Abstract
Objective
To report the incidence of trigeminal neuropathy seen among new patients in a referral center within a period of 1 year (2013). The cause of damage, method of management and treatment outcome was assessed after 1-year follow-up.
Materials and Methods
The records of all new patients visiting the oral and maxillofacial unit of the University hospital of Leuven in 2013 were screened for a history of damage to branches of the trigeminal nerve. The selected records were examined and the duration of nerve damage, received treatment as well as the outcome of the neuropathy after treatment was noted after 1-year follow-up.
Results
56 patients (21 males, 35 females) from 7602 new patients had symptoms of damage to the trigeminal nerve branch. These symptoms persist in more than one-third of the patients [21/56 (37.5 %)] after 1-year follow-up. The least recovery is seen from oral surgery, implant placement, orthognathic surgery and tooth extraction. After 1 year 85 % (12/14) of neuropathic pain cases still have their symptoms as compared to 19 % (5/26) of patients with hypoesthesia.
Conclusion
This study shows a low incidence of nerve damage among the new patients presenting in oral and maxillofacial surgery clinic (<1 %); however, one-third of patients who sustain nerve damage never recover fully. Early diagnosis of the cause of neuropathy is essential. There is a need to objectively assess all patients with symptoms of trigeminal nerve damage before, during and after treatment.
Keywords: Dental care, Disease management, Lingual nerve injuries, Mandibular nerve injuries, Sensation disorder
Introduction
The sensory innervation to the face, mucous membranes, and other structures of the head is supplied by trigeminal nerve through its three branches: the ophthalmic, maxillary, and mandibular branches. The inferior alveolar nerve (IAN), mental nerve as well as the lingual nerve (LN) are the most injured terminal branches of trigeminal nerve during oral and maxillofacial treatment [1, 2]. Damage to the terminal branch of the inferior alveolar nerve is unfortunately a common problem after oral and maxillofacial surgery and even sometimes during routine dental treatment [2, 3].
Injury to branches of the trigeminal nerve can be a result of chemical insult during dental treatment e.g. due to injection of local anesthesia directly into nerve branches, or through direct contact of obturating chemicals with nerve during endodontics management [4, 5]. Another cause of nerve damage can be the manipulation of the nerve and the surrounding structures during surgical osteotomies made for correcting maxillomandibular deformities. Direct injury to the nerve during removal of a tumor or during third molar surgery can also occur [6–8]. Mandibular fracture involving the body and parasymphysis region of mandible result in damage to mandibular and mental nerve [9]. Nerve manipulation can result in nerve elongation, crushing, compression or sectioning of the nerve while manipulation of surrounding structures may result in transient oedema, infection or ischemia. The accompanying complication of nerve injury depends on the severity of the inflicted damage. The resulting effect of insult to the nerve can range from mild complications such as transient hypoesthesia to life changing effects such as neuropathic pain or trigeminal neuralgia [10, 11].
Clinical symptoms of nerve damage vary from hypesthesie to unpleasant altered sensations and pain in the orofacial region, which usually interfere negatively with daily activities. Persistent pain, neuropathic pain, altered sensation such as allodynia, pain and discomfort with occlusion [12] can occur.
The prevalence of injuries of the terminal branch of trigeminal nerve due to dental treatment and oral and maxillofacial surgery is unknown. Severe injuries with permanent disabling symptoms, however, seem rare.
The aim of this study was to report the incidence of trigeminal neuropathy seen among new patients in a tertiary referral center within a period of 1 year (2013). The cause of damage, method of management and the treatment outcome was assessed after a follow up period of 1 year.
Materials and Methods
The case reports of all new patients visiting the oral and maxillofacial outpatient unit of the University hospital of Leuven in 2013 were screened for a history of damage to branches of the trigeminal nerve that existed for at least 6 weeks. Ethical approval for this study was obtained from the Medical Ethical Committee of the University Hospitals of the Catholic University of Leuven.
The records of patients with a history of nerve damage were selected and the patient data, information on previous received treatment, and management of their condition through the 1-year period was entered into a spreadsheet. Patients’ follow-up and the progress of the neuropathy were monitored over a period of 1 year. The nerve damage was grouped according to causative factors or by previous treatment received by the patients: orthognathic surgery, maxillofacial trauma, oral pathology, tooth extraction, implant, local anesthesia, endodontics and unknown (in cases where the cause of neuropathy is not known). The duration of the nerve damage was assessed for each patient, the received treatment as well as the outcome after treatment was noted after 1-year follow-up.
Results
During the 1-year study period the maxillofacial unit of the UZ Leuven hospital had 7602 new patients out of which 56 had symptoms of clinically disturbing damage to the trigeminal nerve branch (21 males, 35 females), age range 16–81 with mean age of 45 ± 14.
In 49 out of 56 (87.5 %) cases the inferior alveolar nerve was affected, the lingual nerve in 5 (8.9 %) cases and the maxillary nerve in 2 (3.6 %).
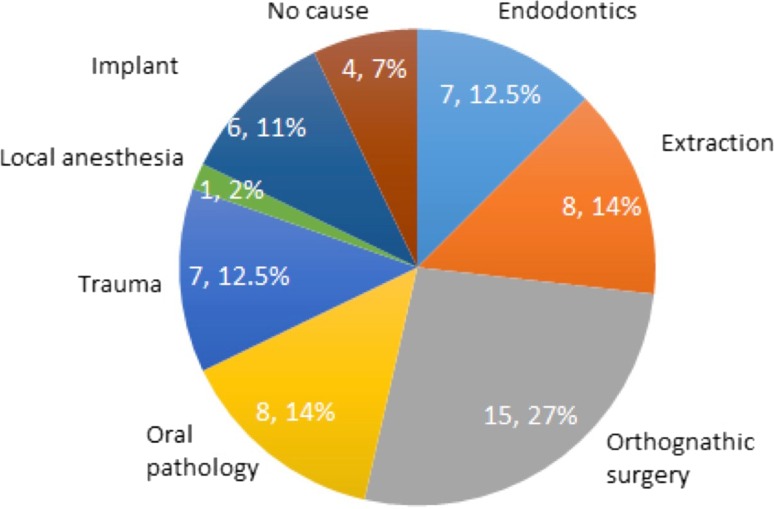
Figure 1 shows the distribution of patients among different causes of nerve damage groups. In 15 patients (27 %) the cause of nerve damage was orthognathic surgery, followed by oral pathology and tooth extraction causing injuries in 8 patients (14 %) per group. In 7 patients (12.5 %) per group, damage was due to maxillofacial trauma and endodontics respectively, 6 patients (11 %) due to implant placement, and in 4 patients (7 %) the cause of damage was not known. Local anaesthesia was the cause of nerve damage in 1 patient (2 %). In Fig. 2 and Table 1 an overview is given of the recovery of patients per group after 1 year.
Fig. 1.
Causes of sensory nerve deficit in 56 patients of UZ Leuven. For each cause the number of patients and the percentage is shown
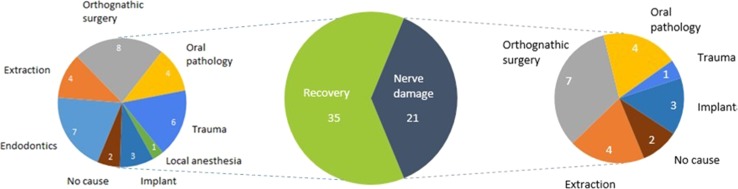
Fig. 2.
Nerve damage and recovery after 1 year of treatment
Table 1.
Percentage of patients grouped by the cause of nerve damage that recovered within 1 year
| Group | Percentage of patients that recovered within 1 year |
|---|---|
| Endodontics | 100 |
| Local anaesthesia | 100 |
| Trauma | 86 |
| Orthognathic surgery | 53 |
| Extraction | 50 |
| Implant | 50 |
| Oral pathology | 50 |
| No cause | 50 |
Table 2 shows the different categories of nerve damages seen among the study subjects. Hypoesthesia is the commonest symptom of nerve damage, it accounts for about half of the symptoms among the study group, followed by neuropathic pain of which one-fourth of the patients suffer.
Table 2.
Different categories of nerve damages seen among the study subjects
| Type of neuropathy | No. of subject | No. of subject with pain after 1 year |
|---|---|---|
| Anaesthesia | 7 (12.5 %) | 1 (14 %) |
| Hypoesthesia | 26 (46 %) | 5 (19 %) |
| Paraesthesia | 3 (5 %) | 1 (33 %) |
| Dysesthesia | 1 (2 %) | 0 (0 %) |
| Hyperesthesia | 2 (3.5 %) | 0 (0 %) |
| Neuropathic pain | 14 (25 %) | 12 (85.5 %) |
| Trigeminal neuralgia | 6 (10.5 %) | 2 (33 %) |
| Unclassified | 2 (3.5 %) | 0 (0 %) |
Five subjects presented with more than one type of neuropathy: four subjects had neuropathic pain and anaesthesia, one subject had neuropathic pain and paraesthesia
Table 3 presents the different diagnostic methods used in the course of managing these patients. Imaging is the main way of assessing the nerve or the region of nerve damage.
Table 3.
Different diagnostic methods used for managing the patients who presented with pain to the UZ Leuven
| No. of patients | Outcomes of the diagnostic method | |
|---|---|---|
| Diagnosis: clinical | ||
| Tissue biopsy | 3 | Close examination of tissue sample |
| Blood sample (WBC control total and differentials, ca, cl, Hco3, total complement) | 2 | To assess general state of health and organ function |
| Diagnosis: others | ||
| Cold detection test | 3 | Temperature test to assess ability to detect cold |
| EMG | 2 | Assess muscle activity |
| Vitality test | 4 | Test the sensibility/vitality of the involved tooth |
| Mechanical pain threshold test | 2 | Test the ability to detect sensation of pricking or stinging |
| Diagnosis: imaging | ||
| OPG | 34 | Gives an overview of the maxillomandibular anatomy |
| Cephalometric radiograph | 6 | Study relationship between bony and soft tissue landmarks |
| CBCT | 23 | Localization of the mandibular canal |
| MRI/fMRI | 20 | Checking state of the nerve and in case of tumor the relation of the tumor with the nerve |
| CT (with/out contrast) | 16 | Using 3D tomographic images obtained from X-ray imaging to examine part of the body with a bigger field of view than CBCT |
| Evoke potential | 3 | Assess nerve activity |
| Peri-apicale RX | 1 | Assess tooth and structures around the tooth |
In this table an overview is given of these methods and the number of patients per method
Few neurosensory tests were done for initial diagnosis and during the follow-up procedure. This includes the cold detection test, evoked potential to monitor nerve activity, mechanical pain threshold test (pinprick) and in some cases tooth vitality test.
The different treatment options and medications are presented in Tables 4 and 5. The treatment that was given varies from physiotherapy to cryosurgery, anaesthetic injection and nerve transection. These treatments are usually in combination with medication (Table 5). Over 60 % of patients are on one form or another of analgesics, while 45 % of patients use vitamins or food supplements and about 40 % of patients with neuropathy are on antidepressant medication.
Table 4.
Treatment options
| Treatment | No. of patients | Duration | Outcome |
|---|---|---|---|
| Cryotherapy | 5 | 1–2× | 0/2 |
| Nerve transection | 1 | 1× | 0/1 |
| Removal of osteosynthesis plate | 8 | 1× | 2 op 8 |
| Relaxation splint | 12 | – | – |
| Epidural electrode application above motor cotex (right) | 3 | – | Little effect |
| Nerve exploration/repair | 5 | 1× | 1/3 |
| Physiotherapy (sometimes with ionoforese and heat application) | 11 | 5–9 sessions | 0/5 |
| Artrocentesis under local anaesthesia | 1 | 1 | 0/1 |
| Soft laser application | 1 | – | – |
| Application of long acting anaesthesia | 8 | 1–2× | Positive effect |
| Acupuncture | 1 | – | No effect |
| BSSO (with freeing of nerve) | 4 | 1 | Improvement but no full recovery |
| Exploration of implant/removal | 4 | 1 | – |
| Exploration/extraction of suspected tooth | 4 | 1× | – |
Table 5.
Groups of medication and corresponding number/percentage of patients taking these
| Type of medication | No. of patients | Percentage | Average duration of treatment |
|---|---|---|---|
| Vitamins/food supplement | 25 | 46 | 1 week to >12 months |
| Antidepressant | 22 | 42 | 2 weeks to >9 months |
| Analgesics/NSAID | 34 | 60 | 2 months to 6 months |
| Antibiotic | 13 | 22 | 5 days to 6 months |
| Anti-epileptic/anti-spasticity/muscle relaxant | 26 | 48 | 2 weeks to 6 months |
| Steroid | 5 | 10 | 1 week to >1 month |
| Antiemetic | 4 | 8 | – |
| Othersa (anti migraine, anti-psychosis) | 3 | 2–4 | – |
The average duration of treatment is also shown
aAntipsychoticum, cardiac medication, antimigraine, antiviral, saliva replacement, antihistaminicum
Symptoms of nerve damage persist in more than one-third of the patients [21/56 (37.5 %)] after 1 year of follow-up, which is shown in Fig. 2. The least recovery is seen from oral surgery, implant placement, orthognathic surgery and the tooth extraction group. At 1 year follow-up 85 % (12/14) of neuropathic pain cases still have their symptoms as compared to 19 % (5/26) of patients with hypoesthesia (Table 2).
Discussion
Damage to branches of the trigeminal nerve following maxillofacial surgery and dental treatment is unfortunately common, in most cases the symptoms are transient and patients fully recover sensation over time. Persistent nerve damage results in severe complications such as neuropathic pain and trigeminal neuralgias.
This study shows low incidence of nerve damage among the new patients presenting in oral and maxillofacial surgery clinic (<1 %); however, one-third of patients who sustain an injury to branches of trigeminal nerve never recover fully.
The most common cause of nerve damage in this study was orthognathic surgery. The post-operative sensory disturbance after bilateral sagittal split osteotomy has been reported to range from 9 to 85 % [13–15]. The higher incidence of nerve damage during orthognathic surgery has been attributed to the manipulation of nerve bundle and structures around the nerve during a surgical procedure. Surgical removal of oral tumors of the jaw inadvertently in some cases results in trauma to neural tissues. The close apposition of an oral tumor to the nerve bundle or the invasion of a nerve bundle by a tumor results in excision of the nerve during tumor removal. This may account for the nerve damage in oral pathology cases, which is the 2nd highest cause together with extractions. Third molar removal, the most important cause of nerve disturbance in the extraction group, is the most common surgical procedure in the oral cavity, and it has been implicated in more than 50 % of nerve damage especially injury to the lingual nerve [16–20]. Other causes of nerve damage are implant placement and endodontic treatment.
Neural damage is characterized by loss or gain in sensation (negative or positive symptoms) as well as other pain conditions (neuropathic pain, trigeminal neuralgias…) [9]. The negative symptoms (sensory deficits) present themselves as anaesthesia or hypoesthesia and positive symptoms as paresthesias, dysesthesias and hyperesthesia among others [9].
Nerve laceration and excessive nerve manipulation are commonest cause of nerve disturbance after oral treatment.
Hypoesthesia is the commonest form of nerve damage seen in this study group (26/56) followed by neuropathic pain (14/56). This finding agrees with previous studies, which show hypoesthesia as the commonest sensory disturbance after BSSO, endodontic treatment, local anaesthesia injection and post-procedural inflammatory process [21–24].
After 1-year follow-up more than one-third of the patients have persistent neuropathy. It is observed that oral pathology, orthognathic surgery, implant placement and tooth extraction have the greatest risk of causing long-term nerve damage. Almost half of the patients from these groups have still sensory disturbance after a 1-year follow-up period (Fig. 2). Nerve damage resulting from trauma, local anaesthesia and endodontics result in transient nerve damage recovering over time [21, 24, 25].
In this study, there is a complete recovery of nerve damage in patients from endodontics and the local anaesthesia group, while 86 % of patients from trauma have full recovery.
As can be deducted from the results, traumatic causes of hypoesthesia are heavily underestimated. This is due to the role of the university hospital as a referral centre for complex trauma. After initial treatment, these patients are transferred to the referring centre for further follow-up.
It is observed that compression of nerve, due to oedema, entrapment or effect of chemicals such as local anaesthesia or irrigation fluid during endodontic treatment, produce a transient inhibition of nerve function, which produce the symptom of hypoesthesia, anaesthesia or muscle weakness (if motor nerve is involve) [26–28]. The removal of cause of entrapment, relieve of oedema and wearing off of chemical effect restores the functionality of the affected nerve; this is different from what is observed in the orthognathic cases in which the nerve can be elongated or bruised, or in oral pathology in which the tumor may be in close apposition to the nerve or even invade it. Surgical removal of the tumor usually results in damage of the neighboring nerve. Placement of implants on nerves or branches of the nerve result in persistent symptoms, removal of such implant may fail to relieve the symptom of nerve damage if not done early [25, 26].
When the recovery of nerve function was assessed based on the symptoms of nerve damage, the recovery of nerve function was seen in over 80 % of those with hypoesthesia while least recovery was seen in patients with neuropathic pain (14.3 % recovery) (Table 2). This finding is in agreement with Politis et al. [10] who stated that once there is onset of neuropathic pain, late intervention (e.g. surgical trigeminal nerve repair) will not improve a patient’s situation.
Varying diagnostic methods were used to assess cause, position and area affected by nerve damage (Table 3). In this study it was found that most assessment was done by imaging. Panoramic radiographs were mostly used as the first line of diagnosis of the cause of damage (34 patients), followed by CBCTs (22 patients), MRIs (20 patients) and CTs (14 patients). X-rays are commonly used despite the fact that the nerve itself cannot be seen on X-ray images. To test the vitality of the nerve, electric pulp tests and carbon dioxide snow tests were done. In rare cases evoked potentials, biopsies, fMRI, teleradiographies were carried out as well. To correctly localize the origin of the pain a local anaesthetic was sometimes used. Previous studies found a relatively good positive correlation between subjective evaluation and objective assessment of the sensitivity of the lower lip and chin after SSO of the mandible [11, 29]. As a result clinical judgments regarding nerve injury-associated sensory dysfunction should not be based on threshold testing results only without the consideration of patients’ subjective reports of altered sensation [30].
Varying treatment modalities exist for managing nerve damage due to maxillofacial intervention. Table 4 presents different therapeutic measures employed to treat the group under study, the treatment method varied from physiotherapy, to application of local anaesthesia, cryotherapy and even nerve transection. These therapies are often in combination with medication. Over 60 % of the patients are on pain relievers while about 50 % are on anti-spasticity/muscle relaxant or on vitamins and food supplement. Vitamins and food supplements are believed to improve nerve health [31]. Anti-epileptic and neuropathic pain drugs stabilize neural membranes, which leads to suppression of hyperexcitability and reduction of neural discharges [32]. A large proportion of the patients are taking antidepressant medication (Table 5). The duration of taking the medication varies, ranging from a few days to several months. Polypharmacotherapy was often used hoping to reduce the adverse effects and enhance the efficacy of treatment [32].
Early treatment of nerve damage has been advocated to ensure remedy and prevent central desensitisation [10]. Early diagnosis of the cause of neuropathy is essential, apart from the clinical examination and nerve function tests, other tests to check for resulting symptoms due to damage and means of assessing these symptoms over time is important. There is a need to objectively assess all patients with symptoms of trigeminal nerve damage before, during and after treatment. Qualitative sensory testing has been shown in reports to be an objective means of assessing and classifying nerve damage [33–35]. The treatment of nerve damage presents a challenge due to the presence of different symptoms. The success of management depends on the understanding of the type of symptoms and the best way to alleviate the specific symptom. Quantitative assessment of all patients using an early management protocol may be of help.
Patient profiling and identification of risk factors for developing neuropathic pain following neural damage should be done. Proper localization of the IAN before maxillofacial and dental treatment is also an essential preventive step. The advent of cone beam computerized tomography has made IAN canal assessment in three dimensions possible.
Conclusion
As a conclusion it need to be said that prevention is still the best option. Since the main cause of nerve damage can be found in orthognathic surgery and that only 50 % of these patients fully recover it will be of utmost importance to obtain better techniques for localization of the inferior alveolar nerve before treatment. During treatment a continuous monitoring of what is happening to the nerve and afterwards an early diagnosis of nerve damage are critical factors for full recovery. In this study it was also clear that there is no standard protocol for treatment of nerve damage. Further studies on setting-up standardized protocols will be beneficial for patients.
Compliance with Ethical Standards
Conflict of interest
The authors report no conflict of interest related to this study.
References
- 1.Jerjes W, Upile T, Shah P, Nhembe F, Gudka D, Kafas P, McCarthy E, Abbas S, Patel S, Hamdoon Z, Abiola J, Vourvachis M, Kalkani M, Al-Khawalde M, Leeson R, Banu B, Rob J, El-Maaytah M, Hopper C. Risk factors associated with injury to the inferior alveolar and lingual nerves following third molar surgery-revisited. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:335–345. doi: 10.1016/j.tripleo.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Pogrel MA, Kaban LB. Injuries to the inferior alveolar and lingual nerves. J Calif Dent Assoc. 1993;21:50–54. [PubMed] [Google Scholar]
- 3.Robert RC, Bacchetti P, Pogrel MA. Frequency of trigeminal nerve injuries following third molar removal. J Oral Maxillofac Surg. 2005;63:732–735. doi: 10.1016/j.joms.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Andrabi SM, Alam S, Zia A, Khan MH, Kumar A. Mental nerve paresthesia secondary to initiation of endodontic therapy: a case report. Restor Dent Endod. 2014;39:215–219. doi: 10.5395/rde.2014.39.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pogrel MA. Damage to the inferior alveolar nerve as the result of root canal therapy. J Am Dent Assoc. 2007;138:65–69. doi: 10.14219/jada.archive.2007.0022. [DOI] [PubMed] [Google Scholar]
- 6.Wijbenga JG, Verlinden CR, Jansma J, Becking AG, Stegenga B. Long-lasting neurosensory disturbance following advancement of the retrognathic mandible: distraction osteogenesis versus bilateral sagittal split osteotomy. Int J Oral Maxillofac Surg. 2009;38:719–725. doi: 10.1016/j.ijom.2009.03.714. [DOI] [PubMed] [Google Scholar]
- 7.Yoshioka I, Tanaka T, Khanal A, Habu M, Kito S, Kodama M, Oda M, Wakasugi-Sato N, Matsumoto-Takeda S, Fukai Y, Tokitsu T, Tomikawa M, Seta Y, Tominaga K, Morimoto Y. Relationship between inferior alveolar nerve canal position at mandibular second molar in patients with prognathism and possible occurrence of neurosensory disturbance after sagittal split ramus osteotomy. J Oral Maxillofac Surg. 2010;68:3022–3027. doi: 10.1016/j.joms.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi K, Takahashi T, Kaneuji T, Nogami S, Yamamoto N, Miyamoto I, Yamashita Y (2011) Risk factors for neurosensory disturbance after bilateral sagittal split osteotomy based on position of mandibular canal and morphology of mandibular angle. J Oral Maxillofac Surg 70:401–406 [DOI] [PubMed]
- 9.Bagheri SC, Meyer RA, Khan HA, Steed MB. Microsurgical repair of peripheral trigeminal nerve injuries from maxillofacial trauma. J Oral Maxillofac Surg. 2009;67:1791–1799. doi: 10.1016/j.joms.2009.04.115. [DOI] [PubMed] [Google Scholar]
- 10.Politis C, Lambrichts I, Agbaje JO. Neuropathic pain after orthognathic surgery. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:e102–e107. doi: 10.1016/j.oooo.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Politis C, Sun Y, Lambrichts I, Agbaje JO. Self-reported hypoesthesia of the lower lip after sagittal split osteotomy. Int J Oral Maxillofac Surg. 2013;42:823–829. doi: 10.1016/j.ijom.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 12.D’Agostino A, Trevisiol L, Gugole F, Bondi V, Nocini PF. Complications of orthognathic surgery: the inferior alveolar nerve. J Craniofac Surg. 2010;21:1189–1195. doi: 10.1097/SCS.0b013e3181e1b5ff. [DOI] [PubMed] [Google Scholar]
- 13.Agbaje JO, Salem AS, Jacobs R, Politis C. Systematic review of the incidence of inferior alveolar nerve injury in bilateral sagittal split osteotomy and the assessment of neurosensory disturbances. Int J Oral Maxillofac Surg. 2015;44:447–451. doi: 10.1016/j.ijom.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Westermark A, Bystedt H, von Konow KL. Inferior alveolar nerve function after mandibular osteotomies. Br J Oral Maxillofac Surg. 1998;36:425–428. doi: 10.1016/S0266-4356(98)90457-0. [DOI] [PubMed] [Google Scholar]
- 15.Monnazzi MS, Real-Gabrielli MF, Passeri LA, Gabrielli MA. Cutaneous sensibility impairment after mandibular sagittal split osteotomy: a prospective clinical study of the spontaneous recovery. J Oral Maxillofac Surg. 2012;70:696–702. doi: 10.1016/j.joms.2011.02.071. [DOI] [PubMed] [Google Scholar]
- 16.Cespedes-Sanchez JM, Ayuso-Montero R, Mari-Roig A, Arranz-Obispo C, Lopez-Lopez J. The importance of a good evaluation in order to prevent oral nerve injuries: a review. Acta Odontol Scand. 2014;72:161–167. doi: 10.3109/00016357.2013.812746. [DOI] [PubMed] [Google Scholar]
- 17.Cheung LK, Leung YY, Chow LK, Wong MC, Chan EK, Fok YH. Incidence of neurosensory deficits and recovery after lower third molar surgery: a prospective clinical study of 4338 cases. Int J Oral Maxillofac Surg. 2010;39:320–326. doi: 10.1016/j.ijom.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Hillerup S. Iatrogenic injury to oral branches of the trigeminal nerve: records of 449 cases. Clin Oral Investig. 2007;11:133–142. doi: 10.1007/s00784-006-0089-5. [DOI] [PubMed] [Google Scholar]
- 19.Penarrocha MA, Penarrocha D, Bagan JV, Penarrocha M. Post-traumatic trigeminal neuropathy. A study of 63 cases. Med Oral Patol Oral Cir Bucal. 2012;17:e297–e300. doi: 10.4317/medoral.17401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagheri SC, Meyer RA, Khan HA, Wallace J, Steed MB. Microsurgical repair of the peripheral trigeminal nerve after mandibular sagittal split ramus osteotomy. J Oral Maxillofac Surg. 2010;68:2770–2782. doi: 10.1016/j.joms.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 21.Degala S, Shetty SK, Bhanumathi M. Evaluation of neurosensory disturbance following orthognathic surgery: a prospective study. J Maxillofac Oral Surg. 2015;14:24–31. doi: 10.1007/s12663-013-0577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scarano A, Di CF, Quaranta A, Piattelli A. Injury of the inferior alveolar nerve after overfilling of the root canal with endodontic cement: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:e56–e59. doi: 10.1016/j.tripleo.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 23.Silbert BI, Kolm S, Silbert PL. Postprocedural inflammatory inferior alveolar neuropathy: an important differential diagnosis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:e1–e3. doi: 10.1016/j.oooo.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Moon S, Lee SJ, Kim E, Lee CY. Hypoesthesia after IAN block anesthesia with lidocaine: management of mild to moderate nerve injury. Restor Dent Endod. 2012;37:232–235. doi: 10.5395/rde.2012.37.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijbenga JG, Verlinden CR, Jansma J, Becking AG, Stegenga B. Long-lasting neurosensory disturbance following advancement of the retrognathic mandible: distraction osteogenesis versus bilateral sagittal split osteotomy. Int J Oral Maxillofac Surg. 2009;38:719–725. doi: 10.1016/j.ijom.2009.03.714. [DOI] [PubMed] [Google Scholar]
- 26.Al-Sabbagh M, Okeson JP, Khalaf MW, Bhavsar I. Persistent pain and neurosensory disturbance after dental implant surgery: pathophysiology, etiology, and diagnosis. Dent Clin N Am. 2015;59:131–142. doi: 10.1016/j.cden.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Tay AB, Zuniga JR. Clinical characteristics of trigeminal nerve injury referrals to a university centre. Int J Oral Maxillofac Surg. 2007;36:922–927. doi: 10.1016/j.ijom.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Ziccardi VB, Assael LA. Mechanisms of trigeminal nerve injuries. Atlas Oral Maxillofac Surg Clin N Am. 2001;9:1–11. [PubMed] [Google Scholar]
- 29.Westermark A, Englesson L, Bongenhielm U. Neurosensory function after sagittal split osteotomy of the mandible: a comparison between subjective evaluation and objective assessment. Int J Adult Orthodon Orthognath Surg. 1999;14:268–275. [PubMed] [Google Scholar]
- 30.Essick GK, Phillips C, Turvey TA, Tucker M. Facial altered sensation and sensory impairment after orthognathic surgery. Int J Oral Maxillofac Surg. 2007;36:577–582. doi: 10.1016/j.ijom.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spencer CJ, Gremillion HA. Neuropathic orofacial pain: proposed mechanisms, diagnosis, and treatment considerations. Dent Clin N Am. 2007;51:209–224. doi: 10.1016/j.cden.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Eisenberg E, River Y, Shifrin A, Krivoy N. Antiepileptic drugs in the treatment of neuropathic pain. Drugs. 2007;67:1265–1289. doi: 10.2165/00003495-200767090-00003. [DOI] [PubMed] [Google Scholar]
- 33.Eliav E, Gracely RH, Nahlieli O, Benoliel R. Quantitative sensory testing in trigeminal nerve damage assessment. J Orofac Pain. 2004;18:339–344. [PubMed] [Google Scholar]
- 34.Svensson P, Baad-Hansen L, Thygesen T, Juhl GI, Jensen TS. Overview on tools and methods to assess neuropathic trigeminal pain. J Orofac Pain. 2004;18:332–338. [PubMed] [Google Scholar]
- 35.Baad-Hansen L, Pigg M, Ivanovic SE, Faris H, List T, Drangsholt M, Svensson P. Intraoral somatosensory abnormalities in patients with atypical odontalgia—a controlled multicenter quantitative sensory testing study. Pain. 2013;154:1287–1294. doi: 10.1016/j.pain.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]