Abstract
PURPOSE
The goal of this study was to determine the prevalence of Insulin Resistance (IR) and Type-2 Diabetes Mellitus (T2DM) in contemporary cardiac rehabilitation (CR) and to compare clinical responses in CR between these subsets of patients with coronary heart disease (CHD).
METHODS
The study cohort included 818 patients enrolled in CR. We separated the cohort into 3 groups: individuals with normal HbA1c (NoIR, glycated hemoglobin, HbA1c<5.7), individuals with insulin resistance (IR, HbA1c≥5.7<6.5), and individuals with T2DM (HbA1c≥6.5).
RESULTS
The combined prevalence of IR (44%) and T2DM (23%) was 67% which paralleled the prevalence of metabolic syndrome (MetSyn), present in 65% of patients. Women had a higher prevalence of IR and MetSyn than men (73% vs. 64%, 72% vs. 63%) and a greater percentage with an elevated waist circumference (71% vs. 60%) (all, p<0.05). All 3 groups experienced decreases in body weight (NoIR= −2.3±4.0, IR= −1.7±4.0, T2DM= −1.0±4.2kg) and increases in maximal METs at exercise testing (NoIR= +2.2±2.5 vs IR= +2.1±2.8 vs T2DM= +1.3±2.3) (all, p<0.05). Individuals with NoIR achieved greater improvements in weight, BMI, and METs compared to patients with T2DM (all, p<0.05). Selected individuals who participated in a 4-session behavioral weight loss program lost more than twice the weight as nonparticipants.
CONCLUSIONS
The combined prevalence of IR and T2DM in patients with CAD enrolled in CR was remarkably high (67%). To reverse the deleterious consequences of IR and T2DM, targeted interventions of exercise and weight loss need to be a central focus of CR programming.
Introduction
Metabolic syndrome (MetSyn), insulin resistance (IR) syndrome, and type 2 diabetes mellitus (T2DM) are highly prevalent conditions among participants in cardiac rehabilitation (CR).1 T2DM is often associated with a cluster of cardiovascular disease (CVD) risk factors that include abdominal obesity, hypertension (HTN), hypertriglyceridemia, and low HDL-C that are frequently termed “metabolic syndrome”. This form of DM, previously referred to as “non-insulin-dependent diabetes” or “adult-onset-diabetes”, accounts for ~90–95% of all diabetes.2 Metabolic syndrome or IR (HbA1c≥5.7<6.5) substantially increases the likelihood of developing T2DM and is associated with the premature development and increased progression of cardiovascular disease.3 The prevalence of T2DM in the general population is increasing with about 29.1 million people or 9.3% of U.S. population diagnosed. Furthermore, an estimated 8.1 million U.S. adults with diabetes are undiagnosed thus increasing their risks for eventual T2DM-related complications and cardiovascular disease.4 Its prevalence in individuals with diagnosed coronary heart disease (CHD) is much higher.5 Patients with T2DM have a 2 to 4 fold increased risk of myocardial infarction, stroke, or death from cardiovascular disease than those without T2DM.6 Co-morbidities such as HTN, elevated BMI, obesity, and dyslipidemia associated with T2DM amplify its role in cardiovascular disease. Hypertension affects 71% of the individuals with T2DM.7 Cardiovascular complications in individuals with HTN and T2DM are substantially more common than with either entity alone.8 With more than 80% of the patients entering CR being overweight, the prevalence of IR and T2DM, and their response to CR needs to be closely assessed.9
While it is well accepted that CR has a favorable impact on exercise tolerance, little data exists on outcomes in individuals with IR, MetSyn, or T2DM in CR.10,11 Whereas behavioral weight loss programs, consisting of exercise and a hypocaloric diet are sometimes offered in CR, the relative effectiveness of CR on body weight and fitness in individuals with IR, MetSyn, or T2DM has not been evaluated. The purpose of this study is to determine the prevalence of IR and T2DM in contemporary CR and to highlight the need for active intervention and management of MetSyn and T2DM in the CR setting.
Methods
The study population included 898 consecutive patients with established coronary artery disease enrolled in CR at the University of Vermont Medical Center, Burlington, Vermont. A total of 80 participants were excluded from the study for missing data pertaining to fasting blood glucose (FBG) or HbA1c. All patients recently experienced a coronary event including coronary artery bypass surgery (CABG), myocardial infarction (MI), percutaneous coronary intervention (PCI), chronic stable angina, or systolic congestive heart failure (CHF). After the overall number of individuals with IR and T2DM was ascertained, the data were analyzed and separated by gender. Additionally, the cohort was classified into 3 groups: individuals with no insulin resistance (NoIR, HbA1c<5.7), IR (HbA1c≥5.7<6.5), and T2DM (HbA1c≥6.5). Type 2 diabetes mellitus classification (fasting serum glucose of ≥126 mg/dL or HbA1c ≥6.5) was also based on the American Diabetes Association (ADA) recommendation.12 The Adult Treatment Panel (ATP) III guideline was utilized for classifying MetSyn13 as follows with 3 of 5 criteria required for a diagnosis of MetSyn.
Abdominal obesity: waist circumference of >102 cm for men and >88 cm for women.
Hypertension: ascertained from patient history of HTN, use of HTN medication, or a medically assessed blood pressure of ≥130 systolic or ≥85 diastolic mmHg.
Hypertriglyceridemia: triglyceride ≥150 mg/dL or use of triglyceride lowering medication.
Low HDL-C: HDL-cholesterol <40 mg/dL in men and <50 mg/dL in women, or use of lipid medication to increase HDL-Cholesterol.
Fasting glucose: use of anti-diabetic medication or fasting serum glucose of ≥110 mg/dL.
The current study included an additional criterion of HbA1c≥5.7 or fasting serum glucose of ≥100 mg/dL for hyperglycemia. Blood lipid analyses were undertaken in the hospital at the time of the index event, the morning after hospital admission. Lipids were done prior to entry to CR. Though not included in the criteria for establishing MetSyn, LDL cholesterol was computed. Patients enrolled in the CR program underwent exercise testing in a standardized manner. At entry to CR aerobic fitness was assessed with a symptom limited exercise tolerance test. Peak metabolic equivalents (METspeak) were estimated based on achieved treadmill speed and elevation. For 517 patients, expired gas was analyzed during the exercise protocol using a Medgraphics Ultima metabolic cart (Saint Paul, MN) with patients exercising to voluntary exhaustion. Peak VO2 was considered to be the highest 30-second average during the test. At the time of entry to CR a digital calibrated scale (Detecto, Webb City, MO) was used to measure body weight with shoes removed and pockets emptied. Body mass index was calculated as weight (kg) divided by height (m2). Waist circumference, measured around the level at the umbilicus, was also obtained. Patient handgrip was assessed using a handgrip dynamometer (Jamar, Bolingbrook, IL).
Self-reported physical functioning was assessed using the Medical Outcomes Study Short Form-36 (MOS SF-36) survey questionnaire (0–100 scale) with 100 representing excellent physical functioning. Depressive symptoms were assessed using the Geriatric Depression Scale (0–15 score) with higher numbers indicating more depressive symptoms. A co-morbidity score was determined by assessing for peripheral vascular disease, cerebrovascular disease, chronic lung disease, or orthopedic limitations. If a co-morbid condition was present, it was quantified by severity as follows: 1, present but not exercising–limiting; 2, present and impacts on exercise performance; and 3, exercising-limiting. A total co-morbidity score ranging from 0 to 12 was thus determined.
The CR exercise intervention has been described in detail previously.14 Briefly, the exercise training program consisted of up to three sessions per week over 3–4 months (maximum, 36 sessions). Individuals exercised for approximately 45 to 60 minutes per CR session on a variety of modalities including treadmills, elliptical trainers, and rowing, cycle, and arm ergometers. This training program is similar to that performed at most CR programs around the country.15 To maximize caloric expenditure, however, overweight participants used treadmill walking and elliptical trainers as the primary exercise modalities rather than weight-supported exercise ergometers.16 Participants were encouraged to exercise aerobically on non-CR days.
All patients participated in 2 classroom teaching sessions on a heart-healthy diet. Overweight individuals were also strongly advised to attend 4 weekly behavioral weight loss sessions. The components of the behavioral weight sessions have been described elsewhere.17 Briefly, behavioral strategies target changes in diet to decrease caloric intake and increase physical activity to improve energy expenditure. These behavioral strategies were designed to assist the patient to acquire the skills and knowledge for short- and long-term weight loss success. Specific behavioral strategies included: establishing a daily caloric goal for dietary intake and activity related energy expenditure, self-monitoring of both eating habits and physical activity, stimulus control, problem solving, and social support.
Statistical analyses were performed using SAS version 9.0 (SAS Institute, Inc. Cary, NC, USA). Baseline characteristics are presented as N, % (based on sex) along with mean ± SD. To compare baseline variables including age, BMI, lipids, HTN, HbA1c, peak VO2, and peak METs, paired and unpaired t-tests were undertaken. ANOVA was used for analyzing differences between the three groups. Variables included in the regression analysis of correlates of baseline HbA1c were baseline body weight, entry BMI, waist circumference, peak METs, peak VO2, total co-morbidity score, and depression and physical function scores. Contingency table analysis was used to compare nominal variables. A p value of <0.05 was used to indicate statistical significance.
Results
The study population included 898 consecutive patients; 241 females (27%) and 657 males (73%). A total of 80 participants were excluded from the study for missing data pertaining to both glucose and HbA1c measures. There were no differences by age, sex, weight, BMI, lipid levels, and peak aerobic exercise capacity when individuals excluded from the study were compared to those included in the study (all, p=NS) (data not shown).
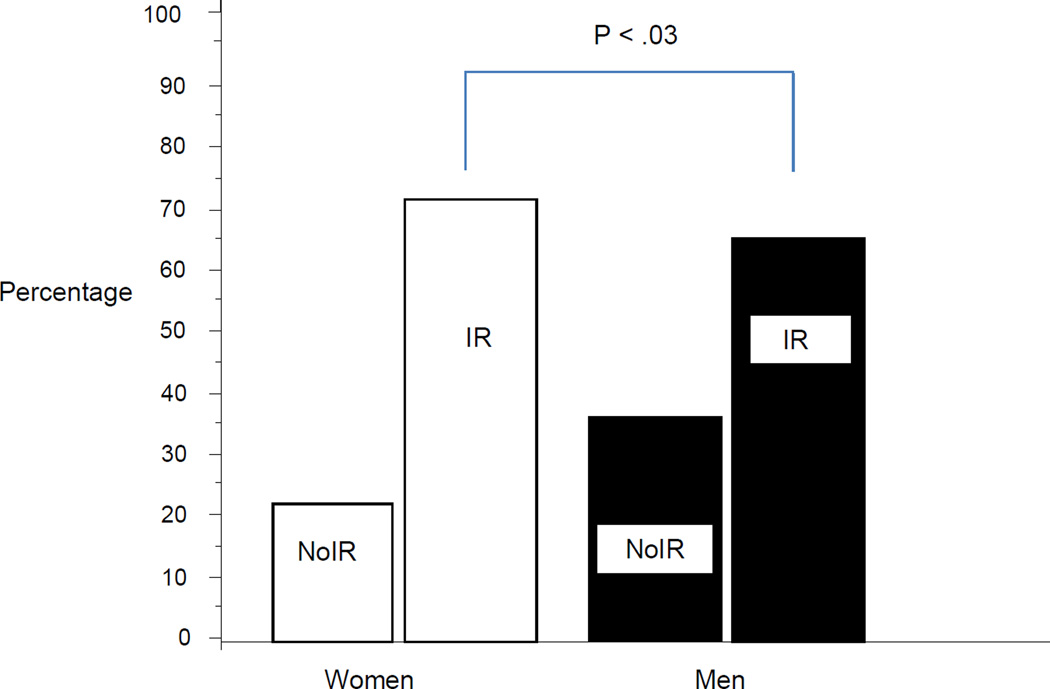
The combined prevalence of IR (44%) and T2DM (23%) was 67% which closely paralleled the prevalence of MetSyn in 65% of patients (Table I). A significantly greater percentage of women had IR than men (73% vs. 64%, respectively, p<0.05, Figure 1). Women also had higher prevalence of MetSyn than men (72% vs. 63%, p<0.05). There were no difference by sex pertaining to prevalence of HTN, hypertriglyceridemia, or low HDL although a greater percentage of women were classified with a high waist circumference compared to men (p<0.05). Combined, 41.2% of men and women were obese (BMI≥30) and 40.9% were overweight (BMI >25 and <30) for a total prevalence of overweight-obese of 82.1%.
Table I.
Baseline Characteristics of Patients Enrolled in CRa
| Total population (N = 818) |
Females (n = 219) |
Males (n = 599) |
P value | |
|---|---|---|---|---|
| Insulin resistanceb | 357 (44) | 100 (46) | 257 (43) | .17 |
| Diabetes mellitusc | 185 (23) | 60 (27) | 125 (21) | .40 |
| Total insulin resistanced | 542 (67) | 160 (73) | 382 (64) | .03 |
| Triglycerides | 283 (35) | 72 (33) | 211 (36) | .50 |
| High blood pressure | 654 (80) | 175 (80) | 479 (80) | .99 |
| Waist circumference | 518 (64) | 156 (71) | 362 (60) | .009 |
| High density lipoprotein | 459 (56) | 133 (61) | 326 (55) | .15 |
| Total metabolic syndrome | 534 (65) | 157 (72) | 377 (63) | .03 |
Abbreviation, CR, cardiac rehabilitation.
Data reported as number (%).
HbA1c ≥5.7% to <6.5% or fasting glucose ≥100 to <126 mg/dL and not on hypoglycemic agent
HbA1c ≥6.5% or fasting glucose ≥126 mg/dL
Insulin resistance + diabetes mellitus
Figure 1.
Insulin Resistance Status
We further analyzed the data by comparing patients by the presence or absence of IR and T2DM (Table II). Overall, 33.7% of CR patients were classified with NoIR, 43.6% had IR, and 22.6% of patients had T2DM. Patients in the NoIR group were younger than the IR and T2DM groups. The percentage of woman was greater in the IR (27.9%) and T2DM (32.6%) groups than the NoIR (21.5%) group (p<0.05). The number of days from hospital discharge to entry into CR for NoIR and IR groups was shorter than the T2DM group (p<0.05). At baseline, significant differences between these three groups of patients were observed for weight (NoIR=84.0±16.4 vs IR=86.4±19.1 vs T2DM=92.8±18.7kg), BMI (NoIR=28.4±5.4 vs IR= 29.8±5.4 vs T2DM=32.2±6.1), and waist circumference (NoIR=99.3±12.7 vs IR= 102.4±14.0 vs T2DM=107.4±13.2cm (all, p<0.001). For weight, BMI and waist circumference, between group comparison revealed lower values for patients with NoIR compared to IR and both NoIR and IR had more favorable body composition measures than individuals with T2DM (all, p<0.05). Patients with T2DM had higher triglycerides, lower HDL-C and LDL-C (all, p<0.05) than patients in the NoIR and IR groups. Comorbidity score varied significantly between the 3 groups being lowest in NoIR, and highest in patients with T2DM (p<0.001). Differences between the three groups were observed for baseline exercise capacity for both peak VO2 (Highest in NoIR=21.8±6.8, intermediate in IR=18.5±5.8, and lowest in T2DM=17.3±5.8 mLO2*kg−1*min−1, p<0.001), and peak METs (NoIR=7.9±2.9 vs IR=6.8±2.6 vs T2DM=6.0±2.4, p<0.001). For both peak VO2 and METs, between group comparison revealed higher fitness levels for patients with NoIR compared to IR and both NoIR and IR were more fit than individuals with T2DM (all, p<0.05). Patients with NoIR had both a higher handgrip and a higher MOS SF-36 physical function score in comparison to patients with IR and T2DM (p<0.001). The Geriatric Depression Scale did not differ between the three groups.
Table 2.
Comparison of CHD Risk Factors by Groupsa
| Total cohort (N = 818) |
No insulin resistance (NoIR) (n = 276) |
Insulin resistance (IR) (n = 357) |
Diabetes mellitus (T2DM) (n = 185) |
P value | |
|---|---|---|---|---|---|
| Age, y | 64.2 ± 11.1 | 62.5 10.8b | 65.5 ± 11.3 | 64.1 ± 10.9 | 0.006 |
| Sex, % female | 26.7 | 21.5b | 27.9 | 32.6 | 0.03 |
| Time to CR entry, days | 38.0 ± 31.3 | 34.7 ± 28.6c | 37.6 ± 28.6 | 43.7 ± 39.4 | 0.003 |
| Weight, kg | 87.0 ± 18.4 | 84.0 ± 16.4c | 86.4 ± 19.1 | 92.8 ± 18.7 | 0.0001 |
| Body mass index, kg/m2 | 29.9 ± 6.0 | 28.4 ± 5.4d | 29.8 ± 5.4 | 32.2 ± 6.1 | 0.0001 |
| Waist circumference, cm | 102.6 ± 13.7 | 99.3 ± 12.7d | 102.4 ± 4.0 | 107.4 ± 13.2 | 0.0001 |
| Total cholesterol, mg/dL | 162.4 ± 43.8 | 164.7 ± 42.2 | 162.9 ± 45.0 | 157.8 ± 43.4 | 0.24 |
| Triglycerides, mg/dL | 145.2 ± 110.0 | 134.0 ± 72.7c | 138.8 ± 72.4 | 173.1 ± 186.3 | 0.0004 |
| HDL-cholesterol, mg/dL | 43.0 ± 12.4 | 44.6 ± 13.3c | 43.2 ± 12.9 | 40.5 ± 9.5 | 0.004 |
| LDL-cholesterol, mg/dL | 90.8 ± 36.3 | 93.4 ± 35.7c | 92.2 ± 38.3 | 84.1 ± 32.6 | 0.02 |
| HbA1c, % | 6.4 ± 1.4 | 5.4 ± 0.2d | 6.0 ± 0.2 | 8.3 ± 1.7 | 0.0001 |
| Fasting glucose, mg/dL | 104.8 ± 25.0 | 91.9 ± 9.5d | 118.8 ± 15.8 | 146.3 ± 31.7 | 0.0001 |
| Peak V̇O2, mLO2/kg/min | 19.4 ± 6.4 | 21.8 ± 6.8d | 18.5 ± 5.8 | 17.3 ± 5.8 | 0.0001 |
| Peak metabolic equivalents, METs | 7.0 ± 2.8 | 7.9 ± 2.9d | 6.8 ± 2.6 | 6.0 ± 2.4 | 0.0001 |
| MOS SF-36 | 67.5 ± 25.0 | 72.9 ± 23.7b | 65.1 ± 24.6 | 62.6 ± 26.4 | 0.0003 |
| Geriatric Depression Scale | 3.0 ± 3.0 | 2.8 ± 3.0 | 3.0 ± 2.8 | 3.5 ± 3.2 | 0.14 |
| Comorbid score | 0.6 ± 1.3 | 0.4 ± 1.0d | 0.7 ± 1.3 | 0.9 ± 1.5 | 0.0001 |
| Handgrip strength, kg | 36.1 ± 11.4 | 38.7 ± 10.8b | 35.0 ± 11.8 | 34.4 ± 11.1 | 0.0004 |
Abbreviations: CHD, coronary heart disease; CR, cardiac rehabilitation; HbA1c, glycated hemoglobin; MOS SF-36; Medical Outcomes Study Short Form 36 items; V̇O2, oxygen uptake.
Data reported as mean ± SD unless otherwise noted.
NoIR significantly different than IR and DM
NoIR and IR significantly different than DM
NoIR, IR and DM all significantly different
Of 818 patients studied, 488 patients (60%) (NoIR=168, IR=196, T2DM=124) completed the CR program (Table III) and had pre and post-program data. Patients with IR were less likely to complete CR than patients with T2DM (p<0.05). Otherwise, completion rates were similar between groups. During CR, all 3 groups of patients experienced improvements in weight (NoIR= −2.3±4.0 vs IR= −1.7±4.0 vs T2DM= −1.0±4.2Kg), BMI (NoIR= −0.7±1.3 vs IR= −0.6±1.4 vs T2DM= −0.4±1.5), and METs (NoIR= +2.2±2.5 vs IR= +2.1±2.8 vs T2DM= +1.3±2.3) (within group comparison, all, p<0.05). Individuals with NoIR achieved greater improvements in weight, BMI, and METs compared to patients with T2DM (all, p<0.05) but experienced similar changes in weight related outcomes compared with subjects with IR.
Table 3.
Risk Factor Responses by Patient Group for CR Completersa
| Total patients (n = 488) |
No insulin resistance (NoIR) (n = 168) |
Insulin resistance (IR) (n = 196) |
Diabetes mellitus (T2DM) (n = 124) |
P valuec | |
|---|---|---|---|---|---|
| Weight, kg | .04 | ||||
| Pre-CR | 85.6 ± 16.9 | 83.7 ± 16.7 | 84.2 ± 16.6 | 90.4 ± 16.7 | |
| Post-CR | 83.9 ± 16.3b | 81.4 ± 16.1b | 82.5 ± 15.5b | 89.4 ± 16.7b | |
| BMI, kg/m2 | .0001 | ||||
| Pre-CR | 29.2 ± 5.3 | 28.1 ± 4.6 | 29.0 ± 5.7 | 31.2 ± 5.2 | |
| Post-CR | 28.7 ± 5.1b | 27.4 ± 4.4b | 28.4 ± 5.3b | 30.8 ± 5.1b | |
| Waist circumference, cm | .02 | ||||
| Pre-CR | 102.4 ± 13.0 | 100.6 ± 13.2 | 101.1 ± 13.0 | 106.7 ± 12.0 | |
| Post-CR | 100.3 ± 13.2b | 97.8 ± 13.2b | 99.1 ± 12.2b | 105.4 ± 12.0b | |
| Peak V̇O2, mLO2kg/min | .25 | ||||
| Pre-CR | 20.0 ± 6.2 | 21.9 ± 6.6 | 19.7 ± 6.2 | 17.6 ± 4.7 | |
| Post-CR | 23.2 ± 7.4b | 25.5 ± 7.8b | 23.0 ± 7.4b | 20.2 ± 5.5b | |
| Peak metabolic equivalents, METs | .006 | ||||
| Pre-CR | 7.2 ± 2.7 | 8.0 ± 2.9 | 7.2 ± 2.7 | 6.2 ± 2.3 | |
| Post-CR | 9.2 ± 3.4b | 10.2 ± 3.4b | 9.3 ± 3.4b | 7.5 ± 2.7b | |
Abbreviations: BMI, body mass index; CR, cardiac rehabilitation; V̇O2, oxygen uptake.
All data reported as mean ± SD.
Within group comparison; all P < .05.
Comparison of response to training by group assignment. See text for post-hoc results.
We reviewed weight loss patterns for 392 of the 488 patients (80%) who completed CR and had a BMI of greater than 25. Weight loss was significantly greater for individuals who participated in our behavioral weight loss (BWL) intervention (N=95) versus patients (N=297) who did not (−5.6±3.5 vs −1.0±3.8kg, respectively, p<0.0001). Among patients participating in the BWL intervention, weight loss was similar for individuals with NoIR=6.6±3.0kg (N=28), IR=5.3±3.0kg (N=39), and T2DM=4.7±4.2kg (N=28) (between group comparison, p=0.10) and substantially greater than our overall body weight response described above.
By univariate linear regression, the baseline correlates of HbA1c included baseline values for peak METs (r= − 0.21, r2=0.04, p=0.0001), peak VO2 (r= − 0.20, r2=0.04, p=0.0001), BMI r=0.19, r2=0.04, p=0.0001), waist circumference (r=0.19, r2=0.04, p=0.0001), weight (r=0.16, r2=0.04, p=0.0001), depression score (r=0.14, r2=0.02, p=0.005) and co-morbidity score r=0.10, r2=0.01, p=0.01). By stepwise multivariate analysis, peak METs and waist circumference were independently associated with baseline HbA1c (cumulative total r=0.21, adjusted R2=0.04, p<0.0001).
Discussion
The rising prevalence of IR and T2DM in CR demands focused attention on outcomes after CR in these individuals. As CR programs have targeted factors critical to reducing cardiovascular risk, a gap exists pertaining to active management of IR and T2DM in the CR setting which should include routine blood glucose monitoring and HbA1c measurement along with an intervention that maximizes fitness and weight reduction. Cardiac rehabilitation presents a unique opportunity to intervene in the course of the IR continuum. In that a measure of insulin sensitivity (i.e. fasting glucose or HbA1c) is almost always obtained during a hospital stay for an acute coronary event, professionals in CR should utilize such information to take advantage of a “teachable” moment when individuals are likely to be motivated to make lifestyle changes. Counseling individuals with IR or “pre-diabetes” that they are at high risk for developing T2DM may motivate some individuals to make significant lifestyle changes. Also, individuals with newly diagnosed T2DM entering CR may be motivated to make the changes necessary to avoid going on a hypoglycemic medication. Studies indicate that intervening early may be key to successfully achieve a partial T2DM remission.18,19 Recently, we reported that 67–80% of overweight/obese subjects with recent (<1 year) onset of T2DM achieved at least a partial T2DM remission at 6 months after undergoing exercise training and behavioral weight loss.20
In 2005, we reported a prevalence of MetSyn in CR that is significantly lower than in our current analysis (50% vs 65%)1. This discrepancy results in part from a differing methodology for defining IR between the 2 studies. The current study, utilizes fasting glucose and HbA1c to categorize IR rather than our previous study which utilized a medical chart verified diagnosis of T2DM, resulting in an under-reporting of total IR. Additionally, rates of obesity in CR since 2005 have increased.5 The present prevalence of MetSyn of 65% is almost 3 times higher than its occurrence in an age matched general US population21, highlighting the deleterious link between MetSyn and the development of CHD. While our previous study focused on the presence of MetSyn,1 the current study separates the diagnoses of IR and T2DM and highlights the differences between subgroups of patients with NoIR, IR, and T2DM. The differences between these groups are of interest and provide a better understanding for managing pre-diabetic and T2DM patients in CR.
Several studies have found that CHD patients with MetSyn and T2DM benefit from CR with weight loss and an improvement in peak VO2.22–24 Our analysis reveals that peak METs and waist circumference at baseline independently correlated with HbA1c, reinforcing the potential importance CR programs integrating exercise and weight loss counseling. The present study not only provides some information relative to the increasing prevalence of IR and T2DM in CR but also describes improvements in body weight, waist circumference, and fitness for individuals completing the program. Our results also indicate that women had a higher prevalence of MetSyn and total IR (IR plus T2DM) than men due in part to greater abdominal obesity. Studies have shown that women are less likely to be referred to and enroll in CR25 despite having more co-morbid conditions than men.26 Our results further highlight the need for programs to target increased participation for women as they experience similar improvement in CR as men.26
In that the rising prevalence of T2DM has been attributed to the obesity epidemic27, it has been generally understood that both the exercise training and weight loss counseling components of CR would be beneficial to CHD patients with T2DM.28–32 In addition, it has been shown that patients with T2DM who complete CR derive similar reductions in mortality and hospitalization to patients without T2DM.33 Achieving weight loss along with improving fitness are key components of CR for these individuals as several studies have linked obesity and IR as risk factors for the progression of CHD.34–36 An exercise approach termed “high caloric expenditure exercise training” along with weight loss counseling improves IR in patients with CHD by 28%.9 Although the current study revealed that improvements in weight were higher for patients with NoIR than the IR and T2DM groups, all three groups experienced favorable improvements in weight. Weight reduction is thus is an attainable goal which is generally perceived to be difficult for patients with IR and T2DM. This change in weight, albeit modest, brings attention to the need for more effective weight management interventions in CR.37,38 Indeed, our results indicate that patients who selected to participate in a BWL program were able to achieve significantly greater weight loss than non-BWL participants. Previous studies have reported minimal reductions in BMI and weight loss in patients with T2DM in CR although these programs did not include a distinct behavioral weight loss component.39,40 In our study, for patients who participated in BWL programming, substantial weight loss was achieved regardless of IR status.
This study has limitations. First, the study cohort was predominantly Caucasian at a single center. Secondly, we lack information regarding dose and type of T2DM medication. We also lacked follow-up HbA1C levels for individuals who completed CR. Thirdly, the use of dyslipidemia medications at the time of entry to CR likely resulted in an underestimation of the prevalence of MetSyn through their effects on blood triglycerides and HDL-C. Finally, including an additional criteria of HbA1c for hyperglycemia could be viewed as a limitation for comparison with other studies using standard ATP III guidelines. Fasting blood glucose and HbA1c are a less precise measure of measuring insulin sensitivity than the “gold standard” hyperinsulinemic-euglycemic clamp technique.41 However, FBG and HbA1c values are utilized in the clinical setting to identify individuals with IR. Further, a newly released study using NHANES data used the same criteria (FBG and HbA1c) to define the prevalence of NoIR, IR, and T2DM.42 Thus, our analysis includes data that are typically available to CR professionals.
Given the improvements pertaining to weight loss, BMI, and METs obtained, particularly in patients who participated in BWL, our study draws attention to the clear need for developing targeted interventions in CR programs to the special population of patients with IR or T2DM. Our current study substantiates that weight management should be an integral component of CR programs alongside exercise training. Additionally, IR should be considered as an important outcome in establishing future CR protocols. The optimization of CR in a population with an increasing prevalence of IR and T2DM draws attention to the need of a multi-dimensional approach beyond traditional CR exercise training alone. As fully two thirds of the patients entering CR have IR or T2DM, protocols that address the underlying causal factors of obesity and low fitness need to be developed and assessed.
Acknowledgments
Dr. Ades and Mr. Savage were supported in part by Centers for Biomedical Research Excellence P20GM103644 award from the National Institute on General Medical Sciences.
References
- 1.Savage PD, Banzer JA, Balady GJ, Ades PA. Prevalence of metabolic syndrome in cardiac rehabilitation/secondary prevention programs. Am Heart J. 2005;149(4):627–631. doi: 10.1016/j.ahj.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Diabetes Care. 2015;38(1):S8–S16. doi: 10.2337/dc15-S005. http://care.diabetesjournals.org/content/38/Supplement_1/S8.full. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Pre-Diabetes, Metabolic Syndrome, and Cardiovascular Risk. J Am Coll Cardiol. 2012;59(7):635–643. doi: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2014 http://www.cdc.gov/diabetes/data/statistics/2014StatisticsReport.html.
- 5.Audelin MC, Savage PD, Ades PA. Changing Clinical Profile of Patients Entering Cardiac Rehabilitation/Secondary Prevention Programs: 1996 to 2006. J Cardiopulm Rehabil Prev. 2008;28:299–306. doi: 10.1097/01.HCR.0000336139.48698.26. [DOI] [PubMed] [Google Scholar]
- 6.National Diabetes Education Program. The Link Between Diabetes and Cardiovascular Disease. http://ndep.nih.gov/media/CVD FactSheet.pdf.
- 7.American Diabetes Association. National Diabetes Statistics Report. 2014 http://www.diabetes.org/diabetes-basics/statistics/ [Google Scholar]
- 8.Almgren T, Wilhelmsen L, Samuelsson O, Rosengren A, Andersson OK. Diabetes in treated hypertension is common and carries a high cardiovascular risk: results from a 28-year follow-up. J Hypertens. 2007;25(6):1311–1317. doi: 10.1097/HJH.0b013e328122dd58. [DOI] [PubMed] [Google Scholar]
- 9.Ades PA, Savage PD, Toth MJ, et al. High-Calorie-Expenditure Exercise. A New Approach to Cardiac Rehabilitation for Overweight Coronary Patients. Circulation. 2009;119(20):2671–2678. doi: 10.1161/CIRCULATIONAHA.108.834184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenger NK, Froelicher ES, Smith LK, et al. Cardiac Rehabilitation as a secondary prevention. Agency for Health Care Policy and Research, and the National Heart, Lung, and Blood Institute. Clin Pract Guidel Quick Ref Guide Clin. 1995;17:1–23. [PubMed] [Google Scholar]
- 11.Lopez-Jimenez F, Kramer VC, Masters B, et al. Recommendations for Managing Patients with Diabetes Mellitus in Cardiopulmonary Rehabilitation: An American Association of Cardiovascular and Pulmonary Rehabilitation Statement. J Cardiopulm Rehabil Prev. 2012;32(2):101–112. doi: 10.1097/HCR.0b013e31823be0bc. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association (ADA) Guidelines. 2015 http://www.ndei.org/ADA-diabetes-management-guidelines-diagnosis-A1C-testing.aspx. [Google Scholar]
- 13.ATP III Guidelines At-A-Glance. National Cholesterol Education Program. https://www.nhlbi.nih.gov/files/docs/guidelines/atglance.pdf. [PubMed]
- 14.Savage PD, Brochu M, Scott P, Ades PA. Low Caloric Expenditure in Cardiac Rehabilitation. Am Heart J. 2000;140(3):527–533. doi: 10.1067/mhj.2000.109219. [DOI] [PubMed] [Google Scholar]
- 15.American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th. Champaign, IL: Human Kinetics; 2013. [Google Scholar]
- 16.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Ades PA, Savage PD. Potential benefits of weight loss in coronary artery disease. Prog Cardiovasc Dis. 2014;56(4):448–456. doi: 10.1016/j.pcad.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Tuomilehto J, Schwarz P, Lindstrom J. Long-Term Benefits From Lifestyle Interventions for Type 2 Diabetes Prevention: Time to expand the efforts. Diabetes Care. 2011;34:S210–S214. doi: 10.2337/dc11-s222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregg EW, Chen H, Wagenknecht LE, et al. Association of an Intensive Lifestyle Intervention With Remission of Type 2 Diabetes. JAMA. 2012;308(23):2489–2496. doi: 10.1001/jama.2012.67929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ades PA, Savage PD, Marney AM, Harvey J, Evans KA. Remission of Recently Diagnosed Type 2 Diabetes Mellitus with Weight Loss and Exercise. J Cardiopulm Rehabil Prev. 2015;35(3):193–197. doi: 10.1097/HCR.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beltran-Sanchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of Metabolic Syndrome in the adult US population, 1990–2010. J Am Coll Cardiol. 2013;63(8):697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavie CJ, Morshedi-Meibodi A, Milani RV. Impact of cardiac rehabilitation on coronary risk factors, inflammation, and the metabolic syndrome in obese coronary patients. J Cardiometab Syndr. 2008;3(3):136–140. doi: 10.1111/j.1559-4572.2008.00002.x. [DOI] [PubMed] [Google Scholar]
- 23.Tjonna AE, Lee SJ, Rognmo O, et al. Aerobic Interval Training Versus Continuous Moderate Exercise as a Treatment for the Metabolic Syndrome-“A Pilot Study.”. Circulation. 2008;118(4):346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soja AM, Zwisler AD, Frederikason M, et al. Use of intensified comprehensive cardiac rehab to improve risk factor control in patients with type 2 diabetes mellitus or impaired glucose tolerance – the randomized DANish StuDy of impaired glucose metabolism in the settings of cardiac rehabilitation (DANSUK) study. Am Heart J. 2007;153(4):621–628. doi: 10.1016/j.ahj.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 25.Gravely S, Anand SS, Stewart DE, Grace SL. Effect of referral strategies on access to cardiac rehabilitation among women. Eur J Prev Cardiol. 2013;21(8):1018–1025. doi: 10.1177/2047487313482280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarrafzadegan N, Rabiei K, Kabir A, Sadeghi M, et al. Gender differences in risk factors and outcomes after cardiac rehabilitation. Acta Cardiol. 2008;63(6):763–770. doi: 10.2143/AC.63.6.2033395. [DOI] [PubMed] [Google Scholar]
- 27.Kwan G, Balady GJ. Cardiac Rehabilitation 2012: Advancing the Field Through Emerging Science. Circulation. 2012;125(7):e369–e373. doi: 10.1161/CIRCULATIONAHA.112.093310. [DOI] [PubMed] [Google Scholar]
- 28.Hordern MD, Coombes JS, Cooney LM, et al. Effects of exercise intervention on myocardial function in type 2 diabetes. Heart. 2009;95(16):1343–1349. doi: 10.1136/hrt.2009.165571. [DOI] [PubMed] [Google Scholar]
- 29.Balducci S, Zanuso S, Cardelli P, et al. Changes in physical fitness predicts improvements in modifiable cardiovascular risk factors independently of body weight loss in subjects with type 2 diabetes participating in the Italian Diabetes and Exercise Study (IDEAS) Diabetes Care. 2012;35(6):1347–1354. doi: 10.2337/dc11-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sixt S, Beer S, Bluher M, Korff N, et al. Long-but not short-term multifactorial intervention with focus on exercise training improves coronary endothelial dysfunction in diabetes mellitus type 2 and coronary artery disease. Eur Heart J. 2010;31(1):112–119. doi: 10.1093/eurheartj/ehp398. [DOI] [PubMed] [Google Scholar]
- 31.Lavie CJ, Thomas RJ, Squires RW, et al. Exercise Training and Cardiac Rehabilitation in Primary and Secondary Prevention of Coronary Heart Disease. Mayo Clin Proc. 2009;84(4):373–383. doi: 10.1016/S0025-6196(11)60548-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong MJ, Sigal RJ, Arena R, et al. Cardiac rehabilitation completion is associated with reduced mortality in patients with diabetes and coronary artery disease. Diabetologia. 2015;58(4):691–698. doi: 10.1007/s00125-015-3491-1. [DOI] [PubMed] [Google Scholar]
- 33.Juraschek SP, Blaha MJ, Blumenthal RS, et al. Cardiorespiratory Fitness and Incident Diabetes: The FIT (Henry Ford Exercise Training) Project. Diabetes Care. 2015 Mar 12; doi: 10.2337/dc14-2714. Pii. dc 142714. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART study Investigators. Obesity and the risk of myocardial infarction in 21000 patients from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 35.Levantesi G, Macchia A, Marfisi R, et al. Metabolic syndrome and the risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. 2005;46:277–283. doi: 10.1016/j.jacc.2005.03.062. [DOI] [PubMed] [Google Scholar]
- 36.Bouchonville M, Armamento-Villareal R, Shah K, Napoli N, et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obes (Lond) 2014 Mar;38(3):423–431. doi: 10.1038/ijo.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sierra-Johnson J, Romero-Corral A, Somers VK, et al. Prognostic importance of weight loss in patients with coronary heart disease regardless of body mass index. Eur J Cardiovasc Prev Rehabil. 2008;15:336–340. doi: 10.1097/HJR.0b013e3282f48348. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Escudero JP, Somers VK, Heath AL, et al. Effect of life style therapy using cardiac rehabilitation resources on metabolic syndrome components. J Cardiopulm Rehabil Prev. 2013;33(6):360–370. doi: 10.1097/HCR.0b013e3182a52762. [DOI] [PubMed] [Google Scholar]
- 39.Hindman L, Falko JM, LaLonde M, et al. Clinical profile and outcomes of diabetic and nondiabetic patients in cardiac rehabilitation. Am Heart J. 2005;150:1046–1051. doi: 10.1016/j.ahj.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Magalhaes S, Viamonte S, Miguel Ribeiro M, et al. Long-term effects of a cardiac rehabilitation program in the control of cardiovascular risk factors. Rev Port Cardiol. 2013;32(3):191–199. doi: 10.1016/j.repc.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Kim SH, Reaven GM. Isolated impaired fasting glucose and peripheral insulin sensitivity: not a simple relationship. Diabetes Care. 2008;31(2):347–352. doi: 10.2337/dc07-1574. [DOI] [PubMed] [Google Scholar]
- 42.Menke A, Casagrande S, Geiss L, Cowie L. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]