Abstract
Depressive disorders are a leading cause of disability in older age. Although the role of psychosocial and behavioural predictors has been well examined, little is known about the biological origins of depression. Findings from animal studies have implicated insulin-like growth factor 1 (IGF-1) in the aetiology of this disorder. A total of 6017 older adults (mean age of 65.7 years; 55% women) from the English Longitudinal Study of Ageing provided serum levels of IGF-1 (mean=15.9 nmol l−1, s.d. 5.7) during a nurse visit in 2008. Depression symptoms were assessed in the same year and again in 2012 using the eight-item Center for Epidemiologic Studies Depression Scale. Self-reports of a physician-diagnosis of depression were also collected at both time points. In separate analyses for men and women, the results from both the cross-sectional and longitudinal analyses revealed a ‘U'-shaped pattern of association, such that lower and higher levels of IGF-1 were associated with a slightly elevated risk of depression, whereas the lowest risk was seen around the median levels. Thus, in men, with the lowest quintile of IGF-1 as the referent, the age-adjusted odds ratios (95% confidence interval) of developing depression symptoms after 4 years of follow-up, for increasing quintiles of IGF-1, were: 0.51 (0.28–0.91), 0.50 (0.27–0.92), 0.63 (0.35–1.15) and 0.63 (0.35–1.13) (P-value for quadratic association 0.002). Some attenuation of these effects was apparent after adjustment for co-morbidity, socioeconomic status and health behaviours. In conclusion, in the present study of older adults, there was some evidence that moderate levels of IGF-1 levels conferred a reduced risk of depression.
Introduction
Depression, a major public health problem, has profound effects on the economy and on individuals. According to the World Health Organization, it is the leading cause of disability worldwide,1 responsible for 65 million disability-adjusted life years in 2008.2 In addition to being an important disease in its own right, depression is also associated with an increased risk of suicide,3, 4 cardiovascular disease,5, 6, 7 some cancers8 and premature mortality.9, 10, 11 Although effective treatments for depression are available, the majority of cases among older people remain undiagnosed; prevention of the disorder is therefore crucial.12, 13 A series of studies have identified a range of behavioural (heavy alcohol use, some dietary characteristics) and social (loneliness, socioeconomic disadvantage, bereavement) predictors of depression in later life.14, 15 Although the aetiological role of weight, height, genetic factors and several plasma markers has been examined,16, 17, 18 relatively less attention has been paid to biological risk factors.
Insulin-like growth factor 1 (IGF-1) is a complex peptide hormone produced in multiple tissue sites whose main function is to mediate cell growth, differentiation and transformation by promoting mitosis and inhibiting apoptosis.19 Evidence from laboratory studies suggests that expression and function of IGF-1 is similar in humans and rodents.20, 21 In humans, elevated IGF-1 levels are associated with increased skeletal muscle and tissue growth,22, 23 bone mineral density,24 risk of selected malignancies25 and cardiovascular disease.26, 27 IGF-1 has also been implicated in psychological functioning of both animals and humans. Thus, rodents whose circulating IGF-1 levels were experimentally perturbed displayed marked changes in mood. For instance, the application of a viral vector to drastically reduce circulating IGF-1 levels resulted in mice showing signs of depression as assessed using the forced swim and tail suspension tests.28 Elsewhere, rodents given IGF-1 infusions experienced a reduction in depression symptoms relative to a no treatment group.29, 30 In laboratory-based experimental studies of humans, through a range of mechanisms such as altered neuroprotection, modulation of neuronal excitability, brain angiogenesis, hippocampal neurogenesis and neuroplasticity,31, 32, 33 alterations of IGF-1 levels have been linked to hippocampal dysfunction, which in turn have been associated with mood disorders.34, 35 IGF-1 expression in the hippocampus has also been found to be reduced in sufferers of depression, and enhanced by the administration of antidepressants.36
Of the two general population-based studies of which we are aware, one showed that low IGF-1 in women, but high levels in men, were predictive of depressive disorder.37 In another, relative to moderate levels, women with high IGF-1 values experienced an elevated risk of minor depression.38 In both the studies, incident depression was rare resulting in low statistical power, and in one,38 the measurement of depression was made using a non-standard scale of unknown validity. Accordingly, against this background of study paucity, discordant findings and methodological concerns, we examined the cross-sectional and longitudinal association between IGF-1 and depression symptoms in a large, well-established general population-based study of older adults in England (UK).
Materials and methods
Study population
The English Longitudinal Study of Ageing is an ongoing prospective cohort study of adults aged 50 years and over who, when recruited, lived in private households in the United Kingdom. Initiated in 2002/3, the original sample was drawn from participants in the Health Surveys for England, a collection of population-based cross-sectional studies. With data collection occurring every 2 years, as of 2012, there have been a total of six waves. As IGF-1 levels were first measured in 2008 (wave 4), this represents our study ‘baseline' for the purposes of the present analyses. Ethical approval for all data collection was granted by the National Research and Ethics Committee,39 and the participants provided written consent.
Measurement of IGF-1
Study members were requested not to eat, smoke, drink alcohol or engage in vigorous exercise for 30 min before blood being drawn. The whole-blood samples were transported to a single laboratory (Royal Victoria Infirmary, Newcastle, UK), where the serum was separated, frozen at 40 °C and batch-assayed (completed in 2008) using the DPC Immulite 2000 method. The inter-assay coefficient of variation for IGF-1 across a range of levels was ⩽3.7%, and the intra-assay coefficient of variation was ⩽5.3%. The IGF-1 values are reported as whole numbers (range: 3–200 nmol l−1).40
Depressive symptoms and physician-diagnosis of depression
Depression symptoms were ascertained during a computer-assisted personal interview using the eight-item Center for Epidemiologic Studies Depressive (CES-D8) scale.41 Each item requires a dichotomous (yes/no) response, and scores range between 0 and 8 (higher score denotes more severe symptoms). Consistent with other analyses, we defined ‘caseness' as anyone scoring 4 or above.42 The CES-D has been widely used in population-based studies of older groups43, 44 and has been validated against clinician-assessed depression.45 Notably, the shortened CES-D8 has good internal consistency (Cronbach's α=0.78) and similar psychometric properties to the full 20-item CES-D.46 The participants also had an opportunity to self-report a physician-diagnosis of depression. During the main English Longitudinal Study of Ageing interview, the participants first reported whether they had ever been diagnosed with any emotional, nervous or psychiatric problems. This was followed by the identification of the actual condition as selected from a list of ailments common to this group, of which depression was one.47 The assessment of depression in this manner has been shown to be valid in a separate study using the Structured Clinical Interview for DSM-IV Axis I Disorders as the gold standard.48 Both these measurements of depression were made in 2008 and 2012.
Measurement of covariates
We grouped covariates, including potential confounders and mediators, according to theme. Anthropometric measures comprised height and weight, which were measured during the nurse visit; body mass index (kg/m2) calculated by dividing each individual's measured weight by height squared. Psychosocial factors were level of education (no qualification, completed secondary (high school) education, educated beyond secondary education but below degree level, and educated beyond degree level); quintiles of net non-pension wealth (derived from an estimation of financial wealth and physical assets reported by study participants and their partners, excluding pension savings and net of debts such as credit cards and loans);49 and marital status (currently living with a partner or not). Health-related behaviours comprised smoking status (current, ex-smoker and never), frequency of alcohol consumption in the past year (less than daily/daily, with consumption on at least 5 days of the week being classed as daily consumption) and leisure time physical activity (low/sedentary, moderate or high activity of exercises such as jogging, cycling, gardening, walking). Co-morbidities were self-reported physician-diagnosis of cancer, diabetes or cardiovascular disease (heart murmur, ischaemic heart disease, abnormal heart rhythm, stroke, valvular heart disease or any other reported heart disease).
Statistical analysis
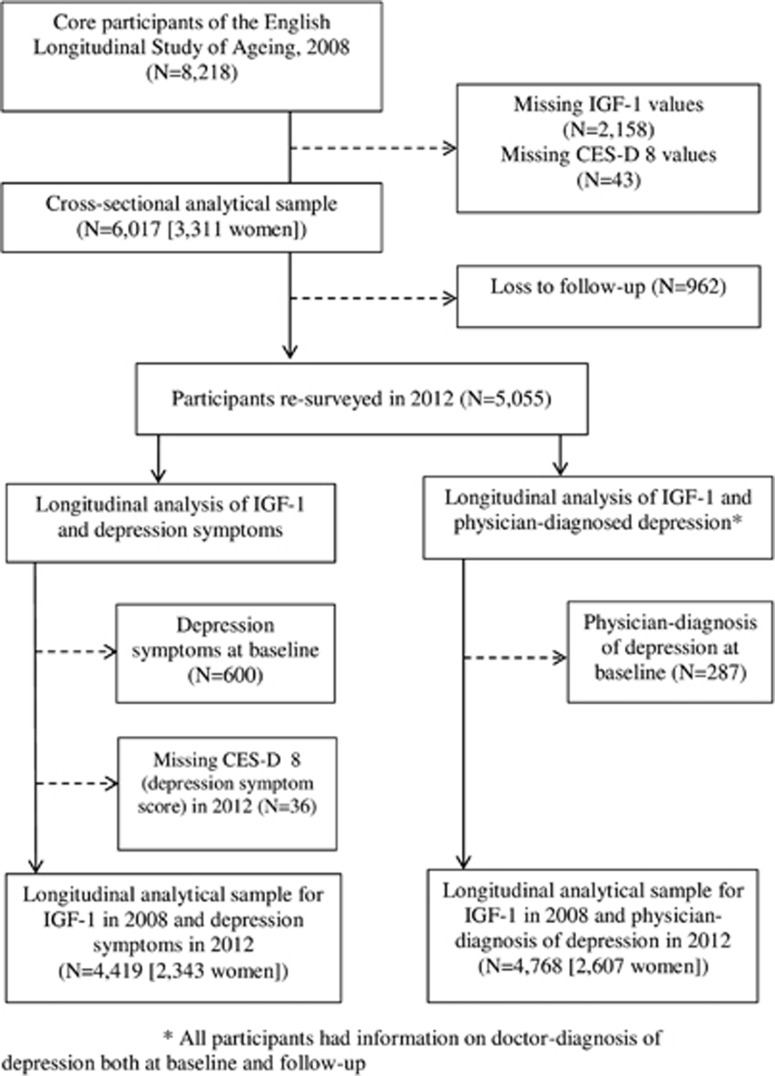
In Figure 1, we illustrate the flow of participants through the study. Of the 8218 participants at baseline in 2008 who had received a nurse visit, we excluded those who had missing values for IGF-1 (N=2158) comprising people who declined to give blood (771), unsuitability or loss of a blood sample (395), or failure to obtain blood sample (992). We also excluded participants with missing values for depression symptoms (43), although none had missing data for physician-diagnosed depression at baseline. The cross-sectional sample therefore comprised 6017 study members from data collection in 2008. We also carried out the longitudinal analyses, again using IGF-1 values from 2008 but relating to new (incident) cases of depression at resurvey in 2012. In deriving new cases of depression, we excluded participants who were classed as depression cases in 2008, resulting in samples of 4419 for the analysis of depression symptoms and 4768 for physician-diagnosed depression.
Figure 1.
Derivation of the analytical sample for cross-sectional (2008) and longitudinal (2012) analyses of the association between serum IGF-1 and depression: the English Longitudinal Study of Ageing. CES-D, Center for Epidemiologic Studies Depressive scale; IGF-1, insulin-like growth factor 1.
Multivariable logistic regression analyses were used to summarize the association of IGF-1 levels with both depression outcomes. The lowest quintile of IGF-1 was used as the referent. There is existing evidence of differential IGF-1-depression relationships in men and women,39, 40, 50 so we present gender-specific analyses here also. We adjusted effect estimates for known covariates in a stepwise manner. In our analyses, depression symptoms were our primary outcome, with physician-diagnosed depression used to test convergence of evidence. All the analyses were carried out using Stata12SE software.51
Results
IGF-1 and baseline characteristics: cross-sectional analyses
In Table 1 (women) and Table 2 (men), we present baseline study participant characteristics according to IGF-1 quintiles. As expected, mean IGF-1 values were higher in men (16.5 nmol l−1) than women (15.2 nmol l−1). In men and women, IGF-1 was inversely associated with age and directly related to height and socioeconomic position. Psychosocial factors, which included social position and cohabiting with a partner, typically occurred at more favourable levels in men and women with higher IGF-1 values. A total of 776 participants (12.9%) had CES-D8 scores of 4 and above at baseline and were therefore denoted as ‘cases' (71.8% were female); 344 (5.7%) participants reported physician-diagnosis of depression. As for some of the somatic conditions such as cancer, there was a suggestion that the greatest proportions of both men and women who reported high depression symptoms and self-declared physician-diagnosed depression were seen in the lowest and highest quintiles of IGF-1, although the differences across groups were not considerable.
Table 1. Baseline characteristics of study participants according to quintiles of serum IGF-1 (nmol l−1): 3311 women in the English Longitudinal Study of Ageing, 2008.
|
IGF-1 quintile (range) |
P-value for difference | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | All | ||
| (2–11 nmol l−1) | (12–14 nmol l−1) | (15–16 nmol l−1) | (17–20 nmol l−1) | (21–65 nmol l−1) | |||
| Subject numbers | 861 | 813 | 479 | 681 | 477 | ||
| IGF-1, nmol l−1, mean (s.e.) | 9.2 (0.06) | 13.0 (0.03) | 15.5 (0.02) | 18.3 (0.04) | 25.2 (0.22) | 15.2 (0.10) | <0.001 |
| Age, years, mean (s.e.) | 68.7 (0.4) | 66.2 (0.3) | 65.1 (0.4) | 64.4 (0.3) | 63.2 (0.4) | 65.9 (0.2) | <0.001 |
| Anthropometry mean (s.e.) | |||||||
| Height, cm | 158.8 (0.3) | 159.9 (0.2) | 160.4 (0.3) | 160.6 (0.2) | 160.9 (0.3) | 160.0 (0.1) | <0.001 |
| BMI, kg/m2 | 28.7 (0.2) | 28.4 (0.2) | 27.6 (0.2) | 27.7 (0.2) | 27.6 (0.2) | 28.1 (0.1) | <0.001 |
| Co-morbidities, % (s.e.) | |||||||
| Diabetes | 8.5 (0.9) | 6.0 (0.8) | 6.9 (1.2) | 5.9 (0.9) | 7.8 (1.2) | 7.0 (0.4) | 0.213 |
| Cancer | 6.6 (0.8) | 4.5 (0.7) | 4.6 (1.0) | 3.2 (0.7) | 6.1 (1.1) | 5.0 (0.4) | 0.029 |
| Cardiovascular disease | 26.6 (1.5) | 24.4 (1.5) | 21.5 (1.9) | 18.6 (1.5) | 23.9 (2.0) | 23.3 (0.7) | 0.005 |
| Psychosocial factors, % (s.e.) | |||||||
| Lowest wealth quintile | 23.1 (1.4) | 16.0 (1.3) | 11.7 (1.5) | 15.0 (1.4) | 14.5 (1.6) | 16.8 (0.7) | <0.001 |
| No educational qualifications | 34.1 (1.6) | 30.3 (1.6) | 29.0 (2.1) | 27.2 (1.7) | 23.1 (1.9) | 29.4 (0.8) | 0.001 |
| Lives alone | 43.1 (1.7) | 37.5 (1.7) | 28.4 (2.1) | 32.8 (1.8) | 36.3 (2.2) | 36.5 (0.8) | <0.001 |
| Behavioural factors, % (s.e.) | |||||||
| Sedentary or low physical activity | 39.4 (1.7) | 30.0 (1.6) | 26.5 (2.0) | 25.8 (1.7) | 30.2 (2.1) | 31.1 (0.8) | <0.001 |
| Current smoking | 12.2 (1.1) | 13.3 (1.2) | 12.7 (1.5) | 13.5 (1.3) | 16.1 (1.7) | 13.4 (0.6) | 0.663 |
| Daily alcohol intake | 15.2 (1.2) | 16.0 (1.3) | 16.5 (1.7) | 15.9 (1.4) | 16.8 (1.7) | 16.0 (0.6) | 0.902 |
| Depression | |||||||
| CES-D score, mean (s.e.) | 1.8 (0.07) | 1.6 (0.07) | 1.3 (0.08) | 1.5 (0.07) | 1.6 (0.09) | 1.6 (0.03) | <0.001 |
| High depression symptoms, % (s.e.) | 20.6 (1.3) | 15.9 (1.3) | 14.4 (1.6) | 14.2 (1.3) | 17.8 (1.5) | 16.8 (0.7) | 0.005 |
| Physician-diagnosed depression, % (s.e.) | 7.1 (0.9) | 6.4 (0.9) | 5.4 (1.0) | 6.5 (0.9) | 6.7 (1.1) | 6.5 (0.4) | 0.837 |
Abbreviations: BMI, body mass index; CES-D, Center for Epidemiologic Studies Depressive scale; IGF-1, insulin-like growth factor 1.
Table 2. Baseline characteristics of study participants according to quintiles of serum IGF-1 (nmol l−1): 2706 men in the English Longitudinal Study of Ageing, 2008.
|
IGF-1 quintile (range) |
P-value for difference | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | All | ||
| (2–11 nmol l−1) | (12–14 nmol l−1) | (15–16 nmol l−1) | (17–20 nmol l−1) | (21–65 nmol l−1) | |||
| Subject number | 483 | 615 | 384 | 667 | 557 | ||
| IGF-I, nmol l−1 (s.e.) | 9.3 (0.08) | 13.1 (0.03) | 15.5 (0.03) | 18.4 (0.04) | 25.1 (0.22) | 16.5 (0.11) | <0.001 |
| Age, years, mean (s.e.) | 68.7 (0.5) | 65.3 (0.4) | 65.0 (0.5) | 64.4 (0.3) | 64.0 (0.3) | 65.4 (0.2) | <0.001 |
| Anthropometry, mean (s.e.) | |||||||
| Height, cm | 171.7 (0.3) | 172.8(0.3) | 173.8 (0.4) | 173.8 (0.3) | 174.2 (0.3) | 173.3 (0.1) | <0.001 |
| BMI, kg/m2 | 28.6 (0.2) | 27.8 (0.2) | 27.9 (0.2) | 28.0 (0.2) | 27.9 (0.2) | 28.0 (0.1) | 0.078 |
| Co-morbidities, % (s.e.) | |||||||
| Diabetes | 12.6 (1.5) | 6.8 (1.0) | 6.3 (1.2) | 10.6 (1.2) | 11.1 (1.3) | 9.6 (5.7) | 0.001 |
| Cancer | 4.8 (1.0) | 5.4 (1.0) | 4.4 (1.1) | 5.2 (0.9) | 4.3 (0.9) | 4.9 (4.1) | 0.897 |
| Cardiovascular disease | 31.5 (2.1) | 24.6 (1.7) | 25.7 (2.2) | 26.3 (1.7) | 27.2 (1.9) | 26.9 (0.9) | 0.121 |
| Psychosocial factors, % (s.e.) | |||||||
| Lowest wealth quintile | 18.0 (1.8) | 13.3 (1.4) | 14.3 (1.8) | 12.0 (1.3) | 13.1 (1.4) | 13.9 (0.7) | 0.167 |
| No educational qualifications | 25.9 (2.0) | 20.0 (1.6) | 23.4 (2.1) | 17.3 (1.5) | 16.3 (1.6) | 20.1 (0.8) | 0.056 |
| Lives alone | 26.7 (2.0) | 20.7 (1.6) | 20.8 (2.1) | 21.4 (1.6) | 16.7 (1.6) | 21.1 (0.8) | 0.003 |
| Behavioural factors, % (s.e.) | |||||||
| Sedentary or low physical activity | 26.9 (2.0) | 18.4 (1.6) | 22.1 (2.1) | 17.4 (1.5) | 21.9 (1.8) | 20.9 (0.8) | 0.005 |
| Current smoking | 13.7 (1.6) | 12.7 (1.3) | 12.8 (1.7) | 11.8 (1.3) | 14.0 (1.5) | 12.9 (0.6) | 0.306 |
| Daily alcohol intake | 28.6 (2.2) | 24.7 (1.7) | 27.6 (2.2) | 26.4 (1.7) | 24.1 (1.8) | 26.1 (0.8) | <0.001 |
| Depression | |||||||
| CES-D8 score, mean (s.e.) | 1.0 (0.08) | 0.9 (0.06) | 0.8 (0.07) | 0.9 (0.06) | 1.1 (0.07) | 0.9 (0.03) | 0.07 |
| High depression symptoms, % (s.e.) | 9.3 (1.3) | 7.5 (1.1) | 5.7 (1.0) | 6.7 (1.0) | 11.0 (1.3) | 8.1 (0.5) | 0.018 |
| Physician-diagnosed depression, % (s.e.) | 5.2 (1.0) | 4.2 (0.8) | 4.2 (1.0) | 3.9 (0.7) | 6.5 (1.0) | 4.8 (0.4) | 0.238 |
Abbreviations: BMI, body mass index; CES-D, Center for Epidemiologic Studies Depressive scale; IGF-1, insulin-like growth factor 1.
IGF-1 and depression: cross-sectional analyses
In Table 3, the computation of odds ratios for the cross-sectional association between IGF-1 and depression symptoms supports earlier evidence of a somewhat higher risk of depression symptoms at opposite ends of the IGF-1 continuum in men and women in this study. Although a similar 'U'-shaped pattern was apparent for physician-diagnosed depression (see Supplementary Table 1), statistical significance at conventional levels was rarely apparent for individual point estimates in these analyses. Adjustment for an array of covariates had little impact on this pattern of association, although taking into account all covariates simultaneously led to some flattening of the IGF-1–depression relationship.
Table 3. Odds ratio (95% confidence interval) for the cross-sectional association between serum IGF-1 and depression symptoms: the English Longitudinal Study of Ageing, 2008.
|
IGF-1 quintile (range)a |
P-value for linearity | P-value for quadratic | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| (2–11) | (12–14) | (15–16) | (17–20) | (21–65) | |||
| Women (analytical sample) | |||||||
| Adjustments | |||||||
| Age (3311) | 1 (ref) | 0.76 (0.58, 0.97) | 0.69 (0.50, 0.93) | 0.68 (0.52, 0.90) | 0.91 (0.68, 1.22) | 0.027 | 0.009 |
| Age, anthropometric measuresb (3181) | 1 | 0.79 (0.61, 1.02) | 0.74 (0.54, 1.01) | 0.72 (0.54, 0.95) | 0.96 (0.71, 1.30) | 0.061 | 0.037 |
| Age, co-morbiditiesc (3308) | 1 | 0.75 (0.58, 0.96) | 0.68 (0.50, 0.92) | 0.69 (0.52, 0.91) | 0.89 (0.66, 1.19) | 0.031 | 0.012 |
| Age, psychosocial factorsd (3162) | 1 | 0.81 (0.62, 1.06) | 0.79 (0.57, 1.09) | 0.73 (0.55, 0.98) | 0.96 (0.70, 1.30) | 0.494 | 0.026 |
| Age, behavioural factorse (2920) | 1 | 0.77 (0.58, 1.02) | 0.76 (0.55, 1.06) | 0.76 (0.56, 1.02) | 0.91 (0.66, 1.25) | 0.08 | 0.078 |
| Multiply adjusted (2691) | 1 | 0.86 (0.63, 1.16) | 0.9 (0.63, 1.29) | 0.85 (0.62, 1.29) | 1 (0.71, 1.41) | 0.756 | 0.199 |
| Men (analytical sample) | |||||||
| Adjustments | |||||||
| Age (2706) | 1 (ref) | 0.75 (0.49, 1.16) | 0.56 (0.33, 0.95) | 0.66 (0.43, 1.02) | 1.12 (0.74, 1.69) | 0.008 | 0.004 |
| Age, anthropometric measuresb (2627) | 1 | 0.85 (0.54, 1.34) | 0.62 (0.35, 1.09) | 0.79 (0.50, 1.25) | 1.26 (0.81, 1.96) | 0.037 | 0.011 |
| Age, co-morbiditiesc (2696) | 1 | 0.76 (0.49, 1.18) | 0.58 (0.34, 0.98) | 0.64 (0.41, 0.99) | 1.11 (0.73, 1.68) | 0.009 | 0.003 |
| Age, psychosocial factorsd (2592) | 1 | 0.85 (0.54, 1.34) | 0.61 (0.35, 1.06) | 0.75 (0.47, 1.19) | 1.3 (0.90, 2.16) | 0.015 | 0.045 |
| Age, behavioural factorse (2377) | 1 | 0.68 (0.40, 1.16) | 0.57 (0.31, 1.06) | 0.71 (0.43, 1.18) | 1.32 (0.83, 2.12) | <0.001 | 0.052 |
| Multiply adjusted (2277) | 1 | 0.84 (0.47, 1.50) | 0.63 (0.32, 1.24) | 0.84 (0.48, 1.47) | 1.54 (0.90, 2.64) | 0.006 | 0.153 |
Abbreviations: BMI, body mass index; IGF-1, insulin-like growth factor 1.
IGF-1 units are nmol l−1.
BMI, height.
Cancer, diabetes, cardiovascular disease.
Own wealth quintile per benefit unit (unit is a couple or single person along with their dependent children), education level.
Alcohol consumption, smoking, physical activity.
IGF-1 and depression: prospective analyses
In Table 4, we depict the association between IGF-1 and depression symptoms after 4 years of follow-up in participants initially free of depression symptoms at baseline (longitudinal analyses). A ‘U'-shaped pattern of risk was again observed for the IGF-1–depression association in both genders based on symptomatology. Similar results were apparent for physician-diagnosis of depression but only among women (Supplementary Table 2). Statistical significance was, again, rarely apparent for individual point estimates. In none of our analyses was there strong statistical evidence that gender modified the IGF-1–depression association (P-values for interaction for the multiply adjusted odds of developing depression symptoms and physician-diagnosed depression in longitudinal analyses are 0.531 and 0.275, respectively).
Table 4. Odds ratio (95% confidence interval) for the longitudinal association between serum IGF-1 in 2008 and new depression symptoms in 2012: the English Longitudinal Study of Ageing.
|
IGF-1 quintile (range)a |
P-value for linearity | P-value for quadratic | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| (2–11) | (12–14) | (15–16) | (17–20) | (21–65) | |||
| Women (analytical sample) | |||||||
| Adjustments | |||||||
| Age (2343) | 1 (ref) | 0.88 (0.60, 1.29) | 0.77 (0.51, 1.16) | 0.84 (0.53, 1.35) | 0.95 (0.60, 1.50) | 0.647 | 0.027 |
| Age, anthropometric measuresb (2276) | 1 | 0.84 (0.57, 1.25) | 0.76 (0.50, 1.16) | 0.84 (0.52, 1.36) | 0.93 (0.58, 1.48) | 0.652 | 0.033 |
| Age, co-morbiditiesc (2342) | 1 | 0.86 (0.58, 1.26) | 0.77 (0.51, 1.16) | 0.84 (0.53, 1.35) | 0.94 (0.60, 1.49) | 0.649 | 0.014 |
| Age, psychosocial factorsd (2243) | 1 | 0.9 (0.60, 1.34) | 0.86 (0.56, 1.30) | 0.9 (0.55, 1.45) | 1.06 (0.66, 1.69) | 0.816 | 0.026 |
| Age, behavioural factorse (2116) | 1 | 0.91 (0.61, 1.35) | 0.76 (0.49, 1.17) | 0.86 (0.52, 1.40) | 0.93 (0.58, 1.51) | 0.915 | 0.029 |
| Multiply adjusted (1969) | 1 | 0.86 (0.56, 1.31) | 0.78 (0.50, 1.23) | 0.87 (0.52, 1.46) | 0.94 (0.56, 1.57) | 0.922 | 0.008 |
| Men (analytical sample) | |||||||
| Adjustments | |||||||
| Age (2076) | 1 (ref) | 0.51 (0.28, 0.91) | 0.5 (0.27, 0.92) | 0.63 (0.35, 1.15) | 0.63 (0.35, 1.13) | 0.126 | 0.002 |
| Age, anthropometric measuresb (2042) | 1 | 0.51 (0.28, 0.94) | 0.5 (0.27, 0.93) | 0.66 (0.36, 1.20) | 0.63 (0.34, 1.14) | 0.142 | 0.002 |
| Age, co-morbiditiesc (2070) | 1 | 0.52 (0.29, 0.94) | 0.56 (0.24, 0.85) | 0.64 (0.35, 1.16) | 0.65 (0.36, 1.16) | 0.098 | 0.002 |
| Age, psychosocial factorsd (2031) | 1 | 0.53 (0.29, 0.97) | 0.54 (0.29, 0.99) | 0.62 (0.33, 1.17) | 0.68 (0.37, 1.24) | 0.173 | 0.001 |
| Age, behavioural factorse (2076) | 1 | 0.52 (0.27, 0.99) | 0.49 (0.25, 0.96) | 0.67 (0.35, 1.30) | 0.58 (0.30, 1.11) | 0.165 | 0.001 |
| Multiply adjusted (1765) | 1 | 0.51 (0.25, 1.02) | 0.5 (0.22, 0.95) | 0.67 (0.33, 1.33) | 0.6 (0.30, 1.19) | 0.072 | 0.003 |
Abbreviations: BMI, body mass index; IGF-1, insulin-like growth factor 1.
IGF-1 units are nmol l−1.
BMI, height.
Cancer, diabetes, cardiovascular disease.
Own wealth quintile per benefit unit (unit is a couple or single person along with their dependent children), education level.
Alcohol consumption, smoking, physical activity.
Discussion
The main finding of this study of older people was that having IGF-1 levels at opposing ends of the continuum was associated with a slightly higher risk of depression symptoms. Similar results were apparent for physician-diagnosis of depression.
Comparison with existing studies
Our results partially accord with the two population studies on IGF-1 and depression of which we are aware. When compared with data from the Study of Health in Pomerania in Germany,38 our results were in agreement with the finding that having a low IGF-1 level was associated with higher risk of developing depression symptoms among women. However, we also found a similar association for men. Our results are also in agreement with the second existent study, which used data from The Longitudinal Aging Study Amsterdam,39 where associations were found between median levels of IGF-1 and lower risk of both prevalent and incident depression symptoms. Once again, the main difference with this present study is that we identified this association among both men and women. The ‘U'-shaped relationship that we identified between IGF-1 and depression symptoms is supported by observations of increased reports of lifetime affective disorders in individuals with lower (pituitary dwarfism) and higher (acromegaly) levels of this growth hormone.52, 53, 54 It may be that this apparent differential result for men and women in these existing studies is due to statistical instability.
To directly compare our findings with some of those already published, we re-categorized IGF-1 levels in our own analyses. In these new analyses, we still found evidence for increased odds of depression symptoms among those with very low and very high IGF-1. Our results after initial re-categorization of IGF-1 levels38 showed that, after 4 years of follow-up, the age-adjusted odds ratios (95% confidence interval) of depression symptoms for the lowest and highest tenth percentiles of IGF-1 among men were 2.26 (1.25, 4.09) and 1.51 (0.86, 2.68), respectively when compared with those with intermediate levels; whereas the corresponding results in women were 1.38 (0.93, 2.07) and 1.27 (0.77, 2.10). Following further re-categorization,39 when compared with the middle tertile, the odds ratios (95% confidence interval) of depression for the lowest and highest tertiles of IGF-1 among men were 1.14 (0.72, 1.80) and 0.89 (0.55, 1.43), respectively, and corresponding results for women were 1.08 (0.79, 1.49) and 1.14 (0.79, 1.65).
Our findings, however, contrast with the results from the handful of case–control studies where IGF-1 levels were found to be elevated in depressed patients compared with healthy controls.55, 56 This may be owing to specific consequences of previous use of anti-depressant medications, such as where they have been seen to improve expression of IGF-1 and other neutrophic and growth factors in the hippocampus.31 Furthermore, there may yet be other unknown biological mechanisms related to the state of being depressed, which cause serum IGF-1 levels to increase, implying reverse causation in the reported case–control studies, where the depression in the cases had, in fact, caused the IGF-1 levels to increase. In this study, we attempted to circumvent the problem of reverse causality by utilizing depression incidence as our outcome in the longitudinal analyses; that is, new cases of depression in participants who, at baseline, were symptom-free and had not previously reported being diagnosed with the condition by a physician.
Strengths and limitations
The main strength of this study is that it has a large, nationally representative sample of people aged 50 years plus in whom there were high rates of follow-up when two standard measures of depression were administered. Our study is not of course without its limitations. The observational nature of our study indicates that we are not able to make any assertions about cause and effect. Although suggestions for mechanisms of action have been posited, it remains possible that IGF-1 levels are a proxy for other factors that are causally related to depression (residual confounding). Although our study was very well characterized, we are not able to control for all possible confounders. Furthermore, although the CES-D8 is a widely used questionnaire in observational studies, it does not provide a diagnosis of depression. Conversely, although self-reported physician-diagnosis of depression does, in principle, do this, many people with depression do not seek medical intervention. The use of anti-depressant medication, which also has some utility in identifying study members with a depression diagnosis, was not gathered in English Longitudinal Study of Ageing. It is also the case, however, that administration of such therapy does not necessarily imply a diagnosis of depression: anti-depressant medication can be used in the treatment of, among other conditions, anxiety and chronic pain disorders. The occurrence of missing data is inevitable in any large-scale study, and about 10% of participants had missing data for one or more of the covariates. However, sensitivity analysis comparing results across the cases with complete information and those with some missing covariates made little difference to outcomes, suggesting that major bias is unlikely. Finally, severe liver and kidney disease may influence IGF-I levels, but we had no such data on these morbidities herein.
In conclusion, taken together, in the present study of older adults, having IGF-1 values at opposite ends of the continuum was associated with a somewhat increased risk of depression symptoms and physician-diagnosis of depression. Further studies are needed to examine whether the observed association is likely to be causal before meaningful discussions about normalizing IGF-1 levels with drugs could be useful in the prevention of depressive symptoms in older people.
Acknowledgments
This work was supported by an ESRC-MRC studentship to SC. GDB is a member of The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative. Funding from the Biotechnology and Biological Sciences Research Council and Medical Research Council is acknowledged. MK is supported by the Medical Research Council (MR/K013351), the National Heart, Lung and Blood Institute (HL36310), the National Institute of Aging (AG034454), NordForsk, the Nordic Programme of Health and Welfare and an ESRC professorial fellowship (ES/J023299/1). Andrew Steptoe is a British Heart Foundation Professor of Psychology.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- World Health Organization. Depression, Fact sheet No. 369, October 2012. Available at http://www.who.int/mediacentre/factsheets/fs369/en/ [accessed 23 January 2015].
- World Health OrganizationThe Global Burden of Disease: 2004 Update. World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Isometsä E, Henriksson M, Marttunen M, Heikkinen M, Aro H, Kuoppasalmi K et al. Mental disorders in young and middle aged men who commit suicide. BMJ 1995; 310: 1366–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S, Russ TC, Kivimäki M, Stamatakis E, Batty GD. Dose-response association between psychological distress and risk of completed suicide in the general population. JAMA Psychiatry 2015; 11: 1–3. [DOI] [PubMed] [Google Scholar]
- Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA 2011; 306: 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalopoulou M, George J, Walters K, Osborn DP, Batty GD, Stogiannis D et al. Depression as a risk factor for the initial presentation of twelve cardiac, cerebrovascular, and peripheral arterial diseases: data linkage study of 1.9 million women and men. PLoS One 2016; 11: e0153838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J 2006; 27: 2763–2774. [DOI] [PubMed] [Google Scholar]
- Currier MB, Nemeroff CB. Depression as a risk factor for cancer: from pathophysiological advances to treatment implications. Annu Rev Med 2014; 65: 203–221. [DOI] [PubMed] [Google Scholar]
- Davidson IA, Dewey ME, Copeland JRM. The relationship between mortality and mental disorder: evidence from the Liverpool Longitudinal Study. Int J Geriatr Psychiatry 1988; 3: 95–98. [Google Scholar]
- Russ TC, Stamatakis E, Hamer M, Starr JM, Kivimaki M, Batty GD. Association between psychological distress and mortality: individual participant pooled analysis of 10 prospective cohort studies. BMJ 2012; 345: e4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med 2000; 160: 1761–1768. [DOI] [PubMed] [Google Scholar]
- Rodda J, Walker Z, Carter J. Depression in older adults. BMJ 2011; 343: d5219. [DOI] [PubMed] [Google Scholar]
- Volkert J, Schulz H, Härter M, Wlodarczyk O, Andreas S. The prevalence of mental disorders in older people in Western countries—a meta-analyiss. Ageing Res Rev 2013; 12: 339–353. [DOI] [PubMed] [Google Scholar]
- Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry 2003; 160: 1147–1156. [DOI] [PubMed] [Google Scholar]
- Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr Scand 2006; 113: 372–387. [DOI] [PubMed] [Google Scholar]
- Blumberger DM, Daskalakis ZJ, Mulsant BH. Biomarkers in geriatric psychiatry: searching for the holy grail? Curr Opin Psychiatry 2008; 21: 533–539. [DOI] [PubMed] [Google Scholar]
- Blazer DG, Hybels CF. Origins of depression in later life. Psychol Med 2005; 35: 1241–1252. [DOI] [PubMed] [Google Scholar]
- Kalia M, Costa E Silva J. Biomarkers of psychiatric diseases: current status and future prospects. Metabolism 2015; 64(3, Supplement 1): S11–S15. [DOI] [PubMed] [Google Scholar]
- Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med 1997; 336: 633–640. [DOI] [PubMed] [Google Scholar]
- Humbel RE. Insulin-like growth factors I and II. Eur J Biochem 1990; 190: 445–462. [DOI] [PubMed] [Google Scholar]
- Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol 2013; 9: 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippou A, Maridaki M, Halapas A, Koutsilieris M. The role of the insulin-like growth factor 1 (IGF-1) in skeletal muscle physiology. In Vivo 2007; 21: 45–54. [PubMed] [Google Scholar]
- Wit JM, Walenkamp MJ. Role of insulin-like growth factors in growth, development and feeding. World Rev Nutr Diet 2013; 106: 60–65. [DOI] [PubMed] [Google Scholar]
- Rosen CJ. Insulin-like growth factor I and bone mineral density: experience from animal models and human observational studies. Best Pract Res Clin Endocrinol Metab 2004; 18: 423–435. [DOI] [PubMed] [Google Scholar]
- Chhabra Y, Waters MJ, Brooks AJ. Role of the growth hormone-IGF-1 axis in cancer. Expert Rev Endocrinol Metab 2011; 6: 71–84. [DOI] [PubMed] [Google Scholar]
- Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol 2004; 24: 435–444. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Quevedo HC, Tiwari S, Sukhanov S, Shai SY, Anwar A et al. The interaction between IGF-1, atherosclerosis and vascular aging. Front Horm Res 2014; 43: 107–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitschelen M, Yan H, Farley JA, Warrington JP, Han S, Hereñú CB et al. Long-term deficiency of circulating and hippocampal insulin-like growth factor I induces depressive behavior in adult mice: a potential model of geriatric depression. Neuroscience 2011; 185: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Terwilliger R, Russell DS, Newton SS, Duman RS. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav Brain Res 2009; 198: 366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res 2005; 1037: 204–208. [DOI] [PubMed] [Google Scholar]
- Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol 2004; 490: 25–31. [DOI] [PubMed] [Google Scholar]
- Szczesny E, Slusarczyk J, Glombik K, Budziszewska B, Kubera M, Lason W et al. Possible contribution of IGF-1 to depressive disorder. Pharmacol Rep 2013; 65: 1622–1631. [DOI] [PubMed] [Google Scholar]
- van Dam PS, Aleman A, de Vries WR, Deijen JB, van der Veen EA, de Haan EHF et al. Growth hormone, insulin-like growth factor I and cognitive function in adults. Growth Horm IGF Res 2000; 10(Suppl B): S69–S73. [DOI] [PubMed] [Google Scholar]
- Schneider HJ, Pagotto U, Stalla GK. Central effects of the somatotropic system. Eur J Endocrinol 2003; 149: 377–392. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, van Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry 2000; 5: 262–269. [DOI] [PubMed] [Google Scholar]
- Torres Aleman I. Role of insulin-like growth factors in neuronal plasticity and neuroprotection. Adv Exp Med Biol 2005; 567: 243–258. [DOI] [PubMed] [Google Scholar]
- Sievers C, Auer MK, Klotsche J, Athanasoulia AP, Schneider HJ, Nauck M et al. IGF-I levels and depressive disorders: results from the Study of Health in Pomerania (SHIP). Eur Neuropsychopharmacol 2014; 24: 890–896. [DOI] [PubMed] [Google Scholar]
- van Varsseveld NC, van Bunderen, Sohl E, Comijs HC, Penninx BWJH, Lips P et al. Serum insulin-like growth factor 1 and late-life depression: a population-based study. Psychoneuroendocrinology 2015; 54: 31–40. [DOI] [PubMed] [Google Scholar]
- NatCen Social Research. English Longitudinal Study of Ageing (ELSA): Wave One to Wave Five—User Guide to the datasets. Available from http://www.ifs.org.uk/elsa/user_guides/waves_1_5_datasets_user_guide.pdf [accessed on 16 February 2015].
- Freeman Laboratories Newcastle (2012). DPC Immulite 2000—IGF-1. (Laboratory summary of methods used up to 02 February 2012, available from laboratory on request).
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1: 385. [Google Scholar]
- Steffick DE. Documentation of Affective Functioning Measures in the Health and Retirement Study. HRS. Survey Research Center University of Michigan: Ann Arbor, MI, USA, 2000. [Google Scholar]
- Karim J, Weisz R, Bibi Z, ur Rehman S. Validation of the eight-item Center for Epidemiologic Studies Depression Scale (CES-D) among older adults. Curr Psychol 2014; 1–20.
- Blake H, Mo P, Malik S, Thomas S. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehab 2009; 23: 873–887. [DOI] [PubMed] [Google Scholar]
- Reinecke MA, Schultz TM. Comparison of self–report and clinician ratings of depression among outpatient adolescents. Depression 1995; 3: 139–145. [Google Scholar]
- Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr 1999; 11: 139–148. [DOI] [PubMed] [Google Scholar]
- UK Data Archive Study Number 5050—English Longitudinal Study of Ageing (2009). English Longitudinal Study of Ageing Wave Four Interview Questionnaire—2008-2009 Draft Version 1.0. Available from http://www.elsa-project.ac.uk/uploads/elsa/docs_w4/questionnaire_main.pdf [accessed on 3 May 2015].
- Sanchez-Villegas A, Schlatter J, Ortuno F, Lahortiga F, Pla J, Benito S et al. Validity of a self-reported diagnosis of depression among participants in a cohort study using the Structured Clinical Interview for DSM-IV (SCID-I). BMC Psychiatry 2008; 8: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks J, Breeze E, Crawford R, Demakakos P, de Oliveira C, Gjonça E et al. Financial Circumstances, Health and Well-Being of the Older Population in England: The 2008 English Longitudinal Study of Ageing (wave 4). Institute of Fiscal Studies: London, UK, 2010. [Google Scholar]
- Goodman-Gruen D, Barrett-Connor E. Epidemiology of insulin-like growth factor-I in elderly men and women: the Rancho Bernardo Study. Am J Epidemiol 1997; 145: 970–976. [DOI] [PubMed] [Google Scholar]
- StataCorp LP. Stata 12StataCorp: College Station, TX, USA, 2011. [Google Scholar]
- Sievers C, Dimopoulou C, Pfister H, Lieb R, Steffin B, Roemmler J et al. Prevalence of mental disorders in acromegaly: a cross-sectional study in 81 acromegalic patients. Clin Endocrinol (Oxf) 2009; 71: 691–701. [DOI] [PubMed] [Google Scholar]
- McGauley GA, Cuneo RC, Salomon F, Sonksen PH. Psychological well-being before and after growth hormone treatment in adults with growth hormone deficiency. Horm Res 1990; 33(Suppl 4): 52–54. [DOI] [PubMed] [Google Scholar]
- Wexler T, Gunnell L, Omer Z, Kuhlthau K, Beauregard C, Graham G et al. Hormone deficiency is associated with decreased quality of life in patients with prior acromegaly. J Clin Endocrinol Metab 2009; 94: 2471–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle M, Blum WF, Strasburger CJ, Schweiger U, Weber B, Korner A et al. Insulin-like growth factor-I (IGF-I) plasma concentrations are increased in depressed patients. Psychoneuroendocrinology 1997; 22: 493–503. [DOI] [PubMed] [Google Scholar]
- Franz B, Buysse DJ, Cherry CR, Gray NS, Grochocinski VJ, Frank E et al. Insulin-like growth factor 1 and growth hormone binding protein in depression: a preliminary communication. J Psychiatr Res 1999; 33: 121–127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.