Abstract
Objective
Examine the association of white matter hyperintensities (WMH) with risk of incident mild cognitive impairment (MCI) and rate of decline in multiple cognitive systems in community‐based older persons.
Methods
Participants (n = 354) were older persons initially free of cognitive impairment from two ongoing longitudinal epidemiologic studies of aging. All underwent brain magnetic resonance imaging (MRI) for quantification of WMH and gray matter volumes and detailed annual clinical evaluations including 17 cognitive tests. Proportional hazards models were used to examine the relationship between WMH and incident MCI, and mixed‐effects models were used to examine the relationship between WMH and decline in global cognition and five specific cognitive systems.
Results
During up to about 6 years of follow‐up (mean = 4.1), 106 (30% of 354) persons developed MCI. In a proportional hazards model adjusted for age, gender, and education, WMH volume was associated with a substantially increased risk of MCI (P < 0.001). Thus, a person with a high WMH volume (90th percentile) was about 2.7 times more likely to develop MCI compared to a person with a low volume (10th percentile). WMH volume also was associated with an increased rate of decline in global cognition (P < 0.001), perceptual speed, working memory, episodic memory, and semantic memory. Associations persisted after adjustment for total gray matter volume, vascular risk factors, and vascular diseases.
Interpretation
WMH contribute to the development of MCI and are associated with progressive decline in multiple cognitive systems in old age.
Introduction
White matter hyperintensities (WMH), alterations in brain white matter identified via magnetic resonance imaging (MRI), are common in old age and associated with cognitive impairment and dementia.1, 2, 3, 4, 5, 6, 7 However, the relation of WMH with the risk of developing mild cognitive impairment (MCI), often the precursor to Alzheimer's disease, remains unclear. Although some evidence suggests that WMH may increase the risk of MCI, data come mainly from studies that included small or highly selected samples (i.e., clinic‐based persons), used semi‐quantitative ratings of WMH, and did not involve annual assessments of cognition.2, 8, 9 Furthermore, findings are inconsistent; whereas one study of 67 cognitively intact persons found that WMH were associated with the development of MCI, a considerably larger study did not, particularly when other MRI indices were included in the analyses.8, 9 The degree to which WMH contribute to progressive cognitive decline among older persons initially free of cognitive impairment also is poorly understood. Cross‐sectional studies have shown that WMH are associated with subtle cognitive decrements, particularly in perceptual speed and executive functions,10, 11, 12 but little data are available on the relation of WMH with cognitive decline among persons initially free of cognitive impairment.7, 13, 14, 15, 16 A better understanding of the degree to which WMH increase the risk of MCI and contribute to progressive decline in multiple cognitive systems is needed to facilitate strategies to promote cognitive health in old age.
In this study, we examined the association of WMH with the risk of developing MCI and rate of decline in multiple cognitive systems using data from 354 well‐characterized community‐based older persons initially free of cognitive impairment. Participants came from two ongoing longitudinal clinical‐pathologic studies of aging, the Rush Memory and Aging Project and the Religious Orders Study.17, 18 All underwent brain MRI for uniform quantification of WMH and gray matter volumes, which allowed us to examine the independent contribution of WMH to cognitive outcomes over and above gray matter volume. Participants also completed detailed annual clinical evaluations including multiple cognitive tests, which allowed for careful clinical classification and precise estimation of rates of decline in specific cognitive systems.10, 17, 18 Proportional hazards models were used to examine the relation of WMH with incident MCI, and mixed‐effects models were used to examine the relation of WMH with rates of decline in global cognition and five specific cognitive systems. Supporting analyses examined whether the effects of WMH on cognition were independent of gray matter volume, vascular risk factors, and vascular diseases.
Methods
Participants
Data came from participants of two ongoing community‐based cohort studies of aging and dementia, the Rush Memory and Aging Project and the Religious Orders Study.10, 17, 18 Both studies were approved by the Institutional Review Board of Rush University Medical Center. Importantly, the two studies are nearly identical in design and implementation. Both studies recruit participants from residential facilities, enroll older persons without known dementia, employ nearly identical detailed annual assessments, and require brain donation. An informed consent and anatomical gift act were obtained from each participant and all agreed to detailed annual evaluations and brain donation.
The Rush Memory and Aging Project and the Religious Orders Study started in 1997 and 1994, respectively. Neuroimaging was initiated in 2009, first in the Memory and Aging Project and subsequently in the Religious Orders Study.10 As a result, this study involves a subset of participants with neuroimaging data. At the time of analyses, 456 participants had completed at least one MRI scan that was processed for WMH data. For participants with multiple scans, data from the first scan were used. Of the 456 participants, we excluded 9 with a prior history of dementia, 83 who were diagnosed with MCI at the time of MRI scan, and 10 yet to have longitudinal cognitive data (2+ evaluations). This left a total of 354 participants eligible for analyses: the vast majority of these (n = 317) are from the Memory and Aging Project due to the earlier start of imaging in that study. The average age of participants was 80.5 years (Standard deviation [SD] = 7.1 years) and average years of education was 15.7 years (SD = 3.2 years). A majority were women (N = 272, 76.8%).
Clinical evaluation and assessment of cognition
All participants underwent structured annual clinical evaluations, which included medical history, detailed cognitive testing (see below), and neurological examinations.17, 18 Annual follow‐up clinical evaluations are identical in all essential details and conducted by clinicians blinded to prior data. Cognitive function is evaluated at each evaluation using a standardized battery of 17 cognitive performance tests.19, 20 These include measures of episodic memory (i.e., word list memory, recall, and recognition, immediate and delayed recall of the East Boston story, and story A from Logical Memory), semantic memory (i.e., verbal fluency, a 15‐item form of the Boston Naming Test, and a modified NAART), working memory (i.e., digit span forward, digit span backward, and digit ordering), perceptual speed (i.e., number comparison and Symbol Digit Modalities Test), and visuospatial ability (i.e., short forms of Judgment of Line Orientation and Standard Progressive Matrices). Composite measures of global cognitive function and five specific cognitive systems (i.e., perceptual speed, working memory, episodic memory, semantic memory, and visuospatial ability) were computed. Raw scores of individual tests were standardized using the baseline mean and standard deviation of the pooled cohorts, and then averaged across systems to derive composite measures.
Briefly, diagnostic procedures are as follows. A neuropsychologist blinded to other data summarized the results of cognitive performance testing and rendered a judgment of impairment. The clinical diagnosis of dementia follows accepted and validated criteria and diagnoses are made by clinicians with expertise in the evaluation of older persons following review of all clinical data. Diagnoses of MCI are rendered for persons judged to have cognitive impairment but not determined to have dementia, as detailed in numerous prior publications.17, 18, 20
Image acquisition, image processing, and quantification of WMH
Brain MRI data were collected at multiple facilities in order to accommodate persons from diverse geographical areas, but all used a 1.5 Tesla General Electric (Waukesha, WI) MRI scanner and the same imaging protocol. High‐resolution T1‐weighted anatomical data were obtained using a 3D magnetization‐prepared rapid acquisition gradient echo (MPRAGE) sequence with the following parameters: echo time (TE) = 2.8 msec, repetition time (TR) = 6.3 msec, preparation time = 1000 msec, flip angle = 8°, field of view (FOV) = 24 cm × 24 cm, 160 sagittal slices, 1 mm slice thickness, no gap, 224 × 192 acquisition matrix reconstructed to a 256 × 256 image matrix, and two repetitions. T2‐weighted fluid‐attenuated inversion recovery (FLAIR) data were collected using a 2D fast spin‐echo sequence with the following parameters: TE = 120 msec, TR = 8 sec, inversion time = 2 sec, FOV = 24 cm × 24 cm, 42 oblique axial slices, slice thickness = 3 mm, no gap, and 256 × 224 acquisition matrix reconstructed to a 256 × 256 image matrix.
WMH and total gray matter volumes were obtained, as previously described.10 Briefly, the two copies of T1‐weighted MPRAGE data collected on each participant were spatially coregistered and averaged. The result was then registered to the T2‐weighted FLAIR data using affine registration (FLIRT, FMRIB [Oxford Centre for Functional Magnetic Resonance Imaging of the Brain], University of Oxford, UK). The brain was extracted from the coregistered MPRAGE and FLAIR image volumes (BET, FMRIB, University of Oxford). WMH were automatically segmented for each participant using a support vector machine classifier using T1‐weighted MPRAGE and T2‐weighted FLAIR information (WMLS, SBIA [Section of Biomedical Image Analysis], University of Pennsylvania). The total WMH volume for each participant was normalized by the corresponding intracranial volume (ICV) generated by FreeSurfer (http://surfer.nmr.mgh.harvard.edu) based on the averaged raw T1‐weighted MPRAGE data. The measure was then logarithm transformed to reduce skewness. Gray matter was automatically segmented for each participant using FreeSurfer (http://surfer.nmr.mgh.harvard.edu) and the total gray matter volume also was normalized by the corresponding ICV generated by FreeSurfer.
Vascular risk factors and disease
Summary scores indicating each participant's vascular risk burden (i.e., the sum of hypertension, diabetes mellitus, and smoking, resulting in a score from 0 to 3) and vascular disease burden (i.e., the sum of heart attack, congestive heart failure, claudication, and stroke, resulting in a score from 0 to 4) were computed on the basis of self‐report questions, clinical examination, and medication inspection, as previously described.20
Statistical analysis
For these analyses, the visit of first MRI scan was used as analytic baseline. Pairwise correlations, t‐tests, and Chi‐square tests described the bivariate associations of the variables of interest at baseline. To examine the association of WMH volume with incident MCI, we fit a series of Cox proportional hazards models. In these models, the outcome variable was time in years to incident MCI (event time), and the predictor was WMH volume. The event time was right censored for participants without a diagnosis of MCI during follow‐up. The primary model was adjusted for age, gender, and education. Subsequent models included terms to investigate whether the association of WMH with MCI was independent of total gray matter volume, as well as vascular diseases and vascular risk factors.
Next, we examined the association of WMH volume with subsequent change in cognition using mixed‐effects models. In this approach, we used linear models to capture individual rates of change in cognition since the MRI scan. The mean baseline level (intercept) and rate of decline (slope) in cognition were functions of WMH volume. Person‐specific deviations from mean change in cognition were estimated by random effects. These models used annual cognitive measures as the continuous longitudinal outcome, and included terms for WMH, time in years since MRI scan, as well as a WMH x time interaction. The coefficient for the term WMH estimated the association with baseline level of cognition, and the coefficient for the interaction estimated the association with rate of decline in cognition. The primary analysis examined the association of WMH with change in global cognition. Subsequent models examined the relation of WMH with change in specific cognitive systems including perceptual speed, working memory, episodic memory, semantic memory, and visuospatial ability. Analyses were performed using SAS/STAT software, version 9.3 [SAS Institute Inc., Cary, NC]. Statistical significance was determined at the nominal level of α = 0.05.
Results
Participant characteristics
Characteristics of the study participants are summarized in Table 1. All were free of cognitive impairment at the analytic baseline. They were subsequently followed for an average of about 4 years (SD = 1.3, range: 1–6 years), and approximately a third (N = 106, 29.9%) developed MCI during the follow‐up period. Greater WMH volume was associated with older age (Spearman correlation r = 0.423, P < 0.001) but not education, and there was a trend toward greater WMH volumes among women compared to men (P = 0.054). Greater WMH volume was associated with lower baseline levels of global cognition (Spearman correlation r = −0.174, P = 0.001), lower total gray matter volumes (Spearman correlation r = −0.407, P < 0.001), and more vascular diseases (Spearman correlation r = 0.220, P < 0.001); WMH was not associated with vascular risk factors.
Table 1.
Participant characteristics at baseline
| Characteristic (n = 354) | Mean, SD, or N, % |
|---|---|
| Age in years | 80.5, 7.1 |
| Female (N, %) | 272, 76.8% |
| Non‐Hispanic Whites (N, %) | 335, 94.6% |
| Education in years | 15.7, 3.2 |
| WMH volume (% ICV) | 0.94% (1.03) |
| WMH volume (% ICV after logarithm) a | −0.222, 0.408 |
| Total gray volume (tenths of % ICV) | 338.1, 41.8 |
| MMSE at baseline (Mean, SD) | 28.7, 1.3 |
| Global cognition at baseline (Mean, SD) | 0.41, 0.43 |
| Episodic memory at baseline (Mean, SD) | 0.51, 0.54 |
| Semantic memory at baseline (Mean, SD) | 0.36, 0.55 |
| Working memory at baseline (Mean, SD) | 0.24, 0.65 |
| Perceptual speed at baseline (Mean, SD) | 0.34, 0.73 |
| Visuospatial ability at baseline (Mean, SD) | 0.43, 0.65 |
| Vascular diseases (N, %) | |
| None | 256, 72.5% |
| 1 | 74, 21.0% |
| 2 | 21, 6.0% |
| 3 | 2, 0.6% |
| Vascular risk factors (N, %) | |
| None | 80, 22.6% |
| 1 | 162, 45.7% |
| 2 | 99, 28.0% |
| 3 | 13, 3.7% |
ICV, intracranial volume, SD, Standard deviation; WMH, white matter hyperintensities
log10 transformed.
WMH and incident MCI
During up to 6 years of follow‐up, 106 (30% of 354) persons developed MCI. We examined the association of WMH volume with incident MCI in a series of proportional hazard models. In the core model (Model 1, Table 2), adjusted for age, gender, and education, WMH volume was associated with a substantially increased risk of incident MCI, such that every 1 SD increase in WMH was associated with a 1.43 increase in the risk of MCI (P = 0.002). To illustrate the magnitude of this result, compared to a typical participant (i.e., female, 80 years of age, 16 years of education) with a low (10th percentile: −0.76) WMH volume at baseline, the risk of incident MCI was 2.67 times higher for a female participant with the same age and education but high (90th percentile: 0.37) WMH volume (Figs. 1, 2).
Table 2.
Association of WMH with incident mild cognitive impairmenta
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Hazard ratios (95% CI) | Hazard ratios (95% CI) | Hazard ratios (95% CI) | |
| Age | 1.0622 (1.0259, 1.0997) | 1.0383 (0.9955, 1.0830) | 1.0352 (0.9916, 1.0808) |
| Male sex | 1.4839 (0.9178, 2.3994) | 1.3705 (0.7999, 2.3481) | 1.3895 (0.8015, 2.4089) |
| Education | 1.0425 (0.9749, 1.1148) | 1.0601 (0.9843, 1.1418) | 1.0599 (0.9821, 1.1438) |
| WMH volume | 1.4287 (1.9090, 3.0084) | 1.3813 (1.6964, 4.1991) | 2.1828 (1.1415, 4.1740) |
| Total gray matter | – | 0.9930 (0.9854, 1.0006) | 0.9933 (0.9857, 1.0009) |
| Vascular risk factors | – | – | 0.9627 (0.7156, 1.2950) |
| Vascular diseases | – | – | 1.1417 (0.8112, 1.6068) |
WMH, white matter hyperintensities
Derived from proportional hazards models adjusted for age, gender, and education.
Figure 1.
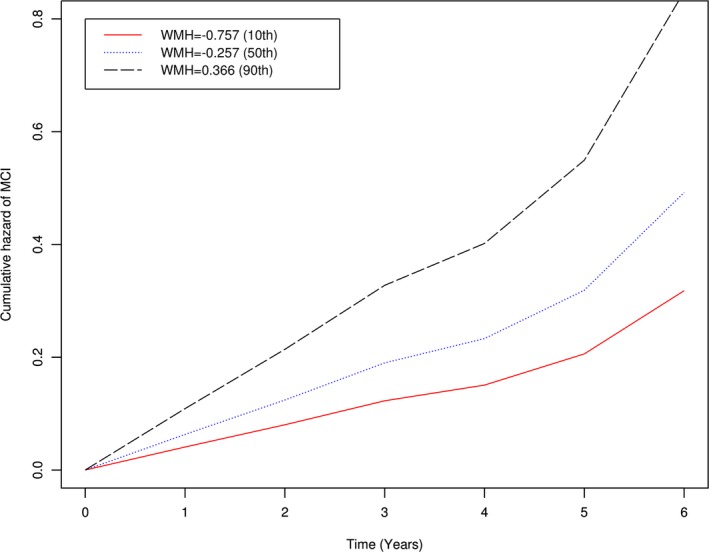
Association of white matter hyperintensities (WMH) with risk of mild cognitive impairment (MCI). Figure shows the cumulative hazard of developing MCI for persons with low (red line), medium (blue), and high (black) burdens of WMH.
Figure 2.
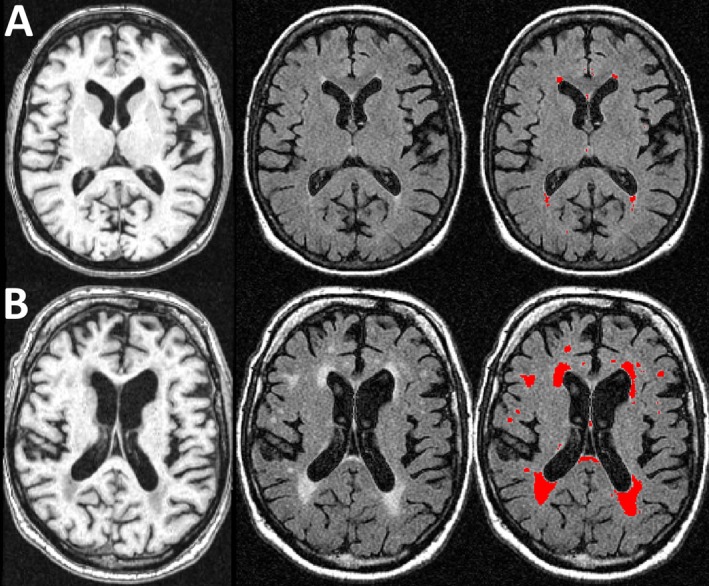
Magnetic resonance imaging (MRI) of persons with low (10th percentile) and high (90th percentile) burdens of white matter hyperintensities (WMH). Figure shows representative images of persons with low (10th percentile, row A) and high (90th percentile, row B) burdens of WMH. The first column (L) shows T1‐weighted MPRAGE axial images, the second (middle) shows T2‐weighted FLAIR images, and the third (R) shows the segmented WMH lesions in red (3rd) column.
Next, as WMH volume was negatively associated with total gray matter volume and this additional measure of brain integrity may account for the association of WMH with MCI, we augmented the core model by further adjusting for total gray matter volume (Model 2, Table 2). In this model, gray matter volume was only marginally related to incidence of MCI, and the association of WMH with MCI was essentially unchanged. This suggests that the association of WMH with MCI is relatively independent of gray matter volume.
Finally, because vascular risk factors and diseases may also confound the association of WMH with MCI, we augmented the core model by including total gray matter volume and two additional terms for summary measures of vascular risk factors and vascular diseases (Model 3, Table 2). In this analysis, gray matter volume was again marginally associated with incident MCI, but vascular risk factors and diseases were not associated with incident MCI. The association of WMH again persisted.
WMH and rate of decline in global cognition
Because cognition deteriorates slowly over years before diagnostic criteria for cognitive impairment are met and the association of WMH with subsequent cognitive decline remains poorly understood, we next examined the association of WMH with the rate of cognitive decline in analyses that simultaneously consider the starting level of cognition. The initial model examined the association of WMH with both the initial level and rate of decline in global cognition (Table 3). After adjustment for age, gender, and education, greater WMH volume was associated with a lower initial level of global cognition (Estimate = −0.118, Standard error [SE] = 0.016, P = 0.033). Furthermore, WMH volume was associated with an increased rate of decline in global cognition (Estimate −0.055, SE = 0.016, P < 0.001), as illustrated in Figure 3. This finding was unchanged after adjustment for total gray matter volume, vascular risk factors, and diseases (data not shown).
Table 3.
Association of white matter hyperintensities with initial level and rate of decline in cognitiona
| Outcome | Initial Level | Rate of Decline |
|---|---|---|
| Estimate (SE, P) | Estimate (SE, P) | |
| Global cognition | −0.1175 (0.0550,0.0333) | −0.0554 (0.0160,0.0005) |
| Episodic memory | −0.0747 (0.0689,0.2785) | −0.0615 (0.0204,0.0027) |
| Semantic memory | −0.0458 (0.0708,0.5188) | −0.0502 (0.0170,0.0032) |
| Working memory | −0.0205 (0.0853,0.8100) | −0.0668 (0.0196,0.0007) |
| Perceptual speed | −0.3842 (0.0928,<0.001) | −0.0542 (0.0191,0.0046) |
| Visuospatial ability | −0.0921 (0.0810,0.2562) | −0.0031 (0.0172,0.8572) |
SE, Standard error
Derived from mixed‐effects models adjusted for age, gender, and education.
Figure 3.
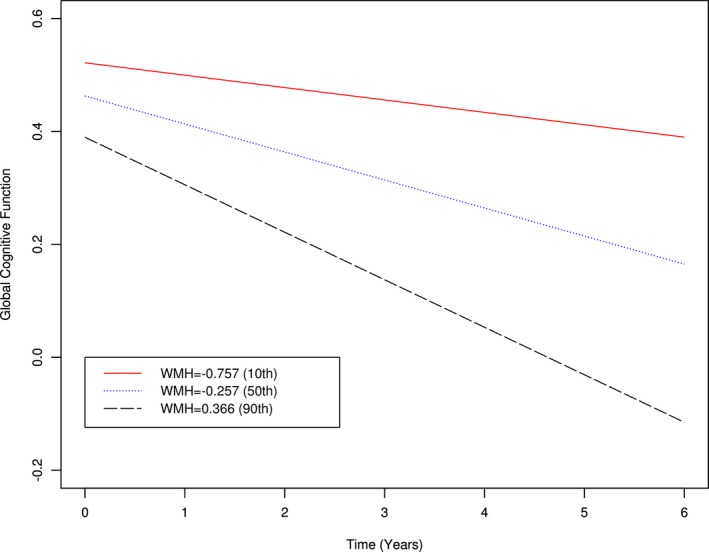
Association of white matter hyperintensities (WMH) with rate of decline in global cognition. Figure shows the rate of decline in global cognition for persons with low (red line), medium (blue), and high (black) burdens of WMH.
WMH and rate of decline in five specific cognitive systems
Next, because the cognitive profile associated with WMH remains unclear, we examined the relation of WMH with the initial level and rate of decline in five specific cognitive systems in a series of mixed‐effects models. Consistent with our prior report, WMH volume was strongly associated with the initial level of perceptual speed (Table 3, Estimate = −0.384, SE = 0.093, P < 0.001), but was not associated with the initial level of any other cognitive system. However, WMH volume was associated with an increased rate of decline in 4 of the 5 specific cognitive systems, including perceptual speed (P = 0.005), working memory (P < 0.001), episodic memory (P = 0.003), and semantic memory (P = 0.003).
The association of WMH with the initial level of perceptual speed but not with the initial level of any of the other systems suggests that WMH affect perceptual speed at relatively younger ages compared to other cognitive systems. To directly examine whether the association of WMH with perceptual speed emerged before other cognitive systems, we added terms for the interaction of age and WMH to the mixed‐effects models examining decline in the four cognitive systems for which WMH volume was significantly associated with decline. In these models, there was evidence of age by WMH interactions on the initial level of cognition, such that the older the baseline age, the stronger the association of WMH with initial level of cognition, particularly for the domain of perceptual speed, with trends for the other domains. By contrast, the associations of the interactions with decline in cognition were not significant (Table 4). Figure 4 illustrates the relations of age and WMH volume with both the initial level and rate of decline for each of the cognitive systems. Each pair of line segments (blue vs. red) represents the predicted trajectories in 5‐year intervals for persons with high (90th percentile) versus low (10th percentile) WMH volumes at different ages (e.g., 70, 75, 80). Notably, the association of WMH volume with perceptual speed emerged at relatively younger ages (i.e., prior to age 80). By contrast, the associations of WMH volume with other cognitive systems emerged at relatively older ages (i.e., after age 80).
Table 4.
Interactions of age and WMH on initial level and rate of decline in cognitiona
| Episodic memory Estimate (SE, P) | Semantic Memory Estimate (SE, P) | Working Memory Estimate (SE, P) | Perceptual Speed Estimate (SE, P) | |
|---|---|---|---|---|
| WMH | −0.0640 (0.0690,0.3547) | −0.0329 (0.0710,0.6429) | −0.0056 (0.0855,0.9478) | −0.3618 (0.0927,0.0001) |
| WMH x time | −0.0592 (0.0204,0.0038) | −0.0499 (0.0171,0.0035) | −0.0665 (0.0197,0.0007) | −0.0549 (0.0192,0.0043) |
| Age x WMH | −0.0143 (0.0093,0.1281) | −0.0172 (0.0096,0.0745) | −0.0203 (0.0116,0.0809) | −0.0306 (0.0125,0.0150) |
| Age x WMH x Time | −0.0043 (0.0029,0.1390) | −0.0007 (0.0024,0.7582) | −0.0009 (0.0028,0.7430) | 0.0015 (0.0028,0.5909) |
SE, Standard error; WMH, white matter hyperintensities
For each column, estimates were derived from a linear mixed model adjusted for age, gender, and education. The term for WMH represents the association of WMH with baseline level of cognition for a typical woman, age 80, and with 16 years of education. The term for WMH x time represents the association of WMH with rate of decline in cognition. The term for age x WMH represents the additional association of WMH with baseline level of cognition with each additional year of age at baseline. The term for age x WMH x time represents the effect of WMH on the rate of decline in cognition with each additional year of age at baseline.
Figure 4.
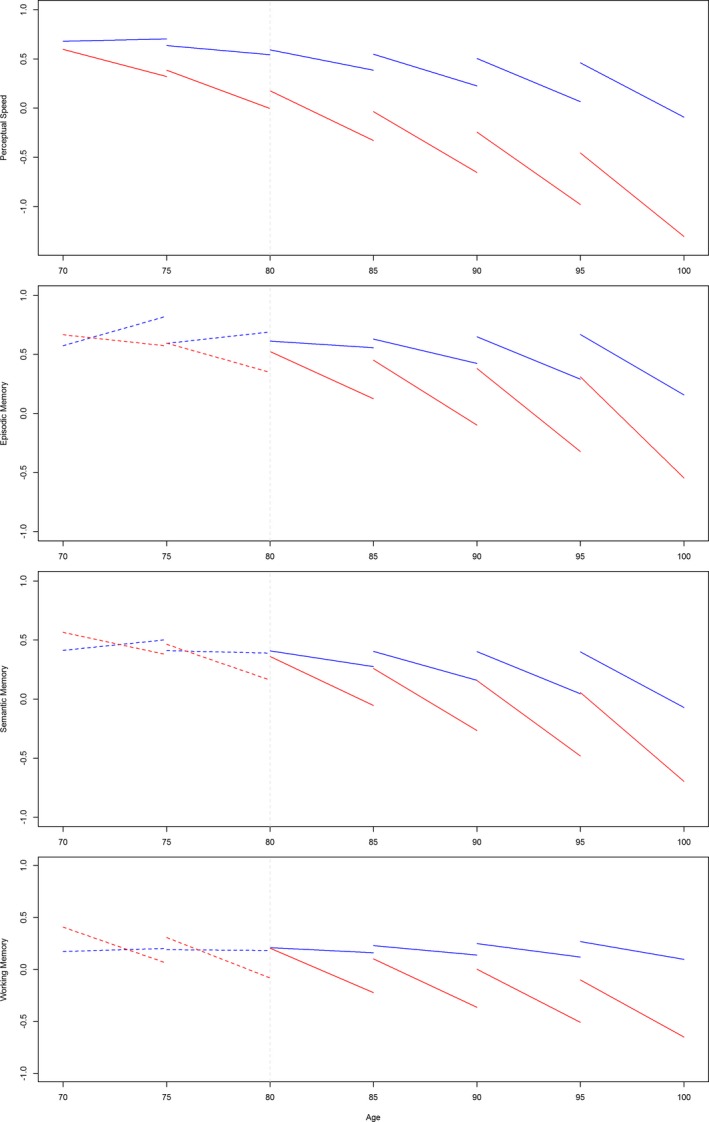
Association of white matter hyperintensities (WMH) with cognitive systems by 5‐year age groups*. Each pair of line segments (blue versus red) represents the predicted trajectories in 5‐year intervals for persons with high (red, 90th percentile) versus low (blue, 10th percentile) WMH volumes at different ages.
Secondary analyses
Because the association of WMH with perceptual speed emerged earlier than other cognitive domains, we sought to examine whether the association of WMH with the rate of decline in the other cognitive systems persisted after adjustment for perceptual speed. Thus, we repeated the analyses examining decline in working memory, semantic memory, and episodic memory with an additional term controlling for the initial level of perceptual speed. Interestingly, the results for decline in the other cognitive domains persisted even after adjustment for the starting level of perceptual speed (all P's <0.01). This suggests that the association of WMH with decline in the other cognitive systems is relatively independent of the initial level of perceptual speed.
Discussion
In this study of more than 350 well‐characterized older persons initially free of cognitive impairment, we found that WMH were associated with a substantially increased risk of incident MCI and that this association was independent of gray matter volume, vascular risk factors, and vascular diseases. Furthermore, in analyses based on cognitive data collected annually for up to 6 years postimaging, WMH were associated with increased rates of decline in global cognition, perceptual speed, working memory, episodic memory, and semantic memory. Finally, the effects of WMH on perceptual speed preceded those on other cognitive systems; that is, WMH‐related deteriorations in perceptual speed were evident at relatively younger ages compared to other cognitive systems. These findings suggest that WMH initially contribute to loss of perceptual speed but ultimately are associated with progressive decline in multiple cognitive systems in old age.
This study extends prior work in three important ways. First, this study shows that WMH contribute to the earliest manifestation of cognitive impairment and this association is relatively independent of other MRI‐based indicators of brain integrity (i.e., total gray matter volume) and several indices of vascular risk and disease. MCI, which is largely recognized as preclinical AD, poses an already large and rapidly increasing public health challenge, and efforts to combat it are primarily focused on strategies to reduce the burden of AD‐related plaques and tangles. These findings suggest that WMH also contribute to preclinical AD. Ultimately, promotion of health and vitality in old age requires successful interventions to prevent the progressive cognitive decline that occurs prior to the onset of overt dementia, and it is important to note that, unlike AD pathology, WMH can be quantified relatively easily and inexpensively in vivo. Thus, older persons without cognitive impairment but in whom WMH are observed may be considered those at relatively higher risk of poor cognitive outcomes and may be among those most likely to benefit from interventions as they are developed. Consideration of WMH in treatment trials therefore may be warranted.
Second, this study greatly extends our understanding of the cognitive profile of WMH. We and others have previously reported cross‐sectional associations of WMH with cognition, most notably perceptual speed and executive functions, and WMH are generally thought to have a relatively domain‐specific effect on cognition.11, 12, 21 In this study, we again observed that the impact of WMH on the initial level of cognition was domain specific, affecting perceptual speed only. However, WMH were associated with an increased rate of decline in multiple cognitive systems over time, including perceptual speed, working memory, episodic memory, and semantic memory; moreover, the findings for working memory, episodic memory, and semantic memory decline persisted even after adjustment for the initial level of perceptual speed, thereby supporting their relatively independent associations with WMH. The disparity between the WMH–cognition associations in initial level and rates of decline underscore the limited utility of one‐time assessments of complex constructs such as cognition as a proxy for rate of change. Use of repeated measures of cognition annually over many years allowed us to precisely capture individual trajectories of change, and results showed that the impact of WMH on cognition is considerably more broad than previously recognized among persons initially free of cognitive impairment. Thus, interventions that target WMH may help preserve multiple cognitive systems in old age.
Third, this study provides evidence that WMH are associated with deteriorations in perceptual speed at relatively younger ages compared to other domains, which are affected at relatively older ages (i.e., age interactions). The earlier, domain‐specific effect of WMH on perceptual speed supports prior findings suggesting that WMH may underlie early cognitive decrements commonly attributed to “normal” aging and may explain the tendency for this cognitive system to decline before other cognitive systems.21, 22, 23 However, our findings suggest that WMH also are associated with deterioration in other cognitive abilities including working memory, semantic memory, and even episodic memory, the hallmark of AD, albeit at relatively older ages. This suggests that cognitively intact older persons with WMH are likely to exhibit subsequent loss of function in multiple cognitive domains, and WMH may serve as a relatively early biomarker for impending and generalized cognitive decline.
There are several potential mechanisms via which WMH may affect the risk of MCI and rate of cognitive decline in old age. WMH are thought in part to represent cerebral microvascular disease due to ischemic injury and gliosis, and these alterations can reflect an evolving pathologic process. Indeed, prior work has shown that WMH are related to indices of white matter pathology derived from the brain and that baseline levels of WMH are related to the rate of progression of WMH.24, 25 Thus, it is possible that persons with greater WMH volumes at baseline were more likely to exhibit poorer cognitive outcomes later as a consequence of more severe small vessel disease. In addition, WMH cooccur with and may also be related to other neurodegenerative processes such as Alzheimer's and Lewy body disease, and the association of WMH with cognitive decline may reflect an increasing burden of pathology in general or the complex interplay between comorbid pathologies. That is, WMH may lower the threshold at which other pathologies impair cognition and thus may decrease cognitive reserve or the ability to tolerate brain injury. Finally, WMH may reflect or be related to separate processes such as inflammation or other forms of vascular pathology and thus may affect cognition via other mechanisms. At present, it remains unknown whether the differential temporal relations of WMH with rates of decline in the cognitive systems (e.g., perceptual speed early vs. episodic memory later) are due to the progression of small vessel disease specifically, the influence of other age‐related pathologies, or decreased cognitive reserve in general. Future research is needed to clarify the basis of the association of WMH with loss of cognitive function in old age.
Strengths of this study include the detailed, uniform clinical evaluations to establish clinical diagnoses and annual cognitive testing using psychometrically sound measures for up to 6 years to quantify individual trajectories. In addition, WMH as well as gray matter volumes were quantified using an automated procedure, and other potentially important confounders of the association of WMH with cognitive outcomes also were considered (i.e., vascular conditions). Limitations include the single assessment of WMH rather than assessment of change in WMH over time. However, we are collecting longitudinal scans and will examine the functional consequences of change in WMH when possible, as such data are needed to more fully elucidate the impact of WMH on late life cognitive outcomes.
Author Contributions
(1) Conception and design of the study: PAB, DF, DAB, RSW, JAS; (2) acquisition and analysis of data: PAB, DAB, LY, JAS, DF, JY, KA, SL, ZA; and (3) drafting a significant portion of the manuscript or figures: LYK.
Conflicts of Interest
The authors have no relevant conflicts to report.
Acknowledgments
This study was supported by the following NIA grants: R01AG17917, P30AG10161, R01AG15819, R01AG34374, R01AG40039, and R01NS084965. The authors gratefully acknowledge the participants and staff of the Religious Orders Study and the Rush Memory and Aging Project.
References
- 1. Brickman AM, Provenzano FA, Muraskin J, et al. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident alzheimer disease in the community. Arch Neurol 2012;69:1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Debette S, Beiser A, Decarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the framingham offspring study. Stroke 2010;41:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology 2003;22:13–22. [DOI] [PubMed] [Google Scholar]
- 4. Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol 2004;61:1531–1534. [DOI] [PubMed] [Google Scholar]
- 5. Tosto G, Zimmerman ME, Carmichael OT, et al. Predicting aggressive decline in mild cognitive impairment: the importance of white matter hyperintensities. JAMA Neurol 2014;71:872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mortamais M, Reynes C, Brickman AM, et al. Spatial distribution of cerebral white matter lesions predicts progression to mild cognitive impairment and dementia. PLoS ONE 2013;8:e56972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benedictus MR, van Harten AC, Leeuwis AE, et al. White matter hyperintensities relate to clinical progression in subjective cognitive decline. Stroke 2015;46:2661–2664. [DOI] [PubMed] [Google Scholar]
- 8. Smith EE, Egorova S, Blacker D, et al. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol 2008;65:94–100. [DOI] [PubMed] [Google Scholar]
- 9. Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the cardiovascular health study cognition study: part 2. Arch Neurol 2003;60:1394–1399. [DOI] [PubMed] [Google Scholar]
- 10. Arvanitakis Z, Fleischman DA, Arfanakis K, et al. Association of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairment. Brain Struct Funct 2016; 221(4):2135–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hedden T, Mormino EC, Amariglio RE, et al. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J Neurosci 2012;32:16233–16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology 2004;63:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Groot JC, De Leeuw FE, Oudkerk M, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 2002;52:335–341. [DOI] [PubMed] [Google Scholar]
- 14. Adak S, Illouz K, Gorman W, et al. Predicting the rate of cognitive decline in aging and early alzheimer disease. Neurology 2004;63:108–114. [DOI] [PubMed] [Google Scholar]
- 15. Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small‐vessel disease and decline in information processing speed, executive function and memory. Brain 2005;128(Pt 9):2034–2041. [DOI] [PubMed] [Google Scholar]
- 16. Vemuri P, Lesnick TG, Przybelski SA, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 2015;138(Pt 3):761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res 2012;9:628–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bennett DA, Schneider JA, Buchman AS, et al. Overview and findings from the rush memory and aging project. Curr Alzheimer Res 2012;9:646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 2002;17:179–193. [PubMed] [Google Scholar]
- 20. Boyle PA, Wilson RS, Aggarwal NT, et al. Mild cognitive impairment: risk of alzheimer disease and rate of cognitive decline. Neurology 2006;67:441–445. [DOI] [PubMed] [Google Scholar]
- 21. Jokinen H, Goncalves N, Vigario R, et al. Early‐stage white matter lesions detected by multispectral MRI segmentation predict progressive cognitive decline. Front Neurosci 2015;9:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith EE, O'Donnell M, Dagenais G, et al. Early cerebral small vessel disease and brain volume, cognition, and gait. Ann Neurol 2015;77:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vannorsdall TD, Waldstein SR, Kraut M, et al. White matter abnormalities and cognition in a community sample. Arch Clin Neuropsychol 2009;24:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jagust WJ, Zheng L, Harvey DJ, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol 2008;63:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silbert LC, Howieson DB, Dodge H, Kaye JA. Cognitive impairment risk: white matter hyperintensity progression matters. Neurology 2009;73:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]


