Abstract
Aim
To investigate if conjugated linoleic acid supplementation (CLA) affects metabolic factors and oxidative stress in non-alcoholic fatty liver disease (NAFLD).
Methods
The study was a randomized, controlled clinical trial conducted in specialized and subspecialized clinics of Tabriz University of Medical Sciences from January 2014 to March 2015. 38 obese NAFLD patients were randomly allocated into either the intervention group, receiving three 1000 mg softgel of CLA with a weight loss diet and 400 IU vitamin E, or into the control group, receiving only weight loss diet and 400 IU vitamin E for eight weeks. Dietary data and physical activity, as well as anthropometric, body composition, metabolic factors, and oxidative stress were assessed at baseline and at the end of the study.
Results
Weight, body composition, and serum oxidative stress, insulin, and lipid profile significantly improved in both groups, while hemoglobin A1c (HbA1c) levels (P = 0.004), total cholesterol to high density lipoprotein ratio (P = 0.008), low density lipoprotein to high density lipoprotein ratio (LDL/HDL) (P = 0.002), and alanine aminotransferase to aspartate aminotransferase (ALT/AST) ratio (P = 0.025) significantly decreased in the intervention group. At the end of the study, fat mass (P = 0.001), muscle mass (P = 0.023), total body water (P = 0.004), HbA1c (P < 0.001), triglycerides (P = 0.006), LDL/HDL ratio (P = 0.027), and ALT/AST ratio (P = 0.046) were significantly better in the CLA group than in the control group.
Conclusion
CLA improved insulin resistance, lipid disturbances, oxidative stress, and liver function in NAFLD. Therefore, it could be considered as an effective complementary treatment in NAFLD.
Registration number: IRCT2014020516491N1.
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease characterized by the accumulation of large droplets of triglycerides within hepatocytes, contributing to more than 5% of liver weight, in the absence of chronic alcohol consumption (1-3). It encompasses a spectrum of pathologic conditions, from simple steatosis to steatohepatitis, cirrhosis, and rarely hepatocellular carcinoma (3). The estimated prevalence of NAFLD in the general population is 20%-30%, increasing to 70%-90% among obese and diabetic patients (3). Its prevalence in Asian countries varies from 9%-40% (4). NAFLD is strongly associated with obesity, type 2 diabetes, dyslipidemia, and hypertension (5) and is thus considered to be the hepatic manifestation of metabolic syndrome (6).
The pathogenesis of NAFLD is not completely understood, but it can be explained by the multi-hit hypothesis, the first hit being steatosis, triggered by insulin resistance, the second hit being oxidative stress and inflammation, resulting in disease progression (7,8), and the third hit being hepatocyte proliferation progenitors impairment (9).
Although there are no specific guidelines for NAFLD treatment, it is recommended to treat the associated factors by weight reduction, glycemic control, and lipid control (7,10). Therefore, functional foods such as bioactive lipids seem to play a role in modulating metabolism and body weight (7). A specific group of 18 carbon poly-unsaturated fatty acids, known as conjugated linoleic acid (CLA) – a mixture of positional and geometric conjugated isomers of linoleic acid (11,12) – has been shown to regulate energy metabolism and is commercially being used as a weight loss supplement (7). Considerable attention has been paid to biological activates of CLA, which act as a potential therapeutic nutrient through their effects on insulin resistance, hyperlipidemia, and controlling oxidative status (13-15).
Recent studies have shown that trans-10, cis-12 CLA isomer reduces body weight and fat accumulation (16) and in some cases increases insulin resistance, impairs blood glucose and lipid profile (15,17-20), and increases oxidative stress and inflammation (21). However, the effects of cis-9, trans-11 and 50:50 isomers are controversial.
Animal studies have shown that CLA supplementation reduces body weight and body fat mass and improves glycemic status and lipid profiles (22-27), but the results in humans are inconsistent (14,22,25-32). CLA has shown no significant effects on lipid profile, fasting blood glucose, insulin resistance, body composition, and body mass index (BMI) among healthy and hyperlipidemic overweight and obese participants (28,29), as well as diabetic patients (14). In diabetic patients, CLA supplementation (3 g/d) showed negative effects on insulin and glucose metabolism and positive effects on serum HDL metabolism (13), triacylglycerol (TAG), and very low density lipoproteins (VLDL), but did not affect any other biochemical parameters (33). However, in another study it improved body composition, serum glucose, and insulin concentrations without having significant effects on lipid profile (34). In addition, CLA supplementation had no significant effects on lipid peroxidation and antioxidant metabolism among healthy volunteers (35), but had beneficial effects on oxidative stress among atherosclerotic patients (36).
Therefore, as NAFLD prevalence is increasing worldwide and studies investigating the effects of CLA supplementation on patients with NAFLD are rare and inconsistent, we aimed to examine whether weight loss diet with and without CLA supplementation had an effect on insulin resistance, lipid profile, and oxidative stress in obese patients with NAFLD.
Methods
Participants and methods
This randomized controlled clinical trial was conducted from January 2014 to March 2015. 234 participants referred to the specialized and subspecialized clinics of Tabriz University of Medical Sciences underwent ultrasonography by a single sonographist for determining fatty liver. According to the Saverymuttu method (37), the degree of fatty liver was classified as: “mild,” “moderate,” and “severe.” The study protocol was approved by the Ethics Committee of Tabriz University of Medical Sciences and was registered at the Iranian Registry of Clinical Trials website (IRCT2014020516491N1). Written informed consent was obtained from each participant.
Sample size was estimated to be 19 participants in each group, considering 20% change in mean Quicki index reported by Shadman et al (14) using Pocock formula and confidence level of 95% (α = 0.05) and power of 90% (β = 0.10).
Patients confirmed as NAFLD were assessed based on the inclusion criteria, ie, 20-50 years of age, BMI between 30-40 kg/m2, and taking 400 international units (IU) of vitamin E supplement daily. Exclusion criteria were alcohol consumption; pregnancy or lactation, being menopausal and athlete; inflammatory conditions such as infection; hypertension; family history of hyperlipidemia; cardiovascular disease, lung, renal or liver disease; liver transplantation; biliary disease; known autoimmune disease; cancer; burns and injuries during the study; surgery in the last 3 months; use of medications such as antihypertensives, insulin sensitivity enhancers, hepatotoxic drugs, statins, contraceptive pills, and estrogens, as well as vitamin and mineral supplements, and antioxidant supplementation in the last two months. This left 28 and 26 participants in the control and CLA group, respectively (totally 54 patients).
Study design
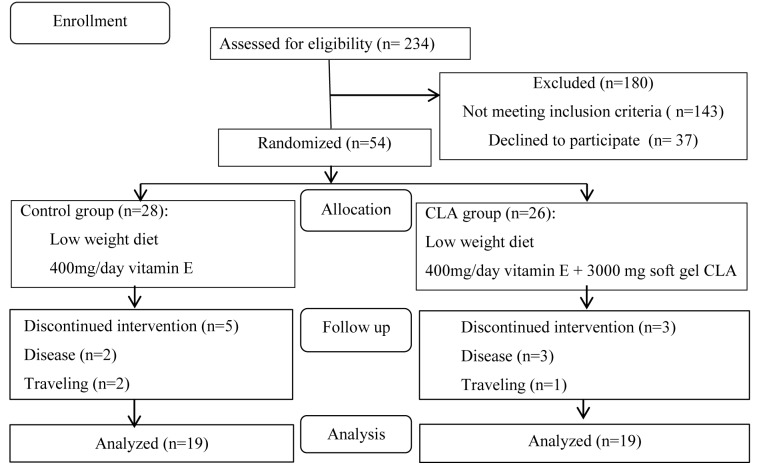
Demographic characteristics and disease history were obtained. All patients received 400 IU/d vitamin E supplement as routine treatment. The patients were randomly allocated into two groups based on BMI, sex, and NAFLD grade using random block (n = 4): the intervention group receiving CLA 80% soft gel 1000 mg supplied by Nutrifit (Nutricentury, Markham, ON, Canada, containing both cis-9, trans-11, and trans-10, cis-12 type CLAs in equal proportion) three times per day with a weight loss diet meal and the control group receiving weight loss diet only for eight weeks (Figure 1). The CLA dosage used in this study has been shown to be non- toxic and without side effects (38-40). Each patient received their supplements in 4 batches, every 2 weeks. The person who determined allocation sequence for the study and those who assigned participants were blinded. The person who analyzed the data was also blinded.
Figure 1.
Flowchart of the study.
A food frequency questionnaire (FFQ) (36) for assessing habitual diet was completed at baseline. For weight loss diet, individual energy requirement was estimated based on the current weight using Harris-Benedict formula (41) minus 700 kcal with the 55:30:15% of energy from C:F:P. All participants were asked to maintain their usual diet and lifestyle habits. 16 participants could not adhere to the protocol because of travel, diseases not related to CLA, discontinued intervention because of failure to follow diet recommendation, and irregular consumption of supplements due to the lack of attention. They were excluded from the study.
Anthropometric and body composition measurement
Weight and height were measured using Seca scale (Hamburg, Germany) and non-stretchable tape to the nearest 100 g and 0.5 cm, respectively, after which BMI was estimated. Waist and hip circumferences were measured after expiration at the midpoint between the lowest rib and the iliac crest and at the widest point between the hip and the buttock to the nearest 0.5 cm, respectively. Waist to hip ratio (WHR) and waist to height ratio (WHtR) were estimated. Body composition was assessed at the beginning and end of the study using body analyzer (Tanita BC-418 Body Composition Analyzer, Arlington Heights, IL, USA)
Dietary assessment
Mean daily dietary intake was assessed through fulfilling a three 3-day food records at baseline, week 4, and week 8. Home measurements and scales were used to quantify the portion sizes. Energy and macronutrients intakes were analyzed using Nutritionist IV software (ver. 3.5.2, San Bruno, CA, USA).
Physical activity measurement
Three physical activity questionnaires (1) were completed at baseline, week 4, and the end of intervention and reported as metabolic equivalents (MET) per day.
Biochemical measurements
10-mL fasting blood samples were taken at the beginning and end of the study. Aliquots of serum were collected in micro tubes and stored at -70◦C until analysis. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), fasting blood glucose (FBS), insulin, lipid profile (total cholesterol [TC], low density lipoprotein cholesterol [LDL-C], high density lipoprotein cholesterol [HDL-C], and triglycerides [TG]), and oxidative stress indices (total antioxidant capacity [TAC], arylesterase [ARE], and malondialdehyde [MDA]). Liver enzymes were measured using International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) method (Biosystem, Barcelona, Spain kit) using autoanalyzer instrument (Hitachi 911 Depok, Indonesia). Lipid profile and FBS were measured using commercial kits and enzymatic colorimetric method (Parsazmun, Tehran, Iran) with autoanalyzer (Abbot, Model Alycon 300, USA). LDL-C was calculated using the Friedwald formula (42). Insulin was measured using enzyme linked immune assay (ELISA) commercial kit (Monobind Inc. Lake Forest, CA, USA). Insulin resistance (IR) was determined using the homeostasis model assessment (HOMA). HOMA-IR and Quantitive Insulin Sensitivity Check Index (Quicki) scores were calculated using the following formulas (14):
HOMA-IR = fasting blood glucose (mmol/L) × serum insulin (μU/mL) /22.5
Quicki = 1/log fasting blood glucose (mmol/L) + log serum insulin (μU/mL)
Spectrophotometry method with phenylacetate as a substrate was applied to measure serum ARE activity. TAC was measured using spectrophotometry method with Randox TAS kit (radical ABTS; Randox Laboratories, Antrim, United Kingdom). However, spectrophotometry technique for MDA assessment was based on its reaction with thiobarbituric acid.
Statistical analysis
All statistical analyses were performed using SPSS software (ver. 16.0, SPSS Inc., Chicago, IL, USA). The normality of distribution of continuous variables was tested using Kolmogorov-Smirnov test. Data for normally distributed variables are shown as mean ± standard deviation. Independent t test was used to compare the variable means between the two groups and analysis of covariance (ANCOVA) was applied for adjusting the covariates. To compare the change in the studied variables over the study period in each group, paired t test and Wilcoxon rank t test were used. A repeated measure analysis was also applied to assess the changes in dietary intakes and physical activity over the study period. P-<0.050 was considered as significant.
Results
38 of 54 patients (19 in each group) completed the study (Figure 1). At baseline, there were no significant differences between the groups in age (36.74 ± 6.87 years and 38.58 ± 8.24 years in intervention and control group, respectively), sex, marital status, education level, and severity of fatty liver disease (Table 1). The control group had significantly greater mean weight, waist and hip circumferences than CLA group (P = 0.031, P = 0.030, and P = 0.015, respectively), Thus, these factors were considered as covariates in the analysis (Table 2). At the end of the study, anthropometric measurements decreased significantly in both groups, but there were no significant differences between the groups. Fat mass, muscle mass, and total body water improved significantly after the study in the CLA group compared to the control group (P = 0.001, P = 0.023, and P = 0.004, respectively).
Table 1.
Baseline characteristics of the group treated with conjugated linoleic acid and controls
| Characteristic | Control (n = 19) (%) | Conjugated linoleic acid (n = 19) (%) | P* |
|---|---|---|---|
| Female |
84.2 |
89.5 |
0.999 |
| Single |
89.5 |
100 |
0.486 |
| Educational level |
|||
| before high school and high school |
57.9 |
57.9 |
0.999 |
| university degree |
42.1 |
42.1 |
|
| Severity of fatty liver |
|||
| mild |
78.9 |
68.4 |
0.461 |
| moderate and severe | 21.1 | 31.6 |
*χ2 test.
Table 2.
Anthropometric measurements and body composition before and after conjugated linoleic acid supplementation (CI – confidence interval, MD – mean difference)
| Variable | Control (n = 19, mean ± standard deviation) | Conjugated linoleic acid (n = 19, mean ± standard deviation) | P |
|---|---|---|---|
| Height (cm) |
159.24 ± 6.67 |
158.81 ± 8.93 |
0.867† |
| Bone |
2.726 ± 0.378 |
2.942 ± 0.373 |
0.105† |
| Weight (kg) |
|||
| before |
89.36 ± 9.34 |
82.10 ± 10.60 |
0.031† |
| after |
84.84 ± 9.56 |
77.30 ± 10.45 |
0.559‡ |
| MD (CI 95%) |
4.5(3.71 to 5.28) |
4.80(4.10 to 5.50) |
|
|
P* |
<0.001 |
<0.001 |
|
| Body mass index (kg/m2) |
|||
| before |
35.27 ± 3.46 |
32.72 ± 4.63 |
0.064† |
| after |
33.50 ± 3.63 |
30.80 ± 4.45 |
0.460‡ |
| MD (CI 95%) |
1.77(1.46 to 2.07) |
1.97(1.26 to 2.22) |
|
|
P* |
<0.001 |
<0.001 |
|
| Waist circumference(cm) |
|||
| before |
109.28 ± 9.92 |
103.18 ± 6.34 |
0.030† |
| after |
103.70 ± 10.34 |
97.34 ± 7.01 |
0.952‡ |
| MD (CI 95%) |
5.58(4.63 to 6.53) |
5.84(4.72 to 6.96) |
|
|
P* |
<0.001 |
<0.001 |
|
| Hip circumference(cm) |
|||
| before |
119.73 ± 7.89 |
113.68 ± 6.75 |
0.015† |
| after |
116.11 ± 8.19 |
109.08 ± 7.09 |
0.342‡ |
| MD (CI 95%) |
4.60(3.45 to 5.57) |
3.63 (2.69 to 5.56) |
|
|
P* |
<0.001 |
<0.001 |
|
| Waist to hip ratio |
|||
| before |
0.91 ± 0.047 |
0.90 ± 0.047 |
0.804† |
| after |
0.89 ± 0.066 |
0.89 ± 0.054 |
0.530‡ |
| MD (CI 95%) |
0.02(0.01 to 0.021) |
0.01(0.00 to 0.02) |
|
|
P* |
<0.001 |
<0.001 |
|
| Waist to height ratio |
|||
| before |
0.68 ± 0.067 |
0.65 ± 0.053 |
0.080† |
| after |
0.65 ± 0.069 |
0.61 ± 0.054 |
0.778‡ |
| MD (CI 95%) |
0.03(0.02 to 0.04) |
0.03 (0.02 to 0.04) |
|
|
P* |
<0.001 |
<0.001 |
|
| Fat mass (%) |
|||
| before |
46.71 ± 6.35 |
46.05 ± 5.71 |
0.737† |
| after |
45.55 ± 6.69 |
41.43 ± 6.46 |
0.001‡ |
| MD (CI 95%) |
1.16(0.39 to 1.93) |
4.61(2.81 to 6.41) |
|
|
P* |
0.005 |
<0.001 |
|
| Muscle mass (%) |
|||
| before |
32.44 ± 4.36 |
33.92 ± 2.57 |
0.210† |
| after |
33.02 ± 4.34 |
35.55 ± 2.79 |
0.023‡ |
| MD (CI 95%) |
-0.63(-0.82 to -0.44) |
-1.63(-2.39 to -0.88) |
|
|
P* |
<0.001 |
<0.001 |
|
| Total body water |
|||
| before |
36.31 ± 4.35 |
36.04 ± 3.90 |
0.846† |
| after |
38.14 ± 4.59 |
38.14 ± 4.41 |
0.004‡ |
| MD (CI 95%) |
-0.45(-1.00 to 0.09) |
-0.094(-3.02 to -1.161) |
|
| P* | 0.102 | <0.001 |
*Paired t- test.
†Independent t test.
‡Analysis of covariance (ANCOVA) adjusted for baseline values and energy intake.
There were no differences in energy and macronutrient intakes between the two groups at baseline, week 4, and week 8, except for fat intake, which was significantly higher at week 8 in the CLA group (P = 0.019). Significant change in total energy intake in the control group was considered as a confounder in advanced analysis. Repeated measure analysis found no significant changes in physical activity (expressed as MET/d) over the study period (Table 3).
Table 3.
Daily total energy and macronutrient intakes and physical activity
| Variable | Control (n = 19, mean ± standard deviation) | Conjugated linoleic acid (n = 19, mean ± standard deviation) | P* |
|---|---|---|---|
| Energy (kcal) |
|||
| week 0 |
1272.71 ± 292.33 |
1120.68 ± 306.25 |
0.126 |
| week 4 |
1124.09 ± 334.92 |
1154.96 ± 252.81 |
0.757 |
| week 8 |
1157.18 ± 340.92 |
1171.45 ± 325.60 |
0.897 |
|
P† |
0.020 |
0.921 |
|
| Carbohydrate (g) |
|||
| week 0 |
192.73 ± 56.51 |
161.67 ± 56.57 |
0.099 |
| week 4 |
176.57 ± 59.45 |
165.09 ± 35.30 |
0.416 |
| week 8 |
182.85 ± 69.23 |
163.72 ± 42.53 |
0.321 |
|
P† |
0.441 |
0.833 |
|
| Protein (g) |
|||
| week 0 |
52.70 ± 14.03 |
49.96 ± 13.92 |
0.510 |
| week 4 |
51.44 ± 14.21 |
49.77 ± 12.20 |
0.708 |
| week 8 |
48.35 ± 10.40 |
48.19 ± 12.55 |
0.887 |
|
P† |
0.298 |
0.808 |
|
| Fat (g) |
|||
| week 0 |
30.71 ± 8.09 |
31.90 ± 9.41 |
0.680 |
| week 4 |
28.20 ± 12.19 |
35.83 ± 13.26 |
0.081 |
| week 8 |
26.88 ± 7.98 |
34.90 ± 11.67 |
0.019 |
|
P† |
0.213 |
0.435 |
|
| Physical activity (metabolic equivalents /d) |
|||
| week 0 |
36.41 ± 4.20 |
38.27 ± 2.69 |
0.117 |
| week 4 |
36.06 ± 4.12 |
38.69 ± 2.61 |
0.027 |
| week 8 |
36.75 ± 3.76 |
38.28 ± 2.44 |
0.148 |
| P† | 0.203 | 0.439 |
*Independent t test.
†Repeated measure analyses.
At baseline, the groups had similar glycemic index and liver enzyme, while CLA group had significantly higher total cholesterol (P = 0.042). At the end of the study, fasting glucose concentration non-significantly decreased in CLA group. HbA1c significantly decreased in the CLA group during the study (P = 0.004) and after the intervention it was lower than in the control group (P < 0.001). Insulin significantly increased during the study in both groups (P = 0.024 and P = 0.020 in the control and CLA group, respectively). HOMA-IR score significantly increased at week 8 compared to baseline in the control group (P < 0.001), while it decreased non-significantly in the CLA group. Quicki index increased non-significantly in both groups. Also, total cholesterol levels, triglycerides, and LDL significantly decreased in both groups, and TC/LDL and LDL/HDL ratios significantly decreased only in the CLA group (P = 0.008 and P = 0.002, respectively). Also, TG and LDL/HDL ratio significantly decreased in the CLA group compared to the control group (P = 0.006 and 0.027, respectively). ALT/AST ratio significantly decreased in the CLA group (P = 0.025). While this ratio non-significantly increased in the control group, the reduction in ALT/AST ratio in CLA group was significantly higher than in the control group (P = 0.046) (Table 4).
Table 4.
Metabolic variables before and after conjugated linoleic acid supplementation (CI – confidence interval; MD – mean difference)
| Variable | Control (n = 19, mean ± standard deviation) | Conjugated linoleic acid (n = 19, mean ± standard deviation) | P |
|---|---|---|---|
| Fasting blood glucose (mg/dL) |
|||
| before |
96.16 ± 10.43 |
102.26 ± 18.05 |
0.210† |
| after |
98.47 ± 11.29 |
98.68 ± 10.26 |
0.683‡ |
| MD (CI 95%) |
1.16 (0.39 to 1.93) |
4.61 (2.81 to 6.41) |
|
|
P* |
0.074 |
0.392 |
|
| Hemoglobin A1c (%) |
|||
| before |
4.57 ± 0.08 |
4.49 ± 0.77 |
0.774† |
| after |
4.74 ± 0.88 |
3.99 ± 0.63 |
<0.001‡ |
| MD (CI 95%) |
-0.17 (-0.36 to 0.02) |
0.50 (0.17 to 0.83) |
|
|
P* |
0.077 |
0.004 |
|
| Insulin (μIU/mL) |
|||
| before |
13.87 ± 7.34 |
13.37 ± 4.04 |
0.797† |
| after |
14.55 ± .7.45 |
13.89 ± 4.25 |
0.423‡ |
| MD (CI95%) |
-0.67 (-1.25 to -1.00) |
-0.51 (-0.94 to -0.09) |
|
|
P* |
0.024 |
0.020 |
|
| Homeostatic model assessment-insulin resistance |
|||
| before |
3.26 ± 1.77 |
3.4 ± 1.33 |
0.795† |
| after |
3.50 ± 1.83 |
3.39 ± 1.12 |
0.178‡ |
| MD (CI 95%) |
-0.23 (-0.03 to -0.12) |
0.006 (-0.26 to 0.27) |
|
|
P* |
<0.001 |
0.096 |
|
| Quantitative insulin sensitivity check index |
|||
| before |
1.57 ± 0.29 |
1.60 ± 0.15 |
0.669† |
| after |
1.59 ± 0.26 |
1.62 ± 0.14 |
0.495‡ |
| MD (CI 95%) |
-0.02 (-0.06 to 0.001) |
-0.01 (-0.09 to 0.00) |
|
|
P* |
0.080 |
0.053 |
|
| Cholesterol(mg/dL) |
|||
| before |
188.74 ± 27.76 |
213.11 ± 41.66 |
0.042† |
| after |
176.95 ± 24.62 |
203.37 ± 39.51 |
0.179‡ |
| MD(CI 95%) |
11.78 (6.26 to 17.31) |
9.737 (1.75 to 17.21) |
|
|
P* |
<0.001 |
0.020 |
|
| Triglyceride(mg/dL) |
|||
| before |
164.05 ± 92.27 |
149.42 ± 63.60 |
0.573† |
| after |
143.95 ± 81.69 |
110.15 ± 48.46 |
0.006‡ |
| MD (CI 95%) |
20.10 (7.098 to 33.11) |
39.26 (27.54 to 50.99) |
|
|
P* |
0.004 |
<0.001 |
|
| Low density lipoprotein (mg/dL) |
|||
| before |
25.23 ± 26.73 |
138.53 ± 33.13 |
0.182† |
| after |
115.58 ± .24.72 |
122.80 ± 29.53 |
0.616‡ |
| MD (CI 95%) |
9.65 (5.02 to 14.28) |
15.72 (6.16 to 25.28) |
|
|
P* |
<0.001 |
0.003 |
|
| High density lipoprotein (mg/dL) |
|||
| before |
45.95 ± 7.46 |
49.63 ± 15.41 |
0.354† |
| after |
44.89 ± 9.86 |
51.95 ± 16.90 |
0.114‡ |
| MD (CI 95%) |
1.05 (-1.20 to 3.31) |
-2.31 (-6.87 to 2.24) |
|
|
P* |
0.34 |
0.300 |
|
| Total cholesterol/high density lipoprotein |
|||
| before |
4.19 ± 0.77 |
4.72 ± 1.88 |
0.259† |
| after |
4.09 ± 0.9 |
4.26 ± 1.52 |
0.150‡ |
| MD (CI 95%) |
0.09 (-0.06 to 0.24) |
0.46 (0.13 to 0.79) |
|
|
P* |
0.277 |
0.008 |
|
| Low density lipoprotein /high density lipoprotein |
|||
| before |
2.79 ± 0.68 |
3.08 ± 1.28 |
0.380† |
| after |
2.70 ± 0.84 |
2.56 ± 0.93 |
0.027‡ |
| MD (CI 95%) |
0.08(-0.07 to 0.23) |
0.52 (0.22 to 0.83) |
|
|
P* |
0.270 |
0.002 |
|
| Aspartate aminotransferase (Iu/L) |
|||
| before |
18.26 ± 3.94 |
19.89 ± 17.36 |
0.405† |
| after |
18.05 ± 3.14 |
17.33 ± 4.21 |
0.375‡ |
| MD (CI 95%) |
0.21 (-1.50 to 1.93) |
2.55 (-0.41 to 5.52) |
|
|
P* |
0.800 |
0.087 |
|
| Alanine aminotransferase (Iu/L) |
|||
| before |
22.37 ± 9.04 |
24.82 ± 23.01 |
0.798† |
| after |
31.74 ± 28.24 |
17.83 ± 10.40 |
0.086‡ |
| MD (CI 95%) |
-8.36 (-22.04 to 5.30) |
7 (-1.16 to 15.16) |
|
|
P* |
0.81 |
0.215 |
|
| Alanine aminotransferase/ aspartate aminotransferase |
|||
| before |
1.24 ± 0.31 |
1.12 ± 0.44 |
0.340† |
| after |
1.81 ± 1.95 |
0.98 ± 0.39 |
0.046|| |
|
P§ |
0.159 |
0.025 |
|
| Total antioxidant capacity (mmol/L) |
|||
| before |
1.36 ± 0.31 |
1.21 ± 0.34 |
0.182† |
| after |
1.58 ± 0.38 |
1.32 ± 0.42 |
0.285‡ |
| MD (CI 95%) |
-0.22 (-0.36 to -0.07) |
-0.0/1 (-0.2 to 0.01) |
|
|
P* |
0.005 |
0.028 |
|
| Aryl esterase (u/L) |
|||
| before |
111.0 ± 29.85 |
118.89 ± 28.41 |
0.409† |
| after |
124.26 ± 30.99 |
133.68 ± 21.54 |
0.694‡ |
| MD (CI 95%) |
-13.26 (-20.05 to -6.47) |
-14.78 (-23.05 to -6.53) |
|
|
P* |
0.001 |
0.001 |
|
| Malondialdehyde (mmol/mL) |
|||
| before |
2.57 ± 0.61 |
2.87 ± 1.10 |
0.314† |
| after |
2.31 ± 0.57 |
2.38 ± 0.59 |
0.437‡ |
| MD (CI 95%) |
021 (0.15 to 0.37) |
0.49 (0.17 to 0.80) |
|
| P* | <0.001 | 0.004 |
*Paired t- test.
†Independent t test.
‡Analysis of covariance (ANCOVA) adjusted for baseline values, energy intake, weight circumference and hip circumference.
§Wilcoxon rank t test.
||Mann-Whitney u test.
Discussion
To our knowledge, this is the first study investigating the effects of CLA in combination with vitamin E and low-calorie diet in patients with NAFLD disease. We found that CLA supplementation improved body composition, lipid profile, oxidative stress, liver function, and serum HbA1c.
In our study, CLA group had decreased insulin resistance assessed by HOMA-IR, which might be attributed to energy expenditure, increasing peroxisome proliferators-activated receptor (PPARγ) expression – acting as a ligand for this receptor (22) and adiponectin production (43). Our findings were similar to a study on sedentary women with metabolic syndrome (44), healthy women (34), and diabetic patients (13). However, in most studies in humans, CLA supplementation had no effect on glycemic status and lipid profile (14,25,29,31,32). In the present study, CLA supplementation improved lipid profile and significantly reduced serum TG levels (12.25%) and LDL/HDL ratio (16.88%), which was shown to be the best single predictor of cardiovascular disease (45). Therefore, the effect of CLA supplementation on LDL/HDL cholesterol in NAFLD patients may be of clinical benefit. Maloney et al (13) showed that a similar dose of CLA in a similar study period as in our study significantly reduced LDL/HDL cholesterol ratio among type 2 diabetic patients. Another study showed that CLA supplementation significantly improved plasma TG levels in normo-lipidemic participants (33), while no changes in serum total cholesterol, HDL, LDL, and triglyceride level were found among healthy women (34). In contrast, 5.5 g/d CLA for 16 weeks increased serum TG and TC/HDL ratio and decreased HDL-C among postmenopausal women (46). The inconsistency in results may be due to differences in study populations, CLA dose, and the intervention period. The hypotriacylglycerol effect of CLA might be explained by the fact that CLAs are potent agonists of PPARs, including PPARα, which is the key transcription factor regulating hepatic lipid metabolism, and PPARγ, which regulates the expression of the genes determining adipogenesis, lipid metabolism, and insulin sensitivity. Some other proposed mechanisms affect peroxisome proliferators-sterol regulatory element-binding proteins (SREBPs), and stearoyl-CoA desaturase (SCD). SREBP1c isoforms regulate fatty acid and TG synthesis (47).
The present study found non-significant reductions in ALT serum levels (28.16%), however the change in ALT/AST ratio (12.5%) was significant. Although there is a limited number of studies examining the effect of CLA mix on liver function, Nagoa et al (43) found that 8-week diet containing 1% CLA significantly decreased serum AST and ALT levels in obese, diabetic Zucker rats. Another study also found that CLA consumption significantly reduced serum ALT concentration in Zucker rats (27). On the other hand, a study in healthy non-obese sedentary women, similarly to our study, failed to show any significant difference in serum ALT levels (48). The effect of CLA on liver function could be due to the enhancement of adiponectin production (43).
ARE is synthesized by the liver and hydrolyzes organophosphate compounds in mammals (49). In our study, serum ARE levels increased significantly in both groups, but no significant difference was shown between the two groups. There is a limited number of studies examining the effects of CLA supplementation on serum ARE levels. Ariyaeian et al (47) reported no significant difference in serum ARE level between patients with rheumatoid arthritis who received supplementation with 2gr CLA + 400 mg vitamin E and those who received corn oil for three months. In the present study, serum MDA levels decreased significantly and TAC increased significantly without any differences between the groups. The studies on Sprague-Dawley rats and mice (15,50) showed that CLA significantly reduced MDA levels. In addition, Aliasghari et al (36) reported a significant reduction of MDA after CLA supplementation in atherosclerosis. The effect of CLA on oxidative stress improvement might be explained by its antioxidant activity (51), induction of the activity of antioxidant enzymes (52), and decreasing lipid peroxidation (53).
This study has several limitations. It was not possible to estimate dietary intake and serum CLA concentration. We also had a relatively small sample size, short follow-up period, and did not use placebo in the control group. Therefore, further long-term placebo-controlled clinical trials are required to study the effect CLA supplementation. Due to insufficient information regarding molecular mechanisms of CLA in humans, human cell culture studies are also recommended.
Accepted: May 2, 2016
Acknowledgment
We thank the study participants for their enthusiasm and commitment to the study protocol.
Funding This study was supported by a research grant from Nutrition Research center, Tabriz University of Medical Sciences, Iran.
Ethical approval received from the Ethics Committee of Tabriz University of Medical Sciences and was registered at the Iranian Registry of Clinical Trials website (IRCT2014020516491N1) and written informed consent was obtained from each subject.
Declaration of authorship All authors take the responsibility for the reported research and approve the submitted version of the manuscript. All authors made a significant contribution to the final version of the manuscript. MEM designed the study protocol, interpreted the data, revised the manuscript, and prepared the final draft. HJ assisted with sampling, data analysis, and preparing the first draft of the manuscript. RM assisted in commenting to the first draft of the manuscript. FK assessed liver ultrasonography for all patients. RA and BKM assisted with sampling and data analysis.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
AUTHOR QUERIES
The citation to Reference 41 appears to be out of order.
The citation to Reference 42 appears to be out of order.
The citation to Reference 34 appears to be out of order.
The citation to Reference 35 appears to be out of order.
The citation to Reference 36 appears to be out of order.
The citation to Reference 37 appears to be out of order.
The citation to Reference 38 appears to be out of order.
The citation to Reference 39 appears to be out of order.
The citation to Reference 40 appears to be out of order.
References
- 1.Ebrahimi-Mameghani M, Aliashrafi S, Javadzadeh Y. AsghariJafarabadi M. The effect of microalgae chlorella vulgaris supplementation on lipid profile and lipid peroxidation in non-alcoholic fatty liver disease: a double- blind randomized clinical trial. Health Promotion Perspectives. 2014;4:107–15. doi: 10.5681/hpp.2014.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118:277–83. doi: 10.1161/CIRCULATIONAHA.107.739920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–50. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 4.Kalra S, Vithalani M, Gulati G, Kulkarni CM, Kadam Y, Pallivathukkal J, et al. Study of Prevalence of Nonalcoholic Fatty Liver Disease (NAFLD) in Type 2 Diabetes Patients in India (SPRINT). J Assoc Physicians India. 2013;61:448–53. [PubMed] [Google Scholar]
- 5.Brea A, Mosquera D, Martín E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Vasc Biol. 2005;25:1045–50. doi: 10.1161/01.ATV.0000160613.57985.18. [DOI] [PubMed] [Google Scholar]
- 6.Vanni E, Bugianesi E, Kotronen A, De Minicis S, Yki-Järvinen H, Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis. 2010;42:320–30. doi: 10.1016/j.dld.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Vyas D, Kadegowda AK, Erdman RA. Dietary conjugated linoleic acid and hepatic steatosis: species-specific effects on liver and adipose lipid metabolism and gene expression. J Nutr Metab. 2012;2012:932928. doi: 10.1155/2012/932928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramesh K, Shyam P, Shruti C, Vikas S, Kaushal M, Datta G, et al. Association of pro-inflammatory cytokines, adipokines & oxidative stress with insulin resistance & non-alcoholic fatty liver disease. Indian J Med Res. 2012;136:229–36. [PMC free article] [PubMed] [Google Scholar]
- 9.Dowman J, Tomlinson J. Newsome pathogenesis of non-alcoholic fatty liver disease. Q J Med. 2010;103:71–83. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Piano A, Prado WL, Caranti DA, Siqueira KO, Stella SG, Lofrano M, et al. Metabolic and nutritional profile of obese adolescents with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2007;44:446–52. doi: 10.1097/MPG.0b013e31803815d9. [DOI] [PubMed] [Google Scholar]
- 11.Lambert E, Goedecke J, Bluett K, Heggie K, Claassen A, Rae D, et al. Conjugated linoleic acid versus high-oleic acid sunflower oil: effects on energy metabolism, glucose tolerance, blood lipids, appetite and body composition in regularly exercising individuals. Br J Nutr. 2007;97:1001–11. doi: 10.1017/S0007114507172822. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin S, Spener F. Conjugated linoleic acids as functional food: an insight into their health benefits. Nutr Metab (Lond) 2009;6:36. doi: 10.1186/1743-7075-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moloney F, Yeow TP, Mullen A, Nolan JJ, Roche HM. Conjugated linoleic acid supplementation, insulin sensitivity, and lipoprotein metabolism in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2004;80:887–95. doi: 10.1093/ajcn/80.4.887. [DOI] [PubMed] [Google Scholar]
- 14.Shadman Zh, Taleban F, Saadat N, Hedayat M. Effect of conjugated linoleic acid and vitamin E on glycemic control, body composition, and inflammatory markers in overweight type2 diabetics. J Diabetes Metab Disord. 2013;12:42. doi: 10.1186/2251-6581-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman MD, Halade GV, Jamali A, Feranandes G. Conjugated linoleic acid (CLA) prevents age associated skeletal muscle loss. Biochem Biophys Res Commun. 2009;383:513–8. doi: 10.1016/j.bbrc.2009.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang YW, Jones PJ. Conjugated linoleic acid and obesity control: efficacy and mechanisms. Int J Obes Relat Metab Disord. 2004;28:941–55. doi: 10.1038/sj.ijo.0802641. [DOI] [PubMed] [Google Scholar]
- 17.Risérus U, Smedman A, Basu S, Vessby B. CLA and body weight regulation in humans. Lipids. 2003;38:133–7. doi: 10.1007/s11745-003-1043-7. [DOI] [PubMed] [Google Scholar]
- 18.Risérus U, Smedman A, Basu S, Vessby B. Metabolic effects of conjugated linoleic acid in humans: the Swedish experience. Am J Clin Nutr. 2004;79(6) Suppl:1146S–8S. [PubMed] [Google Scholar]
- 19.Riserus U, Vessby B, Arner P. Supplementation with trans10cis12-conjugated linoleic acid induces hyperproinsulinaemia in obese men: close association with impaired insulin sensitivity. Diabetologia. 2004;47:1016–9. doi: 10.1007/s00125-004-1421-8. [DOI] [PubMed] [Google Scholar]
- 20.Brown JM, McIntosh MK. Conjugated linoleic acid in humans: regulation of adiposity and insulin sensitivity. J Nutr. 2003;133:3041–6. doi: 10.1093/jn/133.10.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Risérus U, Basu S, Jovinge S, Fredrikson GN, Arnlöv J, Vessby B. Supplementation with conjugated linoleic acid causes isomer-dependent oxidative stress and elevated C-reactive protein: a potential link to fatty acid-induced insulin resistance. Circulation. 2002;106:1925–9. doi: 10.1161/01.CIR.0000033589.15413.48. [DOI] [PubMed] [Google Scholar]
- 22.Xr Z, Ch S, Liu IR, Zhou D. Dietary conjugated linoleic acid increase PPAR gamma gene expression in adipose tissue of obese rat and improve insulin resistance. Growth Horm IGF Res. 2008;18:361–8. doi: 10.1016/j.ghir.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Halade GV, Rahman MM, Fernandes G. Differential effects of conjugated linoleic acid isomers in insulin-resistant female C57Bl/6J mice. J Nutr Biochem. 2010;21:332–7. doi: 10.1016/j.jnutbio.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Parra P, Palou A. Serra. Moderate dose of conjugated linoleic acid reduce fat gain, maintain insulin sensitivity whitout impairing inflammatory adipose tissue status in mice fed a high fat diet. Nutr Metab (Lond) 2010;7:5. doi: 10.1186/1743-7075-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkatramanan S, Joseph SV, Chouinard PY, Jacques H, Farnworth ER, Jones PJ. Milk enriched with conjugated linoleic acid fails to alter blood lipids or body composition in moderately overweight, borderline hyperlipidemic individuals. J Am Coll Nutr. 2010;29:152–9. doi: 10.1080/07315724.2010.10719829. [DOI] [PubMed] [Google Scholar]
- 26.Bouthegourd JC, Even PC, Gripois D, Tiffon B, Blouquit MF, Roseau S, et al. A CLA mixture prevents body triglyceride accumulation without affecting energy expenditure in Syrian hamsters. J Nutr. 2002;132:2682–9. doi: 10.1093/jn/132.9.2682. [DOI] [PubMed] [Google Scholar]
- 27.Noto A, Zahradka P, Yurkova N, Xie X, Nitschmann E, Ogborn M, et al. Conjugated linoleic acid reduces hepatic steatosis, improves liver function, and favorably modifies lipid metabolism in obese insulin-resistant rats. Lipids. 2006;41:179–88. doi: 10.1007/s11745-006-5086-6. [DOI] [PubMed] [Google Scholar]
- 28.Shama V, Jacques H, Me’lanie P, Patricia L, Roger S, Peter J. Conjugated linoleic acid supplementation for 8 weeks does not affect body composition, lipid profile, or safety biomarkers in overweight, hyperlipidemic men. J Nutr. 2011;141:1286–91. doi: 10.3945/jn.110.135087. [DOI] [PubMed] [Google Scholar]
- 29.Taylor JS, Williams SR, Rhys R, James P, Frenneaux MP. Conjugated linoleic acid impairs endothelial function. Arterioscler Thromb Vasc Biol. 2006;26:307–12. doi: 10.1161/01.ATV.0000199679.40501.ac. [DOI] [PubMed] [Google Scholar]
- 30.Pintus S, Murru E, Carta G, Cordeddu L, Batetta B, Accossu S, et al. Sheep cheese naturally enriched in α-linolenic, conjugated linoleic and vaccenic acids improves the lipid profile and reduces anandamide in the plasma of hypercholesterolaemic subjects. Br J Nutr. 2013;109:1453–62. doi: 10.1017/S0007114512003224. [DOI] [PubMed] [Google Scholar]
- 31.Chen SC, Lin YH, Huang HP, Hsu WL, Houng JY, Huang CK. Effect of conjugated linoleic acid supplementation on weight loss and body fat composition in a Chinese population. Nutrition. 2012;28:559–65. doi: 10.1016/j.nut.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Smedman A, Vessby B. Conjugated linoleic acid supplementation in humans–metabolic effects. Lipids. 2001;36:773–81. doi: 10.1007/s11745-001-0784-7. [DOI] [PubMed] [Google Scholar]
- 33.Noone EJ, Roche HM, Nugent AP, Gibney MJ. The effect of dietary supplementation using isomeric blends of conjugated linoleic acid on lipid metabolism in healthy human subjects. Br J Nutr. 2002;88:243–51. doi: 10.1079/BJN2002615. [DOI] [PubMed] [Google Scholar]
- 34.Colakoglu S, Colakoglu M, Taneli F, Cetinoz F, Turkmen M. Cumulative effects of conjugated linoleic acid and exercise on endurance development, body composition, serum leptin and insulin levels. J Sports Med Phys Fitness. 2006;46:570–7. [PubMed] [Google Scholar]
- 35.Kim J, Park HD, Shin MJ, Park E. Eight weeks of conjugated linoleic acid supllementation has no effect on antioxidant status in healthy overweight/obese Korean individuals. Eur J Nutr. 2012;51:135–41. doi: 10.1007/s00394-011-0199-y. [DOI] [PubMed] [Google Scholar]
- 36.Eftekhari M, Aliasghari F, Babaei-Beigi M, Hasanzadeh J. Effect of conjugated linoleic acid and omega-3 fatty acid supplementation on inflammatory and oxidative stress markers in atherosclerotic patients. ARYA Atheroscler. 2013;9:311–8. [PMC free article] [PubMed] [Google Scholar]
- 37.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. BMJ. 1986;292:13–5. doi: 10.1136/bmj.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yavari A, Hamedi M, Heshmati A, Haghbin S. Are conjugated linoleic acid (CLA) isomers good or bad trans fats? Lipid Technology. 2010;22:227–9. doi: 10.1002/lite.201000056. [DOI] [Google Scholar]
- 39.Crumb DJ. Conjugated linoleic acid (CLA)-an overview. Int J App Res in Nat Pro. 2011;4:12–18. [Google Scholar]
- 40.Walsh M, PA-S. What is the safety and efficacy of conjugated linoleic acid used for weight loss supplements?. PCOM. Physician Assistant Studies Student Scholarship. 2011; Paper 2.
- 41.Shils M, Shike M, Ross A, Caballero B, Cousins R. Modern nutrition in health and disease, 10 ed. Philadelphia: Wolters Kluwer; 2006. [Google Scholar]
- 42.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 43.Nagao K, Inoue N, Wang Y, Shirouchi B, Yanagita T. Dietary conjugated linoleic acid alleviates nonalcoholic fatty liver disease in Zucker (fa/fa) rats. J Nutr. 2005;135:9–13. doi: 10.1093/jn/135.1.9. [DOI] [PubMed] [Google Scholar]
- 44.Carvalho RF, Uehara SK, Rosa G. Microencapsulated conjugated linoleic acid associated with hypocaloric diet reduces body fat in sedentary women with metabolic syndrome. Vasc Health Risk Manag. 2012;8:661–7. doi: 10.2147/VHRM.S37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tholstrup T, Raff M, Straarup EM, Lund P, Basu S, Bruun JM. An oil mixture with trans-10, cis-12 conjugated linoleic acid increases markers of inflammation and in vivo lipid peroxidation compared with cis-9, trans-11 conjugated linoleic acid in postmenopausal women. J Nutr. 2008;138:1445–51. doi: 10.1093/jn/138.8.1445. [DOI] [PubMed] [Google Scholar]
- 46.Rader DJ, Davidson MH, Caplan RJ, Pears JS. Lipid and lipoprotein ratios: association with coronary artery disease and effects of rosuvastatin compared with atorvastatin, pravastatin, and simvastatin. Am J Cardiol. 2003;91:20c–3c. doi: 10.1016/S0002-9149(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 47.Aryaeian N, Shahram F, Djalali M, Eshragian M, Djazayeri A, Sarrafnejad A, et al. Effect of conjugated linoleic acid, vitamin E and their combination on lipid profi les and blood pressure of Iranian adults with active rheumatoid arthritis. Vasc Health Risk Manag. 2008;4:1423–32. doi: 10.2147/vhrm.s3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petridou A, Mougios V, Sagredos A. Supplementation with CLA: isomer incorporation into serum lipids and effect on body fat of women. Lipids. 2003;38:805–11. doi: 10.1007/s11745-003-1129-2. [DOI] [PubMed] [Google Scholar]
- 49.Litvinov D, Mahini H, Garelnabi M. Antioxidant and Anti-inflammatory role of paraoxonase 1: Implication in arteriosclerosis diseases. N Am J Med Sci. 2012;4:523–32. doi: 10.4103/1947-2714.103310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi JS, Koh IU, Jung MH, Song J. Effects of three different conjugated linoleic acid preparations on insulin signalling, fat oxidation and mitochondrial function in rats fed a high-fat diet. Br J Nutr. 2007;98:264–75. doi: 10.1017/S000711450770497X. [DOI] [PubMed] [Google Scholar]
- 51.Piergiacomi V, Palacios A. Conjugated linoleic acid and fatty acid binding protein as antioxidants. InVet. 2006;8:139–48. [Google Scholar]
- 52.Chinnadurai K, Kanwal H, Tyagi A, Stanton C, Ross P. High conjugated linoleic acid enriched ghee (clarified butter) increases the antioxidant and antiatherogenic potency in female Wistar rats. Lipids Health Dis. 2013;12:121. doi: 10.1186/1476-511X-12-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ip C, Briggs SP, Haegele AD, Thompson HJ, Strokson J, Scimeca JA. The efficacy of conjugated linoleic acid in mammary cancer prevention is independet of the level or type of fat in the diet. Carcinogenesis. 1996;17:1045–50. doi: 10.1093/carcin/17.5.1045. [DOI] [PubMed] [Google Scholar]