ABSTRACT
Ligand-dependent nuclear receptor compressor-like (LCORL) encodes a transcription factor, and its polymorphisms are associated with measures of skeletal frame size and adult height in several species. Recently, the single nucleotide polymorphism (SNP) BIEC2-808543 located upstream of LCORL was identified as a genetic diagnostic marker associated with withers height in Thoroughbreds. In this study, 322 Thoroughbreds-in-training were genotyped for BIEC2-808543 to evaluate the association between genotype and body composition traits, including body weight, withers height, the ratio of body weight to withers height, chest circumference, and cannon circumference. Of these, withers height and cannon circumference were significantly associated with LCORL genotypes throughout almost the entire training period in males and females. Animals with a C/T genotype had higher withers height (maximum differences of 1.8 cm and 2.1 cm in males and females, respectively) and cannon circumstance (maximum differences of 0.65 cm and 0.48 cm in males and females, respectively) compared with animals with a T/T genotype. These results suggested that the regulation of LCORL expression influences the skeletal frame size in Thoroughbreds and thus, indirectly affects the body weight. Although LCORL and BIEC2-808543 would be useful for selective breeding in Thoroughbreds, the production of genetically modified animals and gene doping based on genetic information should be prohibited in order to maintain racing integrity.
Keywords: BIEC2-808543, cannon circumstance, LCORL, Thoroughbred, withers height
The Thoroughbred is a breed that was developed in the early 18th century. The origin of Thoroughbreds can be traced back to a small number of Arab stallions and native British mares approximately 300 years ago [2, 4, 24]. Current Thoroughbreds have larger body sizes compared with their ancestors. For instance, the withers height of old racehorses, such as the Darley Arabian, was reported to be about 152 cm in old literature, whereas the withers height of current Thoroughbreds is about 160.5 cm [5]. Morphological characteristics of Thoroughbreds have been improved to obtain superior performance by selective breeding [6, 13, 22, 23].
The ligand-dependent nuclear receptor compressor-like (LCORL) is thought to encode a transcription factor associated with measures of skeletal frame size and adult height in humans and several animals [8, 10, 11, 20, 21]. In horses, LCORL has been mapped on Equus caballus autosome 3 (ECA3), and it has been reported that BIEC2-808543, a single nucleotide polymorphism (SNP) located upstream of the gene, is significantly associated with withers height [14, 19]. It is known that height is a quantitative trait controlled by numerous genes in humans; however, the genetic basis of body size in Thoroughbreds is still understudied.
In this study, we investigated the relationships between sequence variants of BIEC2-808543 and measured morphological characteristics, such as body weight, withers height, chest circumference, and cannon circumference, in male and female Thoroughbreds prior to the initiation of training and at monthly intervals during the first seven months of training. We hypothesized that identification of genes controlling body size in Thoroughbreds might be relatively simple, since the breeding population includes a limited number of sires and dams. We also assumed that LCORL might affect the body composition of Thoroughbreds.
Materials and Methods
Thoroughbreds
This study was performed according to the ethical standards in animal research. A total of 322 (165 males and 167 females) Thoroughbreds were used in this study. All horses were born between January and June of 2009–2014, and the majority of them were born in March and April. All horses were purchased at commercial livestock auctions at one year of age, introduced to the Hidaka Training and Research Center (Japan Racing Association, JRA) by the end of September, trained to be ridden (initial training) during October, and trained for racing (full-scale training) from November through April.
Body composition measurement
Body composition assessment was initially performed in September (approximately 18 months of age) and was subsequently performed at monthly intervals during the following six months of training. Measurements included body weight (kg), withers height (cm), chest circumference (cm), and cannon circumference (cm). The ratio of body weight to withers height (kg cm–1) was calculated and defined as the “muscle content”, since the skeletal muscle mass of Thoroughbreds comprises over 55% of their total body mass [7], and thus, it can be used as an indicator of skeletal muscle mass.
SNP genotyping
Blood samples were collected from each animal and stored at −40°C. Genomic DNA was extracted using an MFX-2000 MagExtractor System (Toyobo, Osaka, Japan), according to the manufacturer’s protocol. Genotyping for BIEC2-808543 was performed using a Taqman SNP Genotyping Assay with a 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, U.S.A.), according to the manufacturer’s protocols. The primer and probe sequences used in this study are shown in Table 1.
Table 1. Primer and probe sequences used in this study.
| Sequence | |
|---|---|
| F-primer | CCAAATTTGCCTGGCTAGAGA |
| R-primer | TGTTCCCTGTGATTCTGCCTTT |
| MGB_VIC_C | CATTCCAGCTTATTTCTGTA |
| MGB_FAM_T | CATTCCAGTTTATTTCTGTAC |
Statistical analysis
Genetic association analysis for body weight, withers height, chest circumference, cannon circumference, and the ratio of body weight to withers height was performed using Student’s t-test. In a previous study, we observed differences in body composition between males and females [22]. Thus, phenotypic data and SNP associations were separately evaluated for each sex. In the present study, double blind experiments were designed to avoid errors arising from bias in phenotyping and SNP genotyping. All statistical analyses were performed using IBM SPSS Statistics 19 (IBM, Armonk, NY, U.S.A.).
Results
Growth trends in body composition
To avoid pedigree-based and age-based systematic errors, 59 males and 48 females were selected, excluding animals with full-sibling and half-sibling relationships and/or those born in January, February, May, or June. No significant differences were observed in age between the subpopulations.
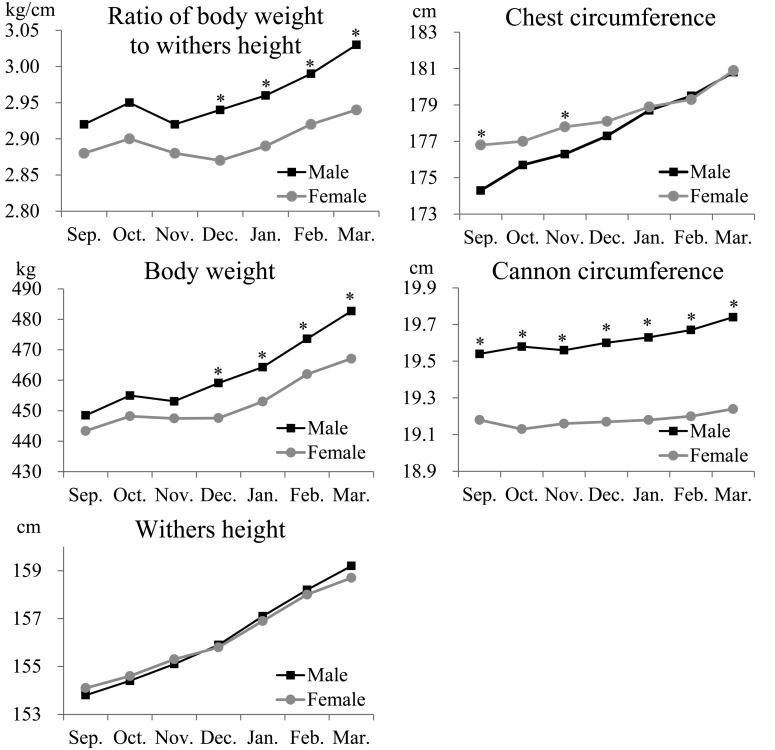
Body weight, withers height, the ratio of body weight to withers height, and chest circumference increased with time in both males and females, although the changes (increase or decrease) were minimal prior to the initiation of full-scale training (Table 2, Fig. 1). Body weight, withers height, and chest circumference of both males and females significantly (P<0.05) increased in March compared with those in October (Table 2, Fig. 1). Body weight and the ratio of body weight to withers height decreased in November, when full-scale training started, and then increased from December through April (Table 2, Fig. 1).
Table 2. Body weight (kg), withers height (cm), ratio of body weight to withers height (kg cm–1), chest circumference (cm), and cannon circumference (cm) (average values ± standard error; SE) from September to March by gender.
| Sep. | Oct. | Nov. | Dec. | Jan. | Feb. | Mar. | |
|---|---|---|---|---|---|---|---|
| Ratio of body weight to withers height (kg·cm–1) | |||||||
| Male (59) | 2.92 ± 0.02 | 2.95 ± 0.02 | 2.92 ± 0.02 | 2.94 ± 0.02 | 2.96 ± 0.02 | 2.99 ± 0.02 | 3.03 ± 0.02 |
| Female (48) | 2.88 ± 0.02 | 2.90 ± 0.02 | 2.88 ± 0.02 | 2.87 ± 0.02 | 2.89 ± 0.02 | 2.92 ± 0.02 | 2.94 ± 0.02 |
| P-values | 0.1631 | 0.0983 | 0.1754 | 0.0160* | 0.0224* | 0.0149* | 0.0018* |
| Body weight (kg) | |||||||
| Male (59) | 448.5 ± 3.0 | 455.0 ± 3.2 | 453.1 ± 3.2 | 459.1 ± 3.2 | 464.3 ± 3.2 | 473.6 ± 3.1 | 482.7 ± 3.0 |
| Female (48) | 443.4 ± 3.3 | 448.2 ± 3.2 | 447.5 ± 3.3 | 447.6 ± 3.4 | 453.0 ± 3.4 | 462.0 ± 3.5 | 467.1 ± 3.4 |
| P-values | 0.2572 | 0.1386 | 0.2355 | 0.0170* | 0.0170* | 0.0143* | 0.0007* |
| Withers height (cm) | |||||||
| Male (59) | 153.8 ± 0.3 | 154.4 ± 0.3 | 155.1 ± 0.3 | 155.9 ± 0.4 | 157.1 ± 0.4 | 158.2 ± 0.4 | 159.2 ± 0.4 |
| Female (48) | 154.1 ± 0.3 | 154.6 ± 0.3 | 155.3 ± 0.3 | 155.8 ± 0.3 | 156.9 ± 0.3 | 158.0 ± 0.3 | 158.7 ± 0.3 |
| P-values | 0.5261 | 0.7230 | 0.7076 | 0.8252 | 0.6340 | 0.7694 | 0.2954 |
| Chest circumference (cm) | |||||||
| Male (59) | 174.3 ± 0.4 | 175.7 ± 0.5 | 176.3 ± 0.5 | 177.3 ± 0.4 | 178.7 ± 0.4 | 179.5 ± 0.4 | 180.8 ± 0.4 |
| Female (48) | 176.8 ± 0.5 | 177.0 ± 0.5 | 177.8 ± 0.5 | 178.1 ± 0.4 | 178.9 ± 0.4 | 179.3 ± 0.4 | 180.9 ± 0.4 |
| P-values | 0.0002* | 0.0769 | 0.0339* | 0.2187 | 0.8319 | 0.7280 | 0.8894 |
| Cannon circumference (cm) | |||||||
| Male (59) | 19.54 ± 0.08 | 19.58 ± 0.08 | 19.56 ± 0.08 | 19.60 ± 0.08 | 19.63 ± 0.08 | 19.67 ± 0.08 | 19.74 ± 0.07 |
| Female (48) | 19.18 ± 0.07 | 19.13 ± 0.07 | 19.16 ± 0.06 | 19.17 ± 0.06 | 19.18 ± 0.06 | 19.20 ± 0.06 | 19.24 ± 0.06 |
| P-values | 0.0015* | <0.0001* | 0.0002* | <0.0001* | <0.0001* | <0.0001* | <0.0001* |
*Statistically significant P-values (<0.05) in a t-test.
Fig. 1.
Changes in the mean ratio of body weight to withers height, body weight, withers height, chest circumference, and cannon circumference from September to the following March in each sex. The black square (■) and gray circle (●) indicate male and female, respectively. An asterisk (*) indicates a statistical difference at P<0.05 by sex.
Differences between males and females
Body weight and the ratio of body weight to withers height were significantly (P<0.05) different between males and females from December through March (Table 2, Fig. 1). Additionally, no significant differences were observed in withers height between males and females throughout the experimental period (Table 2, Fig. 1). Chest circumference was significantly (P<0.05) higher in females in September and November, whereas no differences were observed between males and females from December through March (Table 2, Fig. 1). Cannon circumference was significantly (P<0.05) different between males and females throughout the experimental period (Table 2, Fig. 1); the measured values were significantly (P<0.05) higher in males than in females.
Genotypic distribution
All the animals used in this study were successfully genotyped for BIEC2-808543. No significant differences were observed in genotypic distribution and allele frequency between males and females (Table 3). Since the C/C genotype had a very low frequency in the population, only the C/T and T/T genotypes were used for the genotype-phenotype association study.
Table 3. Genotypic frequency of BIEC2-808543 near the ligand-dependent nuclear receptor compressor-like (LCORL) gene on Equus caballus autosome 3 (ECA3).
| C/C | T/C | T/T | |
|---|---|---|---|
| Male | 0 | 0.15 | 0.85 |
| Female | 0.01 | 0.18 | 0.81 |
Four subpopulations were assigned based on BIEC2-808543 genotype and gender. Animals (n=200) with full and half-sibling relationships and/or those born in January, February, May, or June were excluded from the four subpopulations in order to eliminate pedigree-based and age-based systematic errors. Therefore, the four subpopulations included a total of 67 males and 55 females. These numbers were different from those in the growth trend analysis (59 males and 48 females) due to the classification by sex and genotype. No significant differences were observed in age among the four subpopulations. The four subpopulations were used for the association study.
Genotype association with body composition in males
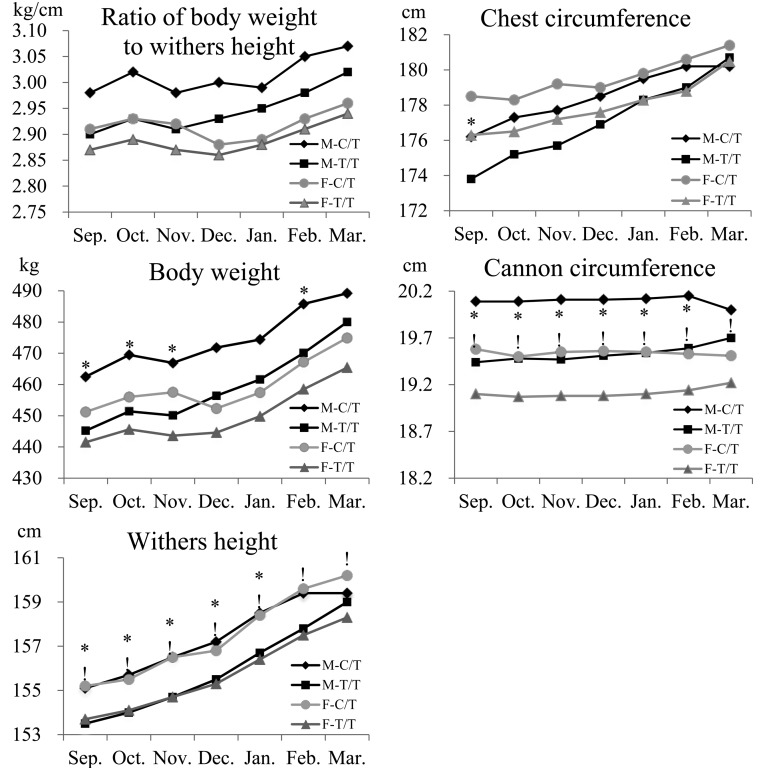
Significant (P<0.05) differences were observed in body weight in September, October, November, and February; in withers height from September to January; in chest circumstance in September; and in cannon circumstance from September to February between genotypes in males (Table 4, Fig. 2). The measured values were significantly (P<0.05) higher in the C/T genotype than in the T/T genotype throughout almost the entire experimental period. However, no significant differences were observed in the ratio of body weight to withers height between genotypes at any point during the experimental period.
Table 4. Body weight (kg), withers height (cm), ratio of body weight to withers height (kg cm–1), chest circumference (cm), and cannon circumference (cm) (average values ± standard error; SE) from September to March by genotype in males and females.
| Sep. | Oct. | Nov. | Dec. | Jan. | Feb. | Mar. | |
|---|---|---|---|---|---|---|---|
| Male | |||||||
| Ratio of body weight to withers height (kg·cm–1) | |||||||
| C/T (13) | 2.98 ± 0.04 | 3.02 ± 0.04 | 2.98 ± 0.05 | 3.00 ± 0.04 | 2.99 ± 0.04 | 3.05 ± 0.04 | 3.07 ± 0.03 |
| T/T (54) | 2.90 ± 0.02 | 2.93 ± 0.02 | 2.91 ± 0.02 | 2.93 ± 0.02 | 2.95 ± 0.02 | 2.98 ± 0.02 | 3.02 ± 0.02 |
| P-values | 0.0582 | 0.0608 | 0.1099 | 0.1715 | 0.2955 | 0.1193 | 0.2389 |
| Body weight (kg) | |||||||
| C/T (13) | 462.5 ± 7.0 | 469.5 ± 7.8 | 466.9 ± 8.0 | 471.8 ± 8.0 | 474.4 ± 7.3 | 485.8 ± 7.1 | 489.2 ± 6.4 |
| T/T (54) | 445.2 ± 3.1 | 451.4 ± 3.1 | 450.1 ± 3.2 | 456.4 ± 3.3 | 461.6 ± 3.2 | 470.1 ± 3.1 | 480.0 ± 3.1 |
| P-values | 0.0175* | 0.0168* | 0.0292* | 0.0502 | 0.0901 | 0.0340* | 0.2012 |
| Withers height (cm) | |||||||
| C/T (13) | 155.1 ± 0.6 | 155.7 ± 0.6 | 156.5 ± 0.6 | 157.2 ± 0.7 | 158.5 ± 0.6 | 159.4 ± 0.6 | 159.4 ± 0.8 |
| T/T (54) | 153.5 ± 0.4 | 154.0 ± 0.3 | 154.7 ± 0.4 | 155.5 ± 0.4 | 156.7 ± 0.4 | 157.8 ± 0.4 | 159.0 ± 0.3 |
| P-values | 0.0434* | 0.0368* | 0.0270* | 0.0382* | 0.0351* | 0.0594 | 0.6640 |
| Chest circumference (cm) | |||||||
| C/T (13) | 176.2 ± 0.8 | 177.3 ± 1.2 | 177.7 ± 1.2 | 178.5 ± 1.1 | 179.5 ± 0.9 | 180.2 ± 0.9 | 180.2 ± 0.6 |
| T/T (54) | 173.8 ± 0.5 | 175.2 ± 0.5 | 175.7 ± 0.5 | 176.9 ± 0.5 | 178.3 ± 0.4 | 179.0 ± 0.4 | 180.7 ± 0.5 |
| P-values | 0.0277* | 0.0797 | 0.1045 | 0.1430 | 0.2563 | 0.2272 | 0.5840 |
| Cannon circumference (cm) | |||||||
| C/T (13) | 20.09 ± 0.18 | 20.09 ± 0.17 | 20.11 ± 0.18 | 20.11 ± 0.18 | 20.12 ± 0.18 | 20.15 ± 0.17 | 20.00 ± 0.19 |
| T/T (54) | 19.44 ± 0.08 | 19.48 ± 0.08 | 19.47 ± 0.08 | 19.51 ± 0.08 | 19.54 ± 0.08 | 19.59 ± 0.07 | 19.70 ± 0.07 |
| P-values | 0.0011* | 0.0015* | 0.0012* | 0.0023* | 0.0021* | 0.0020* | 0.0710 |
| Female | |||||||
| Ratio of body weight to withers height (kg·cm–1) | |||||||
| C/T (11) | 2.91 ± 0.04 | 2.93 ± 0.04 | 2.92 ± 0.04 | 2.88 ± 0.04 | 2.89 ± 0.04 | 2.93 ± 0.04 | 2.96 ± 0.04 |
| T/T (44) | 2.87 ± 0.02 | 2.89 ± 0.02 | 2.87 ± 0.02 | 2.86 ± 0.02 | 2.88 ± 0.02 | 2.91 ± 0.02 | 2.94 ± 0.02 |
| P-values | 0.5163 | 0.4336 | 0.2508 | 0.6778 | 0.8515 | 0.7514 | 0.6298 |
| Body weight (kg) | |||||||
| C/T (11) | 451.2 ± 6.5 | 456.0 ± 6.7 | 457.5 ± 7.1 | 452.3 ± 6.9 | 457.4 ± 6.6 | 467.2 ± 6.7 | 474.9 ± 6.7 |
| T/T (44) | 441.5 ± 3.6 | 445.6 ± 3.5 | 443.6 ± 3.4 | 444.6 ± 3.6 | 449.8 ± 3.7 | 458.5 ± 3.7 | 465.4 ± 3.6 |
| P-values | 0.2218 | 0.1846 | 0.0752 | 0.3440 | 0.3565 | 0.2961 | 0.2351 |
| Withers height (cm) | |||||||
| C/T (11) | 155.2 ± 0.7 | 155.5 ± 0.7 | 156.5 ± 0.9 | 156.8 ± 0.9 | 158.4 ± 0.7 | 159.6 ± 0.8 | 160.2 ± 0.8 |
| T/T (44) | 153.7 ± 0.3 | 154.1 ± 0.2 | 154.7 ± 0.3 | 155.3 ± 0.3 | 156.4 ± 0.2 | 157.5 ± 0.2 | 158.3 ± 0.3 |
| P-values | 0.0131* | 0.0176* | 0.0154* | 0.0347* | 0.0013* | 0.0018* | 0.0059* |
| Chest circumference (cm) | |||||||
| C/T (11) | 178.5 ± 0.7 | 178.3 ± 0.9 | 179.2 ± 1.0 | 179.0 ± 0.9 | 179.8 ± 1.1 | 180.6 ± 0.9 | 181.4 ± 0.8 |
| T/T (44) | 176.3 ± 0.5 | 176.5 ± 0.5 | 177.2 ± 0.5 | 177.6 ± 0.4 | 178.3 ± 0.4 | 178.8 ± 0.5 | 180.5 ± 0.4 |
| P-values | 0.0505 | 0.1142 | 0.0744 | 0.2007 | 0.1226 | 0.0824 | 0.3515 |
| Cannon circumference (cm) | |||||||
| C/T (11) | 19.58 ± 0.13 | 19.50 ± 0.11 | 19.55 ± 0.10 | 19.56 ± 0.10 | 19.55 ± 0.10 | 19.53 ± 0.11 | 19.51 ± 0.12 |
| T/T (44) | 19.10 ± 0.07 | 19.07 ± 0.07 | 19.08 ± 0.06 | 19.08 ± 0.05 | 19.10 ± 0.06 | 19.14 ± 0.05 | 19.22 ± 0.06 |
| P-values | 0.0020* | 0.0038* | 0.0004* | 0.0002* | 0.0006* | 0.0021* | 0.0344* |
*Statistically significant P-values (<0.05) in a t-test.
Fig. 2.
Changes in the mean ratio of body weight to withers height, body weight, withers height, chest circumference, and cannon circumference from September to the following March expressed according to the genotype at BIEC2-808543 in each sex. Black indicates male and gray indicates female, and the rhombus (♦), square (■), circle (●) and triangle (▲) indicate male-C/T, male-T/T, female-C/T and female-T/T, respectively. An asterisk (*) indicates a statistical difference at P<0.05 in males. An exclamation mark (!) indicates a statistical difference at P<0.05 in females.
Genotype association with body composition in females
Withers height and cannon circumference in females were significantly (P<0.05) higher in the C/T genotype than in the T/T genotype throughout the experimental period. Withers height tended to increase during the training period, whereas cannon circumstance did not show any changes (Table 4, Fig. 2). No significant differences were observed in body weight, the ratio of body weight to withers height, and chest circumstance between genotypes at any point during the experimental period, although the measured values tended to be higher in the C/T genotype than in the T/T genotype.
Discussion
To our knowledge, this is the first report of relationships between sequence variants of BIEC2-808543 and measured morphological characteristics in Thoroughbreds under training. Our data demonstrated that BIEC2-808543 was significantly associated with withers height (Table 4, Fig. 2), and the results were consistent with those reported in previous studies on several horse breeds [14, 19]. Animals with a C/T genotype had higher withers heights (maximum differences of 1.8 cm in males; maximum differences of 2.1 cm in females) compared with animals with a T/T genotype. We also demonstrated that BIEC2-808543 was associated with cannon circumstance in Thoroughbreds under training. Animals with a C/T genotype had higher cannon circumstances (maximum differences of 0.65 cm in males; maximum difference of 0.48 in females) compared with animals with a T/T genotype. These findings suggested that LCORL on ECA3 was closely associated with the skeletal frame and body composition in Thoroughbreds. BIEC2-808543 polymorphism disrupts a putative binding site of the transcription factor TFIID [15], which suggests that the influence on the skeletal frame is caused by a regulation mechanism of LCORL expression.
We also observed associations between genotypes and phenotypes when all 322 animals were used for analysis (data not shown). Although the C/C genotype was not evaluated in this study because of a low frequency (Table 3), one female horse with the C/C genotype showed a withers height of 162.0 cm in March. Therefore, it is expected that horses with a C/C genotype would have a much higher withers height and cannon circumstance compared with those with a C/T or T/T genotype. Overall, our findings suggested that the C-allele at BIEC2-808543 increased the skeletal frame size and that the T-allele decreased it.
In the present study, no statistical differences were observed in chest circumference throughout almost the entire experimental period, suggesting that LCORL does not have a strong effect on chest circumference in horses (Table 4, Fig. 2). The differences between genotypes in terms of the association with chest circumference seemed to decrease with growth and/or advanced training. The thoroughbreds used in this study trained almost every day; thus, LCORL was not able to strongly affect their chest circumstance.
Our data showed that cannon circumstance was affected by gender (Table 2, Fig. 1). In the four subpopulations, males with a C/T genotype had the highest cannon circumstance, males with a T/T genotype and females with a C/T genotype had an intermediate cannon circumstance, and females with a T/T genotype had the lowest cannon circumstance (Table 4, Fig. 2). Because it is generally considered that bone size is an important determinant of bone strength, cannon circumstance may play an important role in bone fractures, which are a common cause of loss in Thoroughbreds [17]. Therefore, further studies are needed to investigate the association between bone fracture, LCORL, and sex in order to improve horse health and welfare.
Males with a C/T genotype at LCORL had relatively higher body weights and withers heights. Therefore, body weight was related to withers height in LCORL genotypes, but not to muscle content, since no significant differences were identified in the ratio of body weight to withers height (Table 4, Fig. 2). A similar tendency was also observed in females (Table 4, Fig. 2). In our previous study, racehorses with a C/C genotype at the myostatin (MSTN) gene demonstrated a relatively higher body weights and skeletal muscle masses than racehorses with other genotypes [6, 13, 22, 23].
Based on genotypes at LCORL and MSTN and sex, we may be able to identify the ideal body composition for each racehorse. For instance, male racehorses with a C/C genotype at LCORL and a C/C genotype at MSTN may have large and heavy bodies, whereas female racehorses with a T/T genotype at LCORL and a T/T genotype at MSTN may have small and light bodies. Thus, animal care and training, such feeding and exercise for regulating body weight, could be applied based on individual genotypic information in a manner similar to that in personalized medicine in humans [1]. In addition, genotypic information could contribute to the development of effective breeding strategies for producing Thoroughbreds with favorable body traits. Because of the relatively low frequency of the C-allele at LCORL, withers height could be modified based on the LCORL genotype, leading to improved racing performance.
Although selective breeding based on genetic information is effective for producing racehorses with desired traits, gene doping, which may be defined as the “abuse/misuse of gene therapy”, should be prohibited in order to maintain racing integrity [3]. The CRISPR/Cas system for genome editing [9] and the adeno-associated virus (AAV) vector [12, 18] for gene transfer have been developed for the production of genetically modified animals and gene therapy, respectively. In addition, the combination of both technologies allows for in vivo genome editing that directly modifies the genome in postnatal animals [16]. Therefore, these technologies raise concerns regarding the production of genetically modified animals and gene doping in racehorses. Since LCORL and MSTN influence body phenotype and/or racing performance, the genes should be monitored to protect the genomic and genetic integrity of racehorses.
Acknowledgments
We would like to thank Dr. M. Kurosawa for useful discussions, and we would also like to thank the JRA Hidaka Training and Research Center, Hokkaido, for their assistance in this study. We would like to thank the JRA Equine Department for approving and supporting the study with a grant-in-aid (2014–2016).
References
- 1.Apellaniz-Ruiz M., Gallego C., Ruiz-Pinto S., Carracedo A., Rodríguez-Antona C. 2016. Human genetics: international projects and personalized medicine. Drug. Metabol. Personal. Ther. 31: 3–8. [DOI] [PubMed] [Google Scholar]
- 2.Bower M.A., Campana M.G., Whitten M., Edwards C.J., Jones H., Barrett E., Cassidy R., Nisbet R.E., Hill E.W., Howe C.J., Binns M. 2011. The cosmopolitan maternal heritage of the Thoroughbred racehorse breed shows a significant contribution from British and Irish native mares. Biol. Lett. 7: 316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brzeziańska E., Domańska D., Jegier A. 2014. Gene doping in sport - perspectives and risks. Biol. Sport 31: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham E.P., Dooley J.J., Splan R.K., Bradley D.G. 2001. Microsatellite diversity, pedigree relatedness and the contributions of founder lineages to thoroughbred horses. Anim. Genet. 32: 360–364. [DOI] [PubMed] [Google Scholar]
- 5.Đermanović V., Mitrović S., Đordjević N., Novaković M. 2010. Some significant exterior and reproductive properties of the English Thoroughbred horse population from the stud farm “Ljubicevo”−Serbia. Biotechnol. Anim. Husb. 26: 75–82. [Google Scholar]
- 6.Fonseca R.G., Kenny D.A., Hill E.W., Katz L.M. 2013. The relationship between body composition, training and race performance in a group of Thoroughbred flat racehorses. Equine Vet. J. 45: 552–557. [DOI] [PubMed] [Google Scholar]
- 7.Gunn H.M. 1987. Muscle, bone and fat productions and muscle distribution of thoroughbreds and quarter horses. In: Equine Exercise Physiology 2: Proceedings of the Second International Conference on Equine Exercise Physiology; August 7–11 1986, San Diego. http://www.iceep.org/pdf/iceep2/_1129101114_001.pdf (accessed on May 1, 2016).
- 8.Hirschhorn J.N., Lettre G. 2009. Progress in genome-wide association studies of human height. Horm. Res. 71:(Suppl 2): 5–13. [DOI] [PubMed] [Google Scholar]
- 9.Hsu P.D., Lander E.S., Zhang F. 2014. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157: 1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lettre G. 2011. Recent progress in the study of the genetics of height. Hum. Genet. 129: 465–472. [DOI] [PubMed] [Google Scholar]
- 11.Lindholm-Perry A.K., Sexten A.K., Kuehn L.A., Smith T.P., King D.A., Shackelford S.D., Wheeler T.L., Ferrell C.L., Jenkins T.G., Snelling W.M., Freetly H.C. 2011. Association, effects and validation of polymorphisms within the NCAPG - LCORL locus located on BTA6 with feed intake, gain, meat and carcass traits in beef cattle. BMC Genet. 12: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisowski L., Tay S.S., Alexander I.E. 2015. Adeno-associated virus serotypes for gene therapeutics. Curr. Opin. Pharmacol. 24: 59–67. [DOI] [PubMed] [Google Scholar]
- 13.Love S., Wyse C.A., Stirk A.J., Stear M.J., Calver P., Voute L.C., Mellor D.J. 2006. Prevalence, heritability and significance of musculoskeletal conformational traits in Thoroughbred yearlings. Equine Vet. J. 38: 597–603. [DOI] [PubMed] [Google Scholar]
- 14.Makvandi-Nejad S., Hoffman G.E., Allen J.J., Chu E., Gu E., Chandler A.M., Loredo A.I., Bellone R.R., Mezey J.G., Brooks S.A., Sutter N.B. 2012. Four loci explain 83% of size variation in the horse. PLoS One 7: e39929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metzger J., Schrimpf R., Philipp U., Distl O. 2013. Expression levels of LCORL are associated with body size in horses. PLoS One 8: e56497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ran F.A., Cong L., Yan W.X., Scott D.A., Gootenberg J.S., Kriz A.J., Zetsche B., Shalem O., Wu X., Makarova K.S., Koonin E.V., Sharp P.A., Zhang F. 2015. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520: 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riggs C.M. 2002. Fractures--a preventable hazard of racing thoroughbreds? Vet. J. 163: 19–29. [DOI] [PubMed] [Google Scholar]
- 18.Salganik M., Hirsch M.L., Samulski R.J. 2015. Adeno-associated virus as a mammalian DNA vector. Microbiol. Spectr. 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Signer-Hasler H., Flury C., Haase B., Burger D., Simianer H., Leeb T., Rieder S. 2012. A genome-wide association study reveals loci influencing height and other conformation traits in horses. PLoS One 7: e37282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soranzo N., Rivadeneira F., Chinappen-Horsley U., Malkina I., Richards J.B., Hammond N., Stolk L., Nica A., Inouye M., Hofman A., Stephens J., Wheeler E., Arp P., Gwilliam R., Jhamai P.M., Potter S., Chaney A., Ghori M.J., Ravindrarajah R., Ermakov S., Estrada K., Pols H.A., Williams F.M., McArdle W.L., van Meurs J.B., Loos R.J., Dermitzakis E.T., Ahmadi K.R., Hart D.J., Ouwehand W.H., Wareham N.J., Barroso I., Sandhu M.S., Strachan D.P., Livshits G., Spector T.D., Uitterlinden A.G., Deloukas P. 2009. Meta-analysis of genome-wide scans for human adult stature identifies novel Loci and associations with measures of skeletal frame size. PLoS Genet. 5: e1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takasuga A. 2016. PLAG1 and NCAPG-LCORL in livestock. Anim. Sci. J. 87: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tozaki T., Sato F., Hill E.W., Miyake T., Endo Y., Kakoi H., Gawahara H., Hirota K., Nakano Y., Nambo Y., Kurosawa M. 2011. Sequence variants at the myostatin gene locus influence the body composition of Thoroughbred horses. J. Vet. Med. Sci. 73: 1617–1624. [DOI] [PubMed] [Google Scholar]
- 23.Weller R., Pfau T., May S.A., Wilson A.M. 2006. Variation in conformation in a cohort of National Hunt racehorses. Equine Vet. J. 38: 616–621. [DOI] [PubMed] [Google Scholar]
- 24.Willet P. 1991. A History of the General Stud-Book. Weatherbys, Northants. [Google Scholar]