Abstract
Objective
The King–Devick (KD) test, which is based on rapid number naming speed, is a performance measure that adds vision and eye movement assessments to sideline concussion testing. We performed a laboratory‐based study to characterize ocular motor behavior during the KD test in a patient cohort with chronic concussion to identify features associated with prolonged KD reading times.
Methods
Twenty‐five patients with a concussion history (mean age: 31) were compared to control participants with no concussion history (n = 42, mean age: 32). Participants performed a computerized KD test under infrared‐based video‐oculography.
Results
Average intersaccadic intervals for task‐specific saccades were significantly longer among concussed patients compared to controls (324.4 ± 85.6 msec vs. 286.1 ± 49.7 msec, P = 0.027). Digitized KD reading times were prolonged in concussed participants versus controls (53.43 ± 14.04 sec vs. 43.80 ± 8.55 sec, P = 0.004) and were highly correlated with intersaccadic intervals. Concussion was also associated with a greater number of saccades during number reading and larger average deviations of saccade endpoint distances from the centers of the to‐be‐read numbers (1.22 ± 0.29° vs. 0.98 ± 0.27°, P = 0.002). There were no differences in saccade peak velocity, duration, or amplitude.
Interpretation
Prolonged intersaccadic intervals, greater numbers of saccades, and larger deviations of saccade endpoints underlie prolonged KD reading times in chronic concussion. The KD test relies upon a diffuse neurocognitive network that mediates the fine control of efferent visual function. One sequela of chronic concussion may be disruption of this system, which may produce deficits in spatial target selection and planning of eye movements.
Introduction
Concussion is a change in brain function following a force to the head or body that results in new and otherwise unexplained neurological symptoms.1 Dysfunction may occur in one or more domains, resulting in physical symptoms, behavioral changes, emotional disturbances, and sleep impairment. While up to 90% of concussions resolve in 7–10 days, recovery time may be longer.2 The time to recover may reflect the extent of microscopic neuronal shearing3 and the impairment of cellular and metabolic function.4, 5 While these insights help elucidate the neurophysiology of acute concussive injuries, chronic concussion is still poorly understood and mechanisms explaining the neurobiology over a protracted time scale have not been well characterized.
An estimated 1.6–3.8 million sports‐related concussions occur annually in the United States,6 accounting for 6% and 9% of athletic injuries at the collegiate and high school levels, respectively, though these numbers are conservative, as many concussions are never reported.7, 8 Repeat concussions are associated with greater severity of symptomatology, longer recoveries, and earlier onset of age‐related memory loss and dementia.9 In fact, the probability of repeat concussion increases threefold following the first traumatic event.10 Thus, accurate, rapid, and sensitive sideline measures of concussion detection are critical, as are measures for the clinical assessment of protracted, chronic concussion.
Tools routinely implemented on the sidelines include symptom checklists, the Standardized Assessment of Concussion (SAC), and the Balance Error Scoring System (BESS). In addition, visual performance tests have been shown to be sensitive measures of acute concussion detection. Less emphasis has been given to utilization of these measures to assess chronic concussion. It is estimated that more than half of the brain's pathways are linked to vision and eye movement control11; many of these areas are susceptible to injury by concussion, particularly the frontal and temporal lobes.12, 13 In this regard, the presence of abnormal eye movements has been shown to indicate sub‐optimal brain function in postconcussive states.14, 15, 16, 17, 18 .
The King–Devick (KD) test, a vision‐based rapid number naming task, has been validated as a sensitive and specific sideline performance measure for acute concussion detection.19, 20, 21, 22 The KD test is a pseudoreading task that captures afferent and efferent vision, including saccadic eye movements and their interleaved fixations, along with aspects of cognition. With conventional KD test administration, which consists of reading the card numbers aloud from a spiral‐bound or tablet version, it is not possible to determine the number of saccades generated or to detect any pathology in saccade characteristics.23 Objective metrics include only testing time and error rate. Following a concussive event, total test times are significantly prolonged from baseline,19, 20, 21, 22, 24 but not after physical exertion.25
The mechanisms underlying prolonged KD test times are currently unknown. Several possibilities exist, including saccadic slowing, increased duration of fixations or the pauses between saccades, increased number of saccades, inaccurate saccades, or other superimposed abnormalities of eye movements. It is also possible that the eye movements are normal and that the slowed reading times are cognitive in nature.26, 27 The objective of this study was to perform a detailed temporal, spatial, and kinematic analysis of the saccades and the periods of fixation in between saccades during KD performance following concussion, as well as to determine which eye movement metrics predicted prolonged reading KD times.
Methods
Participants and questionnaire assessments
Concussion subjects were recruited following a clinical visit for a diagnosis of concussion at the NYU Concussion center. Exclusion criteria included abnormal neuroimaging, orbital fracture, or any history of eye movement disorders known to exist prior to injury. Healthy adult volunteers without a history of concussion, neurological impairment, or visual dysfunction (other than refractive error) were recruited as controls. This research was approved by the NYU Institutional Review Board. Informed consent was obtained from each subject.
Vision‐specific quality of life was assessed in all concussed and control subjects with the 25‐item National Eye Institute Visual Functioning Questionnaire (NEI‐VFQ‐25) (perfect score is 100) and 10‐item supplement.28, 29 The self‐administered questionnaires have response gradings utilizing a Likert scale. Each concussion subject also completed the Ohio State University Traumatic Brain Injury Form30 and the Post‐Concussion Symptom Inventory (PCSI).31 The Ohio State University Traumatic Brain Injury Form consists of a 3–5 min structured interview focused on a person's lifetime history of traumatic brain injury and details surrounding each injury. The PCSI assesses symptoms prior to concussion and at the time of form completion (the subject rank symptoms from 0 to 6, with 6 being the most severe). The delta score was calculated as a summation of the pre (y1) to post (y2) differences, where the differences were y2−y1 and the delta score was calculated as the sum of the changes for each symptom listed in the PCSI.
Eye movement recordings and data analysis
All concussed and control subjects completed a 13‐point spatial calibration procedure and one trial of reading three computer‐generated KD test cards (Fig. 1, left panels) while undergoing simultaneous eye movement recording with infrared oculography (Eyelink 1000 + , SR Research, Ontario Canada). The system recorded binocularly with a sampling frequency of 500 Hz and a spatial accuracy of approximately 0.5°. Eye movement data were analyzed offline using custom Matlab software. Data between card presentations were automatically identified for exclusion and manually confirmed, and data within 100 ms of a blink were automatically eliminated.
Figure 1.
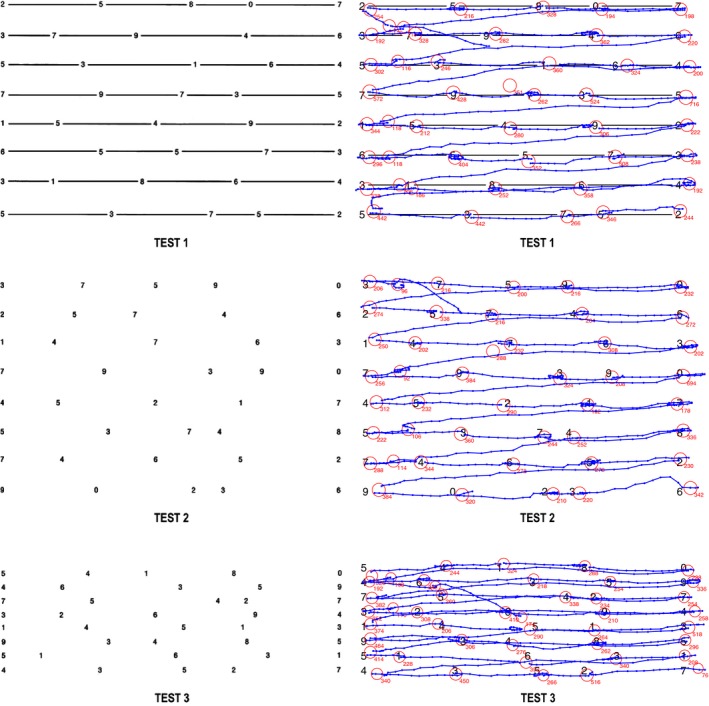
LEFT panels. Test cards 1 (top), 2 (middle), and 3 (bottom) of the King–Devick test (as implemented in digital paradigm and on sideline testing). RIGHT panels. Demonstration of eye tracking tracings of saccades and fixations overlaid on KD test cards 1 (top), 2 (middle), and 3 (bottom) for a representative control subject. The blue lines with dots (data samples) represent “task‐specific” horizontal and oblique saccades and the red circles represent fixations (duration, which is displayed in milliseconds, correlates with the diameter of the circle). KD, King–Devick.
Total reading time and error rate were recorded. Analyses of saccade velocity, acceleration, amplitude, duration, spatial accuracy, and main sequence relationships between these parameters were completed for two sets of saccades: (1) for all saccades performed during the procedure and (2) for “task‐specific” saccades which we define as saccades with a horizontal amplitude of at least 2°. Task‐specific saccades include two subsets: horizontal saccades with horizontal amplitude of at least 2° (the smallest saccade required for number reading progression on the test cards) and saccades with a horizontal amplitude of at least 10° and a vertical amplitude of at least 0.5° (representing oblique saccades functioning to transition between lines during reading) (Fig. 1, right panels). Intersaccadic intervals (ISI), defined as the time between task‐specific saccades, were calculated. Further details, including full methodology of recording procedures and KD eye movement analysis technique, have been previously published.32
Comparisons between subject‐specific mean values in concussed versus control subjects were performed using t‐tests. Product‐moment correlations were computed to assess the relationship between KD test performance and several saccade metrics, including saccade amplitudes and ISI values. Multiple linear regression was performed to assess multidimensional linear correlations. Therefore, only linear terms were included in these regression models.
Results
Demographics and questionnaire assessments
Twenty‐five subjects with a history of concussion (age range: 16–64, mean: 31 years, 52% female) met eligibility criteria and were included. Forty‐two healthy adults (age range: 18–53, mean: 32 years, 71% female) were included in our control population. In the concussed cohort, the median time since the most recent concussion was 54 weeks (interquartile range: 87 weeks). Data from the Ohio State University Traumatic Brain Injury Form is included in Table 1. Ohio State University Traumatic Brain Injury Form interviews were incomplete in two subjects due to poor subject memory of the time course of concussion history.
Table 1.
Demographic and baseline characteristics
| Concussion (n = 25) | Control (n = 42) | |
|---|---|---|
| Age, % | ||
| <18 | 12 | 2 |
| 18–40 | 68 | 84 |
| >40 | 20 | 14 |
| Sex, % | ||
| Male | 48 | 29 |
| Female | 52 | 71 |
| Concussion multiplicity, % | ||
| 1 | 43 | |
| 2 | 26 | |
| 3 | 9 | |
| 4+ | 22 | |
| Mechanism of injury, % | ||
| Sports | 35 | |
| MVA | 26 | |
| Fall | 17 | |
| Assault | 5 | |
| Other trauma | 17 | |
| LOC, % | ||
| No LOC | 56 | |
| <30 min | 39 | |
| 30 min–24 h | 5 | |
| Dazed, % | ||
| Yes | 74 | |
| No | 26 | |
For the NEI‐VFQ‐25, the concussion cohort had a mean unweighted score of 77.19 ± 14.80 versus 95.64 ± 5.5 in healthy controls (P < 0.0001, two‐sample t‐test). For the 10‐item supplement, the concussion cohort had a mean score of 71.39 ± 17.14 versus 92.82 ± 7.20 in healthy controls (P < 0.0001, two‐sample t‐test). For the composite with 10‐item supplement, the concussion cohort had a mean unweighted score of 75.47 ± 14.84 versus 94.82 ± 5.53 in healthy controls (P < 0.0001, two‐sample t‐test).
In the self‐reported PCSI, the preinjury mean was 13.05 ± 16.92, compared to a postinjury mean of 45.60 ± 24.88 (delta mean of 32.55) (Table 2).
Table 2.
Postconcussion symptom inventory total
| Preinjury (%) | Postinjury [Current] (%) | ΔPre > Post change (%) | |
|---|---|---|---|
| 0–10 | 70 | 5 | 10a |
| 11–20 | 5 | 10 | 25 |
| 21–30 | 10 | 20 | 10 |
| 31–40 | 5 | 5 | 10 |
| 41–50 | 5 | 15 | 15 |
| >50 | 5 | 45 | 30 |
one patient had a negative delta value.
King–Devick metrics
The total KD reading time for concussion subjects on average was 53.4 ± 14.04 sec versus 43.80 ± 8.55 sec for healthy controls (P < 0.004, two‐sample t‐test). Per‐card averages are shown in Table 3. Number naming errors included 1 error each in five concussion subjects and three control subjects, two errors in one control subject, and 12 errors in one concussion subject. Of note, the concussion subject with 12 errors had a 20‐year professional boxing history and was currently symptomatic from the most recent concussion 1 year prior.
Table 3.
Comparison of saccade and intersaccadic intervals between concussed subjects and controls
| Variables | Control (n = 42) | Concussion (n = 25) |
|---|---|---|
| Number | ||
| Number of all saccades | 138 ± 18 | 157 ± 50 |
| Number of KD saccades | 124 ± 15 | 137 ± 41 |
| Wrong directional saccade percentage (%) | 10.13% ± 5.33% | 14.43% ± 8.26% |
| Temporal | ||
| Total KD time (sec) | 43.80 ± 8.55 | 53.43 ± 14.04 |
| Card 1 time (sec) | 14.30 ± 2.88 | 16.67 ± 4.30 |
| Card 2 time (sec) | 14.40 ± 2.65 | 17.91 ± 4.40 |
| Card 3 time (sec) | 15.10 ± 3.31 | 18.85 ± 6.01 |
| Duration (msec) | 32.0 ± 4.0 | 31.4 ± 4.5 |
| Peak velocity (°/sec) | 341.6 ± 49.7 | 345.8 ± 43.8 |
| Peak acceleration (°/ss) | 38754.0 ± 6881.6 | 38048.7 ± 7407.2 |
| Peak deceleration (°/ss) | 32828.7 ± 5659.5 | 33076.6 ± 7756.9 |
| Intersaccadic interval (msec) | 286.1 ± 49.7 | 324.4 ± 85.6 |
| Spatial | ||
| Amplitude (°) | 8.5 ± 0.9 | 8.3 ± 1.0 |
| Distance (°) | 1.0 ± 0.3 | 1.2 ± 0.3 |
| X error (°) | 0.1 ± 0.3 | 0.3 ± 0.3 |
| Y error (°) | 0.1 ± 0.2 | 0.0 ± 0.3 |
Values are mean ± standard deviation unless otherwise indicated.
Total KD time is the time used to finish three test cards in King–Devick test.
All other measures were calculated for task‐specific saccades.
KD, King–Devick.
Eye movement analysis
Saccade kinematics
There were no significant differences between saccade peak velocity, acceleration, deceleration, or amplitude between concussed subjects and healthy controls for all saccades and for task‐specific saccades (Table 3). Main sequence relationships of saccade amplitude to peak velocity and duration were similar in concussed and control subjects for all saccades and for task‐specific saccades (Fig. 2A and B).
Figure 2.
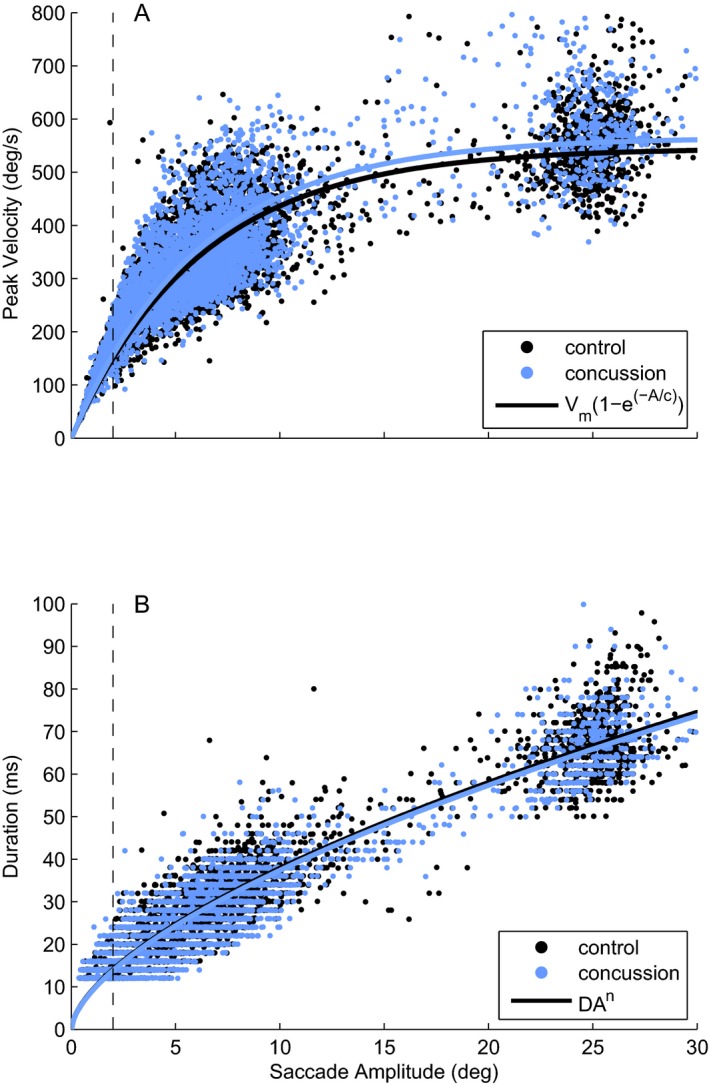
Main sequence plots of concussed and control subjects for all saccades during the KD test. Dotted line demarcates task‐specific saccades (2° or greater). (A). Plot of peak velocity versus amplitude showing that as saccade amplitude increases, the peak velocity increases in an asymptotic distribution. Parameters: Control Vm = 545.41, c = 6.21; Concussion Vm = 565.43, c = 6.17. (B) Plot of duration versus amplitude. No differences are seen in these relationships between concussed and control subjects in A or B. Parameters: Control D = 9.13, n = 0.62; Concussion D = 9.00, n = 0.62. KD, King–Devick.
Intersaccadic interval analysis
ISI was measured for each subject as the median interval between all task‐specific saccades across the three test cards, due to substantial positive skew in the distribution of these values for all subjects (Fig. 3A). The mean ISI across subjects was greater in the concussed compared to the control group (324.4 ± 85.6 msec vs. 286.1 ± 49.7 msec, P = 0.027, one‐sample t‐test) (Table 3). The median ISI value was also significantly prolonged in concussed subjects compared to controls (341.05 vs. 284.03, P = 0.002). The histograms of ISI values (Fig. 3A) highlight the overall pattern of ISI values produced by each subject group, and raster plots (shown below each corresponding histogram) highlight individual differences in the distribution of ISI values.
Figure 3.
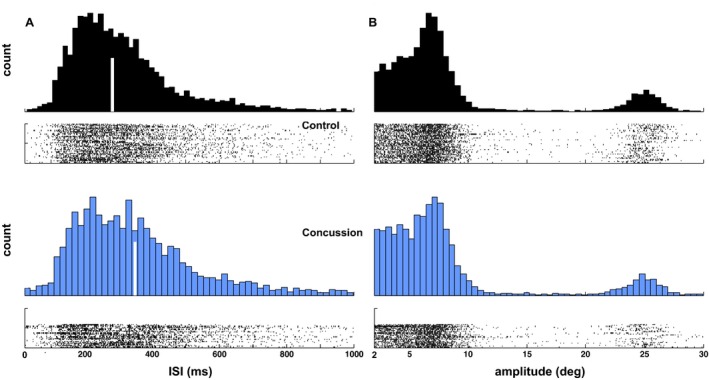
Distributions of saccade amplitudes and intersaccadic intervals. (A) Histograms and subject‐by‐subject raster plots of intersaccadic interval values. (B) Histograms and subject‐by‐subject raster plots of saccade amplitudes. In both panels, histograms for control subjects are plotted in black, and in blue for concussion subjects. Histograms highlight the overall distribution of values, while rasters highlight any individual differences in the pattern of ISI values relative to the group.
Saccade spatial analysis
Distributions of saccade amplitudes are shown in Figure 3B. It appears that concussed and control subjects produced nearly identical patterns of saccade amplitudes for saccades larger than about 20° (saccades made when transitioning from one line to the next), but appeared to produce different patterns of saccade amplitudes for smaller saccades. In particular, control subjects showed a relatively flat distribution (accounting for noise) of amplitudes for small saccades below about 6°, followed by a peak in the distribution between 7° and 8°. Concussed subjects also produced a relatively flat distribution (accounting for noise) for small saccades below 6°, but showed a severely attenuated peak between 7° and 8°. Of note, a higher percentage of saccades occurred in the incorrect direction for proper reading flow (e.g., leftward) in concussed subjects compared to controls (14.43 ± 8.26% vs. 10.13 ± 5.33%, P = 0.028, two‐sample t‐test).
Saccade frequency
Concussed subjects produced a greater number of saccades overall (P = 0.04, one‐sample t‐test), as well as greater numbers of smaller saccades below 20° (P < 0.05). However, both cohorts displayed median saccade endpoints that were horizontally biased (Fig. 4A) relative to the nearest target position (all P < 0.05). Median endpoint errors (Fig. 4B) made by concussed subjects were larger than those produced by control subjects (P < 0.05).
Figure 4.
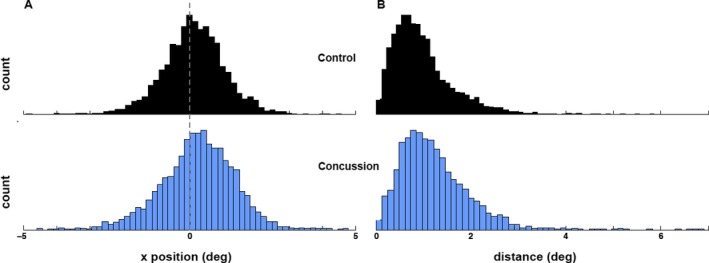
Distributions of spatial errors. For each saccade endpoint, spatial error was defined as the difference between the saccade position and the nearest position on the screen corresponding to a KD number. (A) Horizontal errors. (B) Median endpoint errors. KD, King–Devick.
Correlations
Both median ISI (control: r = 0.79, concussion: r = 0.73, all P < 0.01) and numbers of saccades below 20° (control: r = 0.60, concussion: r = 0.52, all P < 0.05) were significantly correlated with KD test times, as shown graphically in Figure 5. Although these measures were both highly correlated with KD reading times, they were not correlated with one another in either cohort (control: r = 0.09, concussion: r = −0.06, all P > 0.05).
Figure 5.
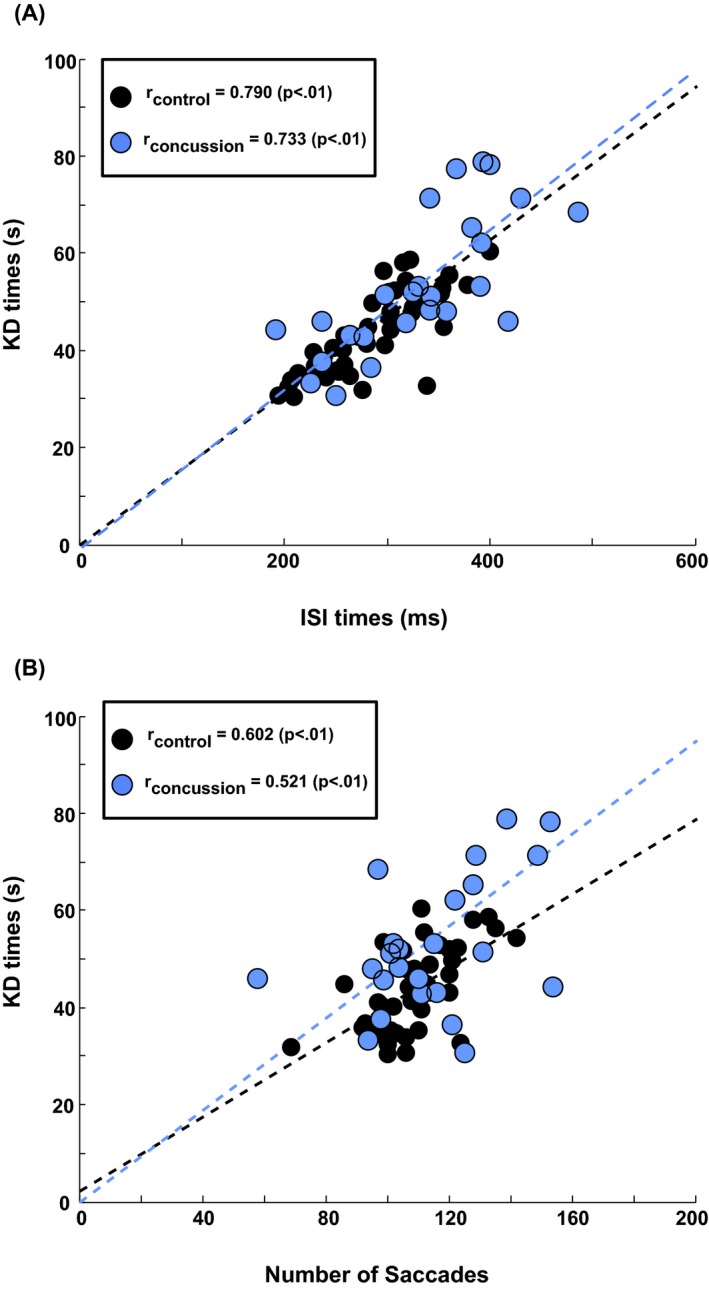
Correlation plots. (A) Correlations between median intersaccadic intervals (ISI) values and King–Devick (KD) test times. (B) Correlations between the median number of saccades below 20° produced by each subject and that subject's KD test time. In both panels, dashed lines are the best fitted line through the origin that minimizes total squared error of the fit to the control data. This fit was chosen on the assumption that the limiting case of a KD test time of zero would also be associated with zero ISI and saccade amplitudes. Fitted lines are displayed to highlight the linear pattern and consistency of results only. Product‐moment correlations are also computed, and are independent of the displayed lines.
Multiple regressions showed that the combination of ISI times and number of saccades was highly predictive of KD times in both control (r = 0.88, F = 69.7, P < 0.01) and concussed subjects (r = 0.89, F = 20.5, P < 0.01). The same parameters were also predictive of the PCSI delta score (r = 0.57, F = 4.0, P < 0.05).
Discussion
In this study, eye movement behavior during reading of the KD test was assessed in individuals with chronic concussion and compared to a cohort of healthy control subjects. Though concussed subjects were outside the 7–10 day window in which most concussions resolve, the concussed cohort largely remained symptomatic from their most recent concussion. As has been reported previously,22 total KD test reading times were prolonged in the concussed subjects as compared to controls. In addition, a variety of deficits in the control of saccades and intersaccadic intervals (ISI) were detected after concussive injury. The most striking of these findings was the prolongation of the ISI, which contributed substantially to KD total test time prolongation, as shown by the strong correlation between ISI durations and total test times. Furthermore, ISI was also predictive of the change in PCSI scores. We discuss each of the deficits in turn and pay particular attention to the possibility that these results may have implications regarding mechanistic dysfunction in mild traumatic brain injury, since the neuroanatomic pathways controlling eye movement behavior are well elucidated.33
Saccade kinematics
Saccade speed is controlled at the level of the brainstem, in horizontal and vertical saccade centers in the pons and midbrain that contain excitatory burst neurons for saccade initiation.34, 35 Lesions in these burst neuron centers lead to saccade slowing. In our study, classical measures of saccade function, including velocity, duration, acceleration, and deceleration showed no differences between concussed and control subjects. Main sequence relationships between saccade amplitude and velocity and between saccade amplitude and duration were also similar between groups.
Our results suggest that brainstem regions responsible for final premotor saccade generation, such as the pontine parapontine reticular formation and the midbrain rostral interstitial medial longitudinal fasciculus, are less prone to injury in those suffering from chronic concussion. Evidence supporting this interpretation has been demonstrated in standard, non‐KD, reflexive saccade (single saccade to a suddenly appearing visual target) paradigms in subacute concussion36 and in chronic postconcussive states,15, 16, 37 in which saccadic velocities were found to be no different than in controls. In fact, normal saccadic velocities following concussion are not a surprising result, as diffuse axonal injury rarely extends to the brainstem in mild traumatic brain injury.38 Rather, saccadic abnormalities are much more likely to result from injuries to higher cortical control regions that are more prone to injury in concussion.
Intersaccadic interval analysis
In classic saccade studies, reaction time or latency is defined as the interval between presentation of a visual stimulus and initiation of a saccade directing gaze to that target.39 The ISI, or the “pause” between saccade events, is simply a measure of the time between saccades. In the context of natural scene viewing, it has been considered a surrogate of latency, though it incorporates both saccade latency and the fixation periods between eye movement events.32, 40 This becomes highly relevant for rapid number naming tasks, such as the KD, given that all the spatial targets or numbers are displayed at the onset of the subtask, that is, card 1, 2, and 3. In this study, ISI durations were significantly prolonged in concussed subjects for all saccades and for task‐specific saccades. Given the pseudoreading structure of this assessment, it is essential to remember that efficient reading requires eye movements that are not only rapid and accurate, but also requires the integration of information obtained continuously from each fixation to read, verbalize, plan the motor movement, and direct attention to the next number.26 We cannot determine in this study if the ISI prolongation during KD reading in concussion is due to prolonged saccadic latency or excessive fixation or if both contribute, however, we hypothesize that both may be contributory.
Saccadic latencies in standard reflexive saccade paradigms have been reported as normal in concussion; however, latencies of saccade paradigms requiring more attention and a higher cognitive load (such as antisaccades) have been shown to be prolonged.36 This distinction makes sense given that certain cortical regions, such as the frontal lobes, are most prone to injury in concussion.41 Though a somewhat simplistic interpretation of anatomic saccade control, the parietal lobe is predominantly responsible for the generation of reflexive saccades,42 whereas frontal lobe structures (i.e., the frontal eye fields and dorsolateral prefrontal cortex) are more involved with cognitively demanding saccade types. These higher cognitively loaded saccades are likely similar to the saccades required to perform the KD test. In the KD framework, shifting attention from a current numerical target to the next sequential target is a three‐step process involving disengagement of attention from the current target, moving attention to the new target and then reengagement with the new target.
In addition to saccadic latency, cognitive factors are involved in mediating the ISI and the continuous flow of integrated information essential to task completion. Mild traumatic brain injury is known to create deficits on the cognitive axis, particularly in the domains of executive function and attention, which have been associated with longer recovery trajectories.43, 44 Of note, prolonged KD reading times have been correlated with worse immediate memory scores on the Standardized Assessment of Concussion, further implicating frontal lobe structures such as the dorsolateral prefrontal cortex.45
Saccade spatial analysis and frequency
Our concussed subjects required a greater number of saccades overall, including smaller saccades below 20°, to complete the KD test. Two possible explanations for this exist. Completing saccades in steps permits sequential direction of gaze toward a visual goal, deconstructing the overall task into simpler or smaller components. On the other hand, the need for more saccades may reflect saccadic dysmetria and the need for additional corrective saccades. Saccade hypometria lesions involving the cortex, pretectum, thalamus, superior colliculus, and cerebellum,46, 47, 48 while saccade hypermetria implicates injury to the cerebellum. Further quantitative study of eye movements should allow a better understanding of how frequently these areas are involved in concussed subjects.
Limitations
The extended duration and variability in time since concussive injury in our subjects, inclusion of more subjects under age 18 in the chronically concussed group, and lack of formal neuropsychological testing are potential limitations of this study. A further limitation is the presence of multiple varied medications with central nervous system (CNS) activity in our concussed cohort, including antidepressants, anxiolytics, sleep aids, and CNS stimulants. It is unclear if, and how, such medications may affect the results, as studies of the effects of these medications on saccade behavior and rapid number naming performance have not been done. We acknowledge that the population studied here may differ neurobiologically from populations with acute concussion and findings from this study may not be generalizable to individuals with acute concussion.
Conclusions
Despite a wealth of literature noting prolonged KD test times following concussion, mechanisms explaining these findings have not been formally examined. We report on a number of eye movement findings in subjects with chronic concussion during performance of the KD test. Prolonged KD test times were associated with prolonged ISI values, an increased number of saccades (particularly at smaller amplitudes), and increased saccadic dysmetria. Although both ISI and the number of saccades were predictive of KD test times, they were not correlated with one another, suggesting that the combination of these two measures may provide superior prediction of concussion over ISI, number of saccades, or perhaps even KD test times alone. Understanding the deficits uncovered in rapid number naming tasks with more granularity may ultimately assist in identifying the neuroanatomic substrates that are disrupted following mild traumatic brain injury. A finer appreciation of the neurocognitive network essential to visual–verbal tasks may assist in optimizing outcome measures to longitudinally track recovery and to design more tailored rehabilitation regimens.
Author Contributions
Conception and design of the study: JRR, TEH, LJB, SLG, JCR. Acquisition and analysis of data: JRR, TEH, WD, JB, RP, IS, JCR. Substantial manuscript drafting: JRR, TEH, WD, LJB, SLG, JCR.
Conflicts of Interest
No author has received any financial compensation or consultant fees from King Devick Test, Inc. No author has other disclosures pertinent to this study.
Acknowledgments
Sources of funding: 5K12HDOO1097 NICHD and NCMRR, National Institutes of Health Rehabilitation Medicine Scientist Training Program (JRR) and Empire Clinical Research Investigator Program (ECRIP). The authors thank the study participants and the NYU Concussion Center, including Mara Sproul and Dina Pagnotta. The authors also thank Moulik Gupta for early involvement with study conception and literature review.
References
- 1. Carney N, Ghajar J, Jagoda A, et al. Concussion guidelines step 1: systematic review of prevalent indicators. Neurosurgery 2014;75(Suppl 1):S3–S15. [DOI] [PubMed] [Google Scholar]
- 2. McCrory P, Meeuwisse W, Aubry M, et al. Consensus statement on Concussion in Sport–the 4th International Conference on Concussion in Sport held in Zurich, November 2012. J Sci Med Sport 2013;16:178–189. [DOI] [PubMed] [Google Scholar]
- 3. Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers' Association position statement: management of sport concussion. J Athl Train 2014;49:245–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery 2014;75(Suppl 4):S24–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vagnozzi R, Signoretti S, Cristofori L, et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain 2010;133:3232–3242. [DOI] [PubMed] [Google Scholar]
- 6. Langlois JA, Rutland‐Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 2006;21:375–378. [DOI] [PubMed] [Google Scholar]
- 7. Gessel LM, Fields SK, Collins CL, et al. Concussions among United States high school and collegiate athletes. J Athl Train 2007;42:495–503. [PMC free article] [PubMed] [Google Scholar]
- 8. Torres DM, Galetta KM, Phillips HW, et al. Sports‐related concussion: anonymous survey of a collegiate cohort. Neurol Clin Pract 2013;3:279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med 2011;30:33–48. [DOI] [PubMed] [Google Scholar]
- 10. Cantu RC. Recurrent athletic head injury: risks and when to retire. Clin Sports Med 2003;22:593–603, x. [DOI] [PubMed] [Google Scholar]
- 11. Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1991;1:1–47. [DOI] [PubMed] [Google Scholar]
- 12. Lipton ML, Kim N, Park YK, et al. Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: intersubject variation, change over time and bidirectional changes in anisotropy. Brain Imaging Behav 2012;6:329–342. [DOI] [PubMed] [Google Scholar]
- 13. Nevin NC. Neuropathological changes in the white matter following head injury. J Neuropathol Exp Neurol 1967;26:77–84. [DOI] [PubMed] [Google Scholar]
- 14. Heitger MH, Jones RD, Anderson TJ. A new approach to predicting postconcussion syndrome after mild traumatic brain injury based upon eye movement function. Conf Proc IEEE Eng Med Biol Soc 2008;2008:3570–3573. [DOI] [PubMed] [Google Scholar]
- 15. Heitger MH, Jones RD, Macleod AD, et al. Impaired eye movements in post‐concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain 2009;132(Pt 10):2850–2870. [DOI] [PubMed] [Google Scholar]
- 16. Kraus MF, Little DM, Donnell AJ, et al. Oculomotor function in chronic traumatic brain injury. Cogn Behav Neurol 2007;20:170–178. [DOI] [PubMed] [Google Scholar]
- 17. Maruta J, Ghajar J. Detecting eye movement abnormalities from concussion. Prog Neurol Surg 2014;28:226–233. [DOI] [PubMed] [Google Scholar]
- 18. Ventura RE, Balcer LJ, Galetta SL, Rucker JC. Ocular motor assessment in concussion: current status and future directions. J Neurol Sci 2016;15:79–86. [DOI] [PubMed] [Google Scholar]
- 19. Galetta KM, Brandes LE, Maki K, et al. The King‐Devick test and sports‐related concussion: study of a rapid visual screening tool in a collegiate cohort. J Neurol Sci 2011;309:34–39. [DOI] [PubMed] [Google Scholar]
- 20. Galetta MS, Galetta KM, McCrossin J, et al. Saccades and memory: baseline associations of the King‐Devick and SCAT2 SAC tests in professional ice hockey players. J Neurol Sci 2013;328:28–31. [DOI] [PubMed] [Google Scholar]
- 21. Galetta KM, Morganroth J, Moehringer N, et al. Adding Vision to Concussion Testing: a Prospective Study of Sideline Testing in Youth and Collegiate Athletes. J Neuroophthalmol 2015; Sep;35(3):235–41. [DOI] [PubMed] [Google Scholar]
- 22. Galetta KM, Liu M, Leong DF, et al. The King‐Devick test of rapid number naming for concussion detection: meta‐ analysis and systematic review of the literature. Concussion 2015;15:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carpenter RH. The neural control of looking. Curr Biol 2000;10:R291–R293. [DOI] [PubMed] [Google Scholar]
- 24. Leong DF, Balcer LJ, Galetta SL, et al. The King‐Devick test for sideline concussion screening in collegiate football. J Optom 2015;8:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galetta KM, Barrett J, Allen M, et al. The King‐Devick test as a determinant of head trauma and concussion in boxers and MMA fighters. Neurology 2011;76:1456–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kulp MT, Schmidt PP. Effect of oculomotor and other visual skills on reading performance: a literature review. Optom Vis Sci 1996;73:283–292. [DOI] [PubMed] [Google Scholar]
- 27. Sachdev PS, Blacker D, Blazer DG, et al. Classifying neurocognitive disorders: the DSM‐5 approach. Nat Rev Neurol 2014;10:634–642. [DOI] [PubMed] [Google Scholar]
- 28. Mangione CM. The National Eye Institute 25‐Item Visual Function Questionnaire (VFQ‐25). Scoring Algorithm. 2000. (online). Available at: https://www.nei.nih.gov/sites/default/files/nei-pdf/manual_cm2000.pdf (accessed March 2016).
- 29. Raphael BA, Galetta KM, Jacobs DA, et al. Validation and test characteristics of a 10‐item neuro‐ophthalmic supplement to the NEI‐VFQ‐25. Am J Ophthalmol 2006;142:1026–1035. [DOI] [PubMed] [Google Scholar]
- 30. Ohio State University TBI Identification Method. Available at: http://www.brainline.org/content/2013/08/new-tbi-screening-tool.html (accessed March 2016).
- 31. Randolph C, Millis S, Barr WB, et al. Concussion symptom inventory: an empirically derived scale for monitoring resolution of symptoms following sport‐related concussion. Arch Clin Neuropsychol 2009;24:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rizzo JR, Hudson TE, Dai W, et al. Objectifying eye movements during rapid number naming: methodology for assessment of normative data for the King‐Devick test. J Neurol Sci 2016;362:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leigh RJ, Zee DS. The Neurology of Eye Movements, 5th ed Oxford: Oxford University Press, 2015. [Google Scholar]
- 34. Horn AK, Buttner‐Ennever JA, Suzuki Y, Henn V. Histological identification of premotor neurons for horizontal saccades in monkey and man by parvalbumin immunostaining. J Comp Neurol 1995;359:350–363. [DOI] [PubMed] [Google Scholar]
- 35. Horn AK, Helmchen C, Wahle P. GABAergic neurons in the rostral mesencephalon of the macaque monkey that control vertical eye movements. Ann N Y Acad Sci 2003;1004:19–28. [DOI] [PubMed] [Google Scholar]
- 36. Heitger MH, Anderson TJ, Jones RD, et al. Eye movement and visuomotor arm movement deficits following mild closed head injury. Brain 2004;127(Pt 3):575–590. [DOI] [PubMed] [Google Scholar]
- 37. Heitger MH, Jones RD, Dalrymple‐Alford JC, et al. Motor deficits and recovery during the first year following mild closed head injury. Brain Inj 2006;20:807–824. [DOI] [PubMed] [Google Scholar]
- 38. Servadei P, Vergoni G, Pasini A, et al. Diffuse axonal injury with brainstem localisation: report of a case in a mild head injured patient. J Neurosurg Sci 1994;38:129–130. [PubMed] [Google Scholar]
- 39. Leigh RJ, Kennard C. Using saccades as a research tool in the clinical neurosciences. Brain 2004;127(Pt 3):460–477. [DOI] [PubMed] [Google Scholar]
- 40. Roos JC, Calandrini DM, Carpenter RH. A single mechanism for the timing of spontaneous and evoked saccades. Exp Brain Res 2008;187:283–293. [DOI] [PubMed] [Google Scholar]
- 41. Williams IM, Ponsford JL, Gibson KL, et al. Cerebral control of saccades and neuropsychological test results after head injury. J Clin Neurosci 1997;4:186–196. [DOI] [PubMed] [Google Scholar]
- 42. Pierrot‐Deseilligny C, Rivaud SF, Gaymard B, Agid Y. Cortical control of reflexive visually‐guided saccades. Brain. 1991. Jun;114 ( Pt 3):1473–85.19910815 DCOM‐ 19910815(0006‐8950 (Print)). [DOI] [PubMed] [Google Scholar]
- 43. Barker‐Collo S, Jones K, Theadom A, et al. Neuropsychological outcome and its correlates in the first year after adult mild traumatic brain injury: a population‐based New Zealand study. Brain Inj. 2015;29(13‐14):1604–16. 20151216(1362‐301X (Electronic)). [DOI] [PubMed] [Google Scholar]
- 44. Clarke LA, Genat RC, Anderson JF. Long‐term cognitive complaint and post‐concussive symptoms following mild traumatic brain injury: the role of cognitive and affective factors. Brain Inj. 2012;26(3):298–307.20120229 DCOM‐ 20120521(1362‐301X (Electronic)). [DOI] [PubMed] [Google Scholar]
- 45. Benedict PA, Baner NV, Harrold GK, et al. Gender and age predict outcomes of cognitive, balance and vision testing in a multidisciplinary concussion center. J Neurol Sci 2015;353:111–115. [DOI] [PubMed] [Google Scholar]
- 46. Ohtsuka K, Noda H. The effect of microstimulation of the oculomotor vermis on discharges of fastigial neurons and visually‐directed saccades in macaques. Neurosci Res. 1991. May;10(4):290–5.19910927 DCOM‐ 19910927(0168‐0102 (Print)). [DOI] [PubMed] [Google Scholar]
- 47. Keating EF, Kenney DV, Gooley SG, et al. Targeting errors and reduced oculomotor range following ablations of the superior colliculus or pretectum/thalamus. Behav Brain Res. 1986. Dec;22(3):191–210. 19870211 DCOM‐ 19870211(0166‐4328 (Print)). [DOI] [PubMed] [Google Scholar]
- 48. Pierrot‐Deseilligny C, Rosa A, Masmoudi K, et al. Saccade deficits after a unilateral lesion affecting the superior colliculus. J Neurol Neurosurg Psychiatry. 1991. Dec;54(12):1106–9. 19920319 DCOM‐ 19920319(0022‐3050 (Print)). [DOI] [PMC free article] [PubMed] [Google Scholar]


