Abstract
We examined the impact of expiratory muscle strength training on maximum expiratory pressure, cough spirometry, and disease progression in a 71‐year‐old male with amyotrophic lateral sclerosis. Maximum expiratory pressure declined 9% over an 8‐week sham training period, but subsequently improved by 102% following 8 weeks of expiratory muscle strength training. Improvements in cough spirometry and mitigated disease progression were also observed post expiratory muscle strength training. Improvements in maximum expiratory pressures were maintained 6 months following expiratory muscle strength training and were 79% higher than baseline data obtained 301 days prior. In this spinal‐onset amyotrophic lateral sclerosis patient, respiratory training improved subglottic air pressure generation and sequential cough generation.
Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive, fatal neurodegenerative disease resulting from degeneration of upper and lower motor neurons.1 Approximately 85% of individuals with ALS experience dysphagia, or swallowing impairment, leading to malnutrition, dehydration, aspiration, pneumonia, reduced psychosocial health, and morbidity.2, 3 Progressive rigidity and weakness of the diaphragm, internal intercostals and abdominal muscles also contribute to impairments in the ability to generate adequate subglottic air pressure to produce a protective and effective cough.4, 5 We recently documented impaired airway defense mechanisms in ALS patients who aspirated on videofluoroscopic exam with no patient able to produce an effective cough response to remove tracheal aspirate.6 Given the recent findings denoting a predictive relationship between peak cough flow and cough volume acceleration on airway safety during swallowing in ALS,7 management strategies to improve or maintain subglottic air pressure generation and cough strength have been recommended to prolong pulmonary health and nutritional intake.5
Current ALS management strategies are primarily palliative and include assistive communication devices, placement of percutaneous endoscopic gastronomy (PEG) tubes, assistive ventilation, and pharmacological management.8 Although historically controversial, recent animal9 and human data5 indicate a potential role for active interventions, performed early, at low intensities, to improve or maintain physiologic function and slow the rate of diminishing physiologic reserve in individuals with ALS. Expiratory muscle strength training (EMST) has been shown to improve subglottic air pressure generation, cough strength, and swallowing function in other neurodegenerative populations (see10 for review). Given the high prevalence of dysphagia and dystussia in ALS, we examined the impact of EMST in an individual with ALS.
Participant and Methods
JR is a 71‐year‐old male with definite spinal‐onset ALS (Revised El‐Escorial criteria) who was recruited as part of a randomized, sham‐control trial. Baseline forced vital capacity (FVC) was 76% of predicted, disease duration from symptom onset was 21 months, and baseline ALS Functional Rating Scale‐Revised (ALSFRS‐R)11 score at entry was 32. JR was not on riluzole and reported negative smoking history. This study was approved by our university IRB and the subject completed an informed consent (IRB#00009178).
Expiratory muscle strength training was completed using a calibrated threshold resistance device (EMST 150; Aspire Products, Atlanta, GA, USA), which contains a one‐way, spring‐loaded valve. Threshold load was set at 50% of the individual's 1‐repetition maximum expiratory pressure (MEP), representing a moderate training load. An experienced home therapist visited weekly to re‐evaluate and adjust the training threshold to maintain consistent resistance relative to the trainees' current MEP. The sham training device was created by removing the spring‐valve from the EMST device, allowing the participant to exhale against atmospheric pressure (~7 cm H20). Although every attempt was made to blind the patient to treatment condition (visually identical trainers), there is the possibility that JR perceived the sham device as easier.
The experimental training protocol consisted of 25 repetitions per day, 5 days per week, for 8 weeks. This training protocol is based on a previous trial in individuals with Parkinson's disease with an extended training length.12 Training occurred in the patient's home, with nose clips in place to avoid nasal air emissions. To maximize compliance with training, the participant was provided with a log to track exercise, which the home therapist reviewed. Training compliance was 100%.
The patient attended a total of three evaluation sessions that included an initial baseline evaluation, 8‐week post‐sham and post‐EMST evaluations. During each evaluation, MEP and voluntary cough spirometry data were collected and the ALSFRS‐R survey was administered to index global disease progression. MEP data were obtained using a handheld digital manometer (MP01; MicroDirect, Manchester). Voluntary cough was assessed using an oral pneumotachograph (MLT 1000; ADInstruments, Inc. CO, USA) connected to a spirometry filter (MQ 304 Spirometer Filter; Vacumed, Ventura, CA, USA) as previously described.7 While seated upright with nose clips in place, the subject was instructed to take a deep breath in and “Cough hard like you have something stuck in your throat.” Three trials were recorded, low‐pass filtered at 150 Hz and digitized at 1000 Hz for analysis in Lab Chart Version 7 (ADInstruments, Inc). Each epoch, or completed cough trial, consisted of sequential coughing. Cough inspired volume (CIV), defined as the amount of air inspired prior to the cough and cough total (CrTot), defined as number of coughs within an epoch, were primary outcome measures.
Following the 8‐week EMST regimen, JR reported continued use of the trainer at home, keeping it at the final training load (~50 cm H20). Three‐ and six‐month follow‐up data were collected for MEPs and global disease progression.
Results
Maximum expiratory pressure
Descriptive statistics (percent change) were utilized to determine change in primary outcome variables across the three time points (Table 1). Following 8 weeks of sham training, JR's MEP values decreased by 9%, from 56 to 51 cmH20. Following 8 weeks of EMST, MEP increased by 102%, from 51 to 103 cmH20 (see Fig. 1). JR retained therapeutic gains at the 3‐ and 6‐month follow‐up evaluations with MEPs of 84 and 100 cm H20, respectively.
Table 1.
Physiologic data pre‐ and post‐EMST
| Timeline day | Baseline (0) | Post‐sham (60) | Post‐EMST (120) | Change Post‐sham (%) | Change Post‐EMST (%) |
|---|---|---|---|---|---|
| MEP (cm H20) | 56 | 51 | 103 | −9 | +102 |
| CIV (L) | 1.01 | 1.44 | 1.87 | +43 | +30 |
| CrTot | 1 | 2 | 5 | +100 | +150 |
| ALSFRS‐R | 32 | 29 | 30 | −9 | +3 |
|
ALSFRS‐ R Subscores |
R: 10 B: 12 |
R: 10 B: 11 |
R: 12 B: 11 |
R: 0 B: −8 |
R: +20 B: 0 |
Physiologic data over a 4‐month period for respiratory, cough, and global disease progression in a 71‐year‐old male with ALS who participated in a randomized clinical trial. He completed 8 weeks of sham training (Baseline–Post‐sham) followed by 8 weeks of active EMST respiratory training.
MEP, maximum expiratory pressure; CIV, cough inspired volume; CrTot, total coughs produced within an epoch; ALSFRS‐R, ALS functional rating scale‐revised; R, respiratory subscore; B, bulbar subscore; EMST, expiratory muscle strength.
Figure 1.
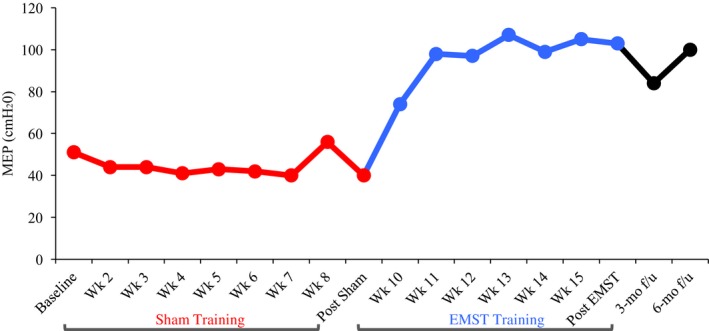
Maximum expiratory pressure (MEPs cm H20) in a 71‐year‐old male patient with amyotrophic lateral sclerosis who underwent 8 weeks of sham training followed by 8 weeks of expiratory muscle strength training at 50% of individualized MEP. Three‐ and six‐month follow‐up MEP data are also included. MEPs decreased by 9% between baseline and post‐sham and increased by 102% pre‐ versus post‐EMST. Six‐month follow‐up data indicate that treatment gains were maintained at 100 cm H20, representing a 3% decrease from the immediate post‐EMST assessment and a 79% increase from his initial baseline (pre‐sham) MEPs taken 10 months earlier.
Voluntary cough
Mean CIV across trials increased from 1.01 L at baseline to 1.44 L post‐sham treatment period and to 1.87 L post‐EMST. Median cough total within an epoch (CrTot) increased from 1 at baseline, to 2 post‐sham and to 5 coughs post‐EMST (see Fig. 2).
Figure 2.
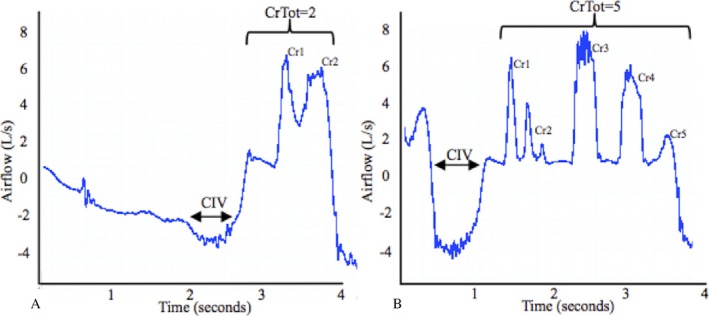
A cough epoch, or sequential cough flow waveform depicting cough total (CrTot), defined as number of coughs within an epoch and cough inspired volume (CIV), defined as the amount of air inspired prior to cough at baseline (A) and post‐EMST (B) evaluations in a 71‐year‐old male with amyotrophic lateral sclerosis. Cr1, Cr2, etc. represent individual coughs within the epoch, which make up the CrTot value.
Global disease progression
Total ALSFRS‐R scores were 32, 29, and 30 across baseline, post‐sham and post‐EMST time points, respectively. ALSFRS‐R respiratory subscores were 10 at baseline and post‐sham time points and increased by 2 points following EMST (12). Three‐month post‐EMST ALSFRS‐R follow‐up score was 25.
Discussion
Active interventions targeting maintenance of subglottic pressure generation have recently been suggested in early affected ALS patients to prolong the vital functions of breathing, swallowing, and airway protection.7, 13, 14 In this study of a patient with spinal‐onset ALS, 8 weeks of EMST led to immediate improvements in expiratory force generating pressures (MEPs) and sequential cough generation. EMST was well tolerated with no reported adverse effects.
JR's baseline MEPs (56 cm H2O) were less than half of expected for his age and gender (115.5+/−21.0 cm H2O).15 EMST was noted to improve JR's MEPs by 52 cm H2O to within the normal range thereby increasing the respiratory reserve between expiratory capability and demand. Reduced respiratory reserve in the presence of a compromised system, such as that in ALS, reduces the ability to compensate for physiologic stressors including airway invasion, secretion management, and exacerbation of disease.16 Improvements in subglottic air pressure generation and the number of coughs generated in a trial may have implications for airway safety; specifically, the ability to eject tracheal aspirate during swallowing through forceful, sequential cough generation, as well as removing endogenously produced material.17 Interestingly, cough inspired volume increased following both sham and active respiratory training, which may lend explanation to increased CrTot, as more coughs can be produced in succession with greater starting lung volume. An additional physiologic explanation may be that incorporating daily breathing exercises in a patient with restrictive lung disease facilitated active stretching of the diaphragm through greater inspiratory volume recruitment. These findings may place an individual at a greater mechanical advantage to produce a more forceful cough, or a greater number of coughs.
MEP gains were maintained at the 6‐month follow‐up and were 79% higher than baseline MEPs. This finding is of great clinical significance given the rapidly degenerating nature of ALS. Maintaining MEP, maximum inspiratory pressure, and FVC have been shown to be highly predictive of 1‐year survival and are associated with tracheostomy‐free survival in ALS.18 Additionally, expiratory muscle weakness has been indicated as a causative factor in the inability to generate adequate cough flow rates and has been associated with concomitant inspiratory muscle weakness in ALS.19 Training and improving the force‐generating ability of the muscles involved in expiration is a direct physiologic goal of targeted expiratory training; however, the impact of targeting both inspiratory and expiratory pressure generation may yield improved outcomes related to both airway protection and ventilator dependence and has yet to be investigated in individuals with ALS. Further, early intervention for respiratory insufficiency in ALS may be beneficial in managing respiratory function and tracking disease progression.20 It is important to note potential limitations of these findings, including the possibility that JR could distinguish the sham from the active treatment trainer and concern for testing effect of cough spirometry measurements on cough volume outcomes. Given that the latter was tested only three times over a 10‐month time period, we believe this potential was minimized.
This case report highlights the potential of EMST in the maintenance of subglottic pressure‐generation capacity, cough function, and disease progression. Here, we present data of the first ALS patient who completed all treatment arms of this study (baseline, sham/EMST training, 3/6‐month f/u) constituting a time period of 301 days from baseline to the 6‐month follow‐up evaluation, which began following the post‐EMST evaluation. Given the lack of effective treatments in this challenging patient population and the results herein, we wish to disseminate these data in a timely fashion, with results from the larger randomized trial to follow upon completion of all 48 patients. Future directions include the role of a combined inspiratory and expiratory training regimen in patients with ALS.
Authorship Contributions
LCT and KR were involved in the clinical treatment and data collection. LCT, KH, RR, IH, and EKP were involved in the data analysis. LCT and EKP drafted the manuscript. EKP was involved in study conceptualization and data management. All authors reviewed the manuscript.
Conflict of Interest
None declared.
Acknowledgments
This study was funded by grant R21HD075327 from the National Institute of Child Health and Development and by the NIH National Center for Medical Rehabilitation Research T32 Neuromuscular Plasticity Training Program grant T32HDO43730. The authors thank the University of South Florida ALS Clinic and the University of Florida for their support. Expert advice was also provided by Drs. Christine Sapienza, Paul Davenport, and Teresa Pitts.
References
- 1. Brooks BR, Miller RG, Swash M, Munsat TL. El escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Scler Other Motor Neuron Disord 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 2. Chen A, Garrett CG. Original articles: otolaryngologic presentations of amyotrophic lateralsclerosis. Otolaryngol Head Neck Surg 2005;132:500–504. [DOI] [PubMed] [Google Scholar]
- 3. Tabor L. Defining swallowing‐related quality of life in individuals with amyotrophic lateral sclerosis. Dysphagia 2015;2015:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenbek JCT. M.S. in: Postma PB Gregory N.,Easterling Caryn, Belafsky Peter C., Shaker Reza, eds. Manual of diagnostic and therapeutic techniques for disorders of deglutition. Springer, New York, 2013. [Google Scholar]
- 5. Plowman EK. Is there a role for exercise in the management of bulbar dysfunction in amyotrophic lateral sclerosis? J Speech Lang Hear Res. 2015;1103–1395. [DOI] [PubMed] [Google Scholar]
- 6. Gaziano J. Prevalence, timing and source of aspiration in individuals with ALS. Dysphagia 2015;2015:Published abstract; Dysphagia Research Society 2014. [Google Scholar]
- 7. Plowman EK, Watts SA, Robison R, et al. Voluntary cough airflow differentiates safe versus unsafe swallowing in amyotrophic lateral sclerosis. Dysphagia 2016;383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ‘Albert SM, Murphy PL, Del Bene ML, Rowland LP. Prospective study of palliative care in ALS: choice, timing, outcomes. J Neurol Sci 1999;169(1–2):108–113. [DOI] [PubMed] [Google Scholar]
- 9. Carreras I, Yuruker S, Aytan N, et al. Moderate exercise delays the motor performance decline in a transgenic model of ALS. Brain Res 2010;1313:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laciuga H, Rosenbek JC, Davenport PW, Sapienza CM. Functional outcomes associated with expiratory muscle strength training: narrative review. J Rehabil Res Dev 2014;51:535–546. [DOI] [PubMed] [Google Scholar]
- 11. Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS‐R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci 1999;169:13–21. [DOI] [PubMed] [Google Scholar]
- 12. Troche MS, Okun MS, Rosenbek JC, et al. Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: a randomized trial. Neurology 2010;75:1912–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plowman EK, Watts SA, Tabor L, et al. Impact of expiratory strength training in amyotrophic lateral sclerosis. Muscle Nerve 2015;48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheah BC, Boland RA, Brodaty NE, et al. Inspirational – Inspiratory muscle training in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis 2009;10:384–392. [DOI] [PubMed] [Google Scholar]
- 15. Neder JA, Andreoni S, Lerario MC, Nery LE. Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res. 1999;32:719–727. [DOI] [PubMed] [Google Scholar]
- 16. Haas CF, Loik PS, Gay SE. Airway clearance applications in the elderly and in patients with neurologic or neuromuscular compromise. Respir. Care 2007;52:1362–1381; discussion 81. [PubMed] [Google Scholar]
- 17. Hegland KW, Troche MS, Davenport PW. Cough expired volume and airflow rates during sequential induced cough. Front Physiol 2013;4:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lyall RA, Donaldson N, Polkey MI, et al. Respiratory muscle strength and ventilatory failure in amyotrophic lateral sclerosis. Brain 2001;124:2000–2013. [DOI] [PubMed] [Google Scholar]
- 19. Polkey MI, Lyall RA, Green M, et al. Expiratory muscle function in amyotrophic lateral sclerosis. Am J Respir Crit Care Med 1998;158:734–741. [DOI] [PubMed] [Google Scholar]
- 20. Schiffman PL, Belsh JM. Pulmonary function at diagnosis of amyotrophic lateral sclerosis. Rate of deterioration. Chest 1993;103:508–513. [DOI] [PubMed] [Google Scholar]


