PubMed] [Google Scholar]
- Adams G. B. et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599–603, doi: 10.1038/nature04247 (2006). [DOI] [PubMed] [Google Scholar]
- Yasuda Y., Kamaguchi A. & Saito T. In vitro evaluation of the antimicrobial activity of a new resin-based endodontic sealer against endodontic pathogens. Journal of oral science 50, 309–313 (2008). [DOI] [PubMed] [Google Scholar]
- Torabinejad M., Hong C. U., Pitt Ford T. R. & Kettering J. D. Antibacterial effects of some root end filling materials. Journal of endodontics 21, 403–406 (1995). [DOI] [PubMed] [Google Scholar]
- Estrela C., Bammann L. L., Estrela C. R., Silva R. S. & Pecora J. D. Antimicrobial and chemical study of MTA, Portland cement, calcium hydroxide paste, Sealapex and Dycal. Brazilian dental journal 11, 3–9 (2000). [PubMed] [Google Scholar]
- Murray P. E. & Garcia-Godoy F. The incidence of pulp healing defects with direct capping materials. American journal of dentistry 19, 171–177 (2006). [PubMed] [Google Scholar]
- Kakehashi S., Stanley H. R. & Fitzgerald R. J. The Effects Of Surgical Exposures Of Dental Pulps In Germ-Free And Conventional Laboratory Rats. Oral surgery, oral medicine, and oral pathology 20, 340–349 (1965). [DOI] [PubMed] [Google Scholar]
- Zarrabi M. H., Javidi M., Jafarian A. H. & Joushan B. Histologic assessment of human pulp response to capping with mineral trioxide aggregate and a novel endodontic cement. Journal of endodontics 36, 1778–1781, doi: 10.1016/j.joen.2010.08.024 (2010). [DOI] [PubMed] [Google Scholar]
- Cox C. F., Keall C. L., Keall H. J., Ostro E. & Bergenholtz G. Biocompatibility of surface-sealed dental materials against exposed pulps. The Journal of prosthetic dentistry 57, 1–8 (1987). [DOI] [PubMed] [Google Scholar]