Abstract
Importance
Structural brain abnormalities are prominent in psychotic disorders including schizophrenia. However, it is unclear when aberrations emerge in the disease process, and if such deficits are present in association with less severe psychosis-spectrum (PS) symptoms in youth.
Objective
To investigate the presence of structural brain abnormalities in youth with PS symptoms.
Design
The Philadelphia Neurodevelopmental Cohort (PNC) is a prospectively accrued community-based sample of nearly 10,000 youths who received a structured psychiatric evaluation. A subsample of 1,601 subjects underwent neuroimaging including structural magnetic resonance imaging.
Setting
The PNC is a collaboration between The Children’s Hospital of Philadelphia and the Hospital of the University of Pennsylvania.
Participants
Youths ages 8–22 years identified through structured interview as having psychosis-spectrum features (PS, n=391), and typically developing comparison subjects without significant psychopathology (TD, n=400).
Main Outcomes and Measures
Measures of brain volume derived from T1-weighted structural neuroimaging at 3T. Analyses were conducted at global, regional, and voxelwise levels. Regional volumes were estimated with an advanced multi-atlas regional segmentation procedure; voxelwise volumetric analyses were conducted as well. Nonlinear developmental patterns were examined using penalized splines within a general additive model. PS symptom severity was summarized using factor analysis and evaluated dimensionally.
Results
Compared to the TD group, the PS group had diminished whole brain gray matter volume and expanded white matter volume. Voxelwise analyses revealed significantly lower gray matter volume in the medial temporal lobes as well as in frontal, temporal, and parietal cortex. Reduction of medial temporal lobe volume was correlated with PS symptom severity.
Conclusions and Relevance
Structural brain abnormalities that have been commonly reported in adults with psychosis are present early in life in youth with PS symptoms, and are not due to medication effects. Future longitudinal studies could use the presence of such abnormalities in conjunction with clinical presentation, cognitive profile, and genomics to predict risk and aid in stratification to guide early interventions.
Keywords: psychosis, development, neuroimaging, MRI, adolescence, brain
INTRODUCTION
Psychotic disorders have a devastating impact on the lives of patients and families, producing substantial morbidity and mortality.1–3 Based on the early age of onset and convergent evidence from animal models, psychosis is increasingly conceptualized as a downstream product of abnormal neurodevelopment.4–7 A better understanding of the developmental antecedents of psychosis may lead to both early identification and novel targeted interventions.8–10
Adults with psychotic disorders such as schizophrenia have significant abnormalities of brain structure.11–18 While initial studies examined relatively modest samples, large recent meta-analyses have yielded consistent findings.11,13,15,16 Recently, the ENIGMA-SZ consortium19 examined subcortical volumes from a sample totaling over 2,000 patients with schizophrenia, and found reduced volume that was maximal in the hippocampus.20 These results accord with a long line of research documenting structural and functional medial temporal lobe abnormalities.21–27 Both meta-analyses and large-scale single site studies examining cortical deficits have additionally provided evidence for diminished volume in frontal, temporal, and parietal brain regions.11,13,15,16 Sub-cortical and cortical volume reduction have also been reported in unaffected family members,28–30 suggesting that structural deficits are a heritable intermediate phenotype.
In contrast to large studies of adults with psychosis, studies of youth remain smaller. Most studies have examined either first-episode psychosis10,12,17,18,31–33 or youth at clinical high risk.10,12,31–33 These studies typically document attenuated patterns of gray matter volume reductions similar to those seen in adults with schizophrenia. Recently, the NAPLS9 consortium demonstrated that clinical high risk youth who later convert to psychosis have accelerated gray matter loss in frontal cortex compared to non-converters and healthy comparators.34 Beyond such studies of high-risk youth, it is also increasingly recognized that subtle psychosis-spectrum (PS) symptoms are prevalent (5–10%) in the general population.35,36 PS symptoms can impact functioning,36,37 are associated with increased risk of conversion to a psychotic disorder,38 and have been associated neuroimaging abnormalities.39–42
Here we used a community-based approach to examine brain structure in a large sample of non-help seeking youth with PS symptoms imaged as part of the Philadelphia Neurodevelopmental Cohort (PNC).43 While such a design will likely produce lower rates of transition to frank psychosis than studying help-seeking clinical high risk youth, understanding early sub-clinical psychotic symptoms may be valuable for elucidating the neurodevelopmental etiology of psychosis, as it allows investigation of brain abnormalities at an earlier stage.44–47 To our knowledge there has been only one small study of developmental structural abnormalities in community youth with PS symptoms,42 and it is unknown whether PS symptoms in this population demonstrate patterns of volume reduction similar to those found in clinical risk and adult psychosis samples. Similarities to adult clinical phenotypes would provide support for a dimensional view of psychosis symptomatology,4,48,49 and support examination of these phenotypes at younger ages and milder severity levels.44–47
We hypothesized that youth with PS symptoms would demonstrate abnormalities of structural brain development. Specifically, we expected that PS youth would show reduced gray matter volume in similar regions to those impacted in adults with frank psychosis, such as the medial temporal lobe. We used nonlinear analyses of developmental patterns to investigate structural deficits on multiple scales, including analyses of global volumes, lobar volumes, and high-resolution voxelwise analyses. As described below, this approach yielded novel evidence for structural brain abnormalities in PS youth that show parallels to those seen in adults with clinically diagnosed psychotic disorders.
METHODS
Participants
Of the 1,601 participants imaged as part of the PNC, 172 were excluded due to co-morbidity including medical illness that could impact brain function (n=73), incomplete data (n=1), non-psychiatric medication with potential CNS effects (n=78), or an incidentally-encountered structural abnormality that distorted normal brain anatomy (n=20).50 Of the remaining n=1,429, psychosis-spectrum (PS) symptoms were present in 408 participants, which was defined as in prior reports36,37,39,40,51 using the GOASSESS interview,36 which includes elements of the K-SADS, PRIME screen, and SOPS (see eMethods).52–54 Notably, this community-based ascertainment strategy is distinct from studies of help-seeking ultra-high risk youth. A minority of the PS youths (n=69) were being treated psychoactive medication at the time of scan (see eMethods for details). PS youths were compared to 416 typically developing (TD) youths, who had no significant psychiatric symptoms, were not taking psychotropic medication, and no history of psychiatric hospitalization. Following image quality assurance, the final sample was comprised of 391 PS youths and 400 TD youths. Cognition was assessed using the Penn computerized neurocognitive battery, and summarized as a general cognitive factor as previously reported.52,56,57 Dimensional psychosis severity in the PS group was estimated using a previously described factor analysis of psychosis assessments.37
Demographic characteristics are detailed in Table 1. While TD and PS samples were matched on sex (p>0.9), TD and PS samples differed on age, race, and maternal education (p<0.01); these variables were included as covariates in group-level analyses and further evaluated in supplementary analyses (see below). All study procedures were approved by the Institutional Review Boards of the University of Pennsylvania and Children’s Hospital of Philadelphia. Adult participants provided informed consent; minors provided assent and their parent or guardian provided informed consent.
Table 1.
Sample demographics.
| Typically Developing | Psychosis Spectrum | |
|---|---|---|
| n | 400 | 391 |
| Number Female (%) | 195 (49%) | 198 (51%) |
| Number Caucasian (%) | 221 (55%) | 121 (31%) |
| Mean Years Age (SD) | 14.57 (4.03) | 15.73 (3.11) |
| Mean Years Maternal Education (SD) | 14.68 (2.56) | 13.78 (2.23) |
| Mean Psychosis Score (SD) | −0.56 (0.73) | 1.12 (0.81) |
| Mean Cognitive Performance (SD) | 0.23 (0.76) | −0.18 (0.99) |
| Psychoactive Medication (n) | 0 | 69 |
| Mean ICV, cubic mm (SD) | 1485.69 (138.55) | 1436.7 (155.04) |
Image processing
All data were acquired on the same scanner using the same imaging sequences.43,55 To maximize sensitivity to detect effects in PS youth, advanced structural image processing and registration procedures were employed. The T1 image was skull-stripped using a multi-atlas procedure56 followed by multiplicative intrinsic component optimization for bias correction.57 Multi-atlas regional segmentation (MARS)58 was performed, which yields regional, lobar, and tissue class volume estimates. Voxelwise analyses were conducted using regional analysis of volumes in normalized space (RAVENS maps). 59 A deformable registration (DRAMMS)60 was used to register images to a study-specific template.61 RAVENS maps were down-sampled to 2mm and smoothed (8 mm FWHM) prior to voxelwise analyses.
Group-level analyses
Prior work has demonstrated that brain development is not a linear process.62–64 Accordingly, group-level analyses of regional and voxelwise data were flexibly modeled using penalized splines within a general additive model (GAM, see eMethods).65,66 The GAM assesses a penalty (using restricted maximum likelihood) on nonlinearity in order to avoid over-fitting. This approach allowed us to estimate group differences and also ascertain whether the pattern of age-related changes was significantly different between groups.64,67 We examined group differences and group-by-age interactions for global, lobar, and voxelwise volume measurements. In all models, we controlled for potentially confounding covariates including sex, race, and maternal education. Intracranial volume (ICV) was included as a covariate in all regional and voxelwise models;68,69 tissue class volumes were modeled with and without ICV.
In addition to group differences, we examined the relationship between overall dimensional psychosis symptom severity (as defined by a previously-completed factor analysis of PS symptoms)37 and voxelwise gray matter volume within the PS group, while controlling for covariates as above (sex, race, maternal education, and spline of age). Clusters demonstrating a significant association with overall psychosis symptom severity were further evaluated versus previously defined factors corresponding to positive and negative symptoms.37 In order to enhance interpretability, dimensional associations with symptom severity were limited to voxels where a nominal (p<0.05, uncorrected) group difference was present. Type I error for voxelwise analyses was controlled using AFNI AlphaSim70 (cluster height z>2.3, corrected cluster significance p<0.01). Cortical projections were displayed using Caret;71 subcortical images were projected to the Montreal Neurological Institute 1mm template for display.
Supplementary analyses
We conducted several supplementary analyses to ensure that potentially confounding variables did not drive the observed results. Specifically, we conducted analysis of global, lobar, and regional medial temporal lobe volumes in three sub-samples. In the first sub-sample (n=722), we excluded PS youth who were taking psychoactive medication. In the second sub-sample (n=665), as prior,39,40 we excluded PS youth younger than age 11 whose status was assessed only by collateral interview. In the third sub-sample (n=478), we used propensity score matching72 to create groups that were exactly matched on age, sex, race, and maternal education. Finally, in order to examine the specificity of PS results,51 we compared TD participants to 591 youth imaged as part of the PNC who had other psychopathology (and not PS symptoms) while controlling for covariates as above.
RESULTS
PS youth have lower gray matter volume and greater white matter volume
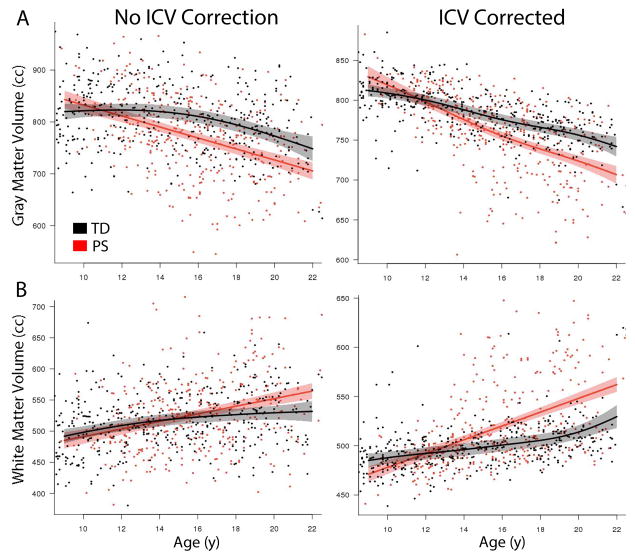
PS youths had reduced ICV compared to TD youth (p=0.002). Global gray, white, and CSF volumes were thus evaluated with and without ICV correction; ICV was included as a covariate in all other analyses. PS youth had marked reductions in total gray matter volume (Figure 1A), regardless of whether ICV was included as a covariate (ICV-corrected: p=1.8x10−10; without ICV correction: p=6.3x10−9). This difference was larger in older participants, producing a significant group-by-age interaction (ICV-corrected: p=1.13x10−5; without ICV correction: 1.58x10−5). When accounting for diminished ICV, PS youth had greater white matter volume than TD comparators (p=2.8x10−11; Figure 1B), which was larger at older ages (p=6.6x10−8). When ICV was not included in the model, the main white matter effect was present as a trend (p=0.10), while the age by group interaction remained significant (p=0.02). When examined on a lobar level while accounting for ICV, gray matter differences were widespread and most significant within frontal cortex (eFigure 1).
Figure 1.
Psychosis spectrum youth have volumetric deficits that progress with age. PS youth have diminished gray matter volume (A) regardless of whether ICV is included as a model covariate. Additionally, PS youth have expanded white matter volume (B) when smaller head size is accounted by covarying for ICV. In both gray and white matter, these abnormalities become more marked at later ages in adolescence and young adulthood. Shaded regions represent 95% confidence intervals.
Distributed gray matter volume reduction is maximal in medial temporal lobes
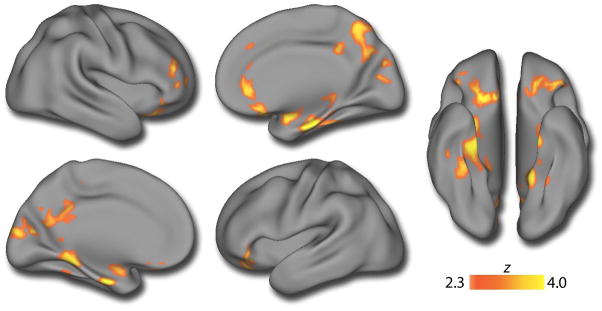
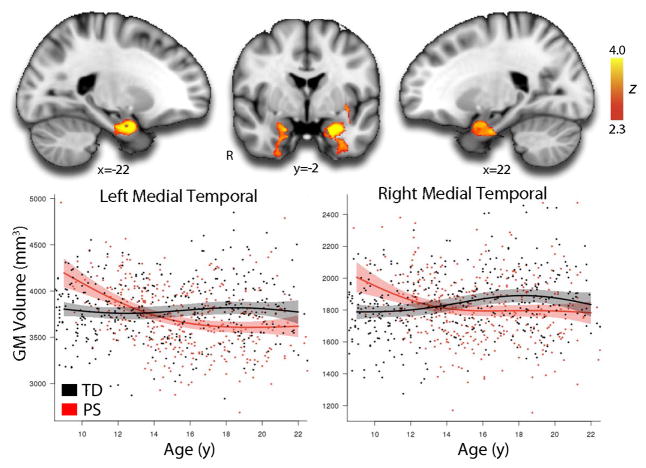
In order to delineate the spatial pattern of volumetric deficits in more detail, we next conducted whole-brain voxelwise analyses. As displayed in Figure 2 and detailed in eTable 1, voxelwise analyses revealed significant clusters of reduced volume in PS youth in a network of regions including bilateral medial temporal lobe, ventromedial prefrontal cortex, orbitofrontal cortex, and posterior cingulate. Reduced volume was also seen in right dorsolateral prefrontal cortex and superior parietal cortex. Peak deficits were found in the medial temporal lobe. There was a single cluster in the right inferior cerebellum where the PS group had larger gray matter volume. Significant group-by-age interactions were present in bilateral medial temporal lobe (Figure 3; eTable 2), where higher volume in PS youth during childhood is followed by lower volume in adolescence. No other regions demonstrated a significant age by group interaction.
Figure 2.
Multifocal gray matter volume reduction in psychosis-spectrum youth. A voxelwise between-group analysis of gray matter volume reveals that PS youth have diminished GM volume across multiple brain regions, including the precuneus, posterior cingulate, bilateral medial temporal lobes, frontal pole, and orbitofrontal cortex. Image thresholded at z>2.3, corrected p<0.01; minimum cluster size: k>255 voxels. See eTable 1 for further details.
Figure 3.
Reduced medial temporal volume in PS youth relative to TD youth develops in early adolescence. Voxelwise examination of nonlinear group-by-age interactions reveals significant differences in the developmental pattern of bilateral medial temporal lobes. Whereas ICV-adjusted medial temporal volumes are stable in TD youth, PS youth lose medial temporal volumes in late childhood and early adolescence, resulting in lower MTL volumes bilaterally by mid-adolescence. Image thresholded at z>2.3, corrected p<0.01; minimum cluster size: k>255 voxels. See eTable 2 for further details. Shaded regions represent 95% confidence intervals.
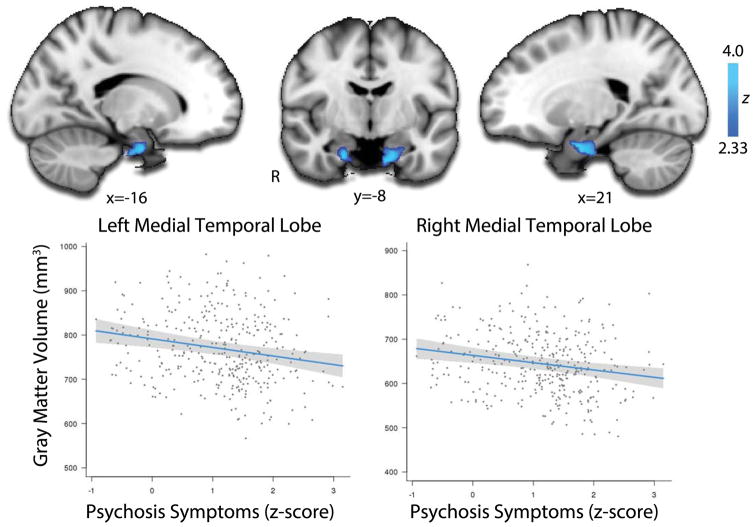
Dimensional psychosis severity is associated with medial temporal volume loss
Having identified regions of reduced volume in PS youth, we next evaluated whether the severity of symptoms in PS participants was related to the magnitude of structural abnormalities. Greater severity of PS symptoms was associated with volume reduction in bilateral medial temporal lobe (Figure 4, see also eTable 3). Follow-up analyses revealed that these medial temporal effects were driven by positive (p<0.001) but not negative symptoms (NS). These results suggest that lower volume in the medial temporal lobe is related not just to the presence of PS symptoms, but also their severity.
Figure 4.
Dimensional severity of psychosis-spectrum symptoms is associated with diminished volume in bilateral medial temporal lobe in PS youth. Voxelwise regression of dimensional PS symptoms within the PS sample reveals bilateral clusters of diminished volume in the medial temporal lobe (top panels). Image thresholded at z>2.3, corrected p<0.01; minimum cluster size: k>150 voxels. Bottom panels plot the mean volume within these clusters versus PS symptom severity, while adjusting for covariates. Shaded regions represent 95% confidence intervals. See eTable 3 for details.
Supplementary analyses
Supplementary analyses in three sub-samples that excluded medicated subjects, removed subjects under age 11, or used groups matched on demographic covariates provided convergent results (eTable 4). Effect sizes in all samples were small (d =0.4 or lower; see eTable 4). Finally, PNC participants with other psychopathology did not show similar volumetric deficits to those seen in PS youths (eTable 5).
DISCUSSION
In a large, community-based sample of PS youth, we identified abnormalities of brain structure. Gray matter volume loss was globally reduced and focused in regions including medial temporal lobe, ventromedial and orbital frontal cortex, posterior cingulate, and dorsolateral prefrontal cortex. Volume reduction was maximal in the medial temporal lobe, where deficits became apparent in mid-adolescence and were correlated with the severity of PS symptoms. Taken together, these findings delineate a pattern of abnormal structural brain development in PS youth that in part mirrors that seen in both adults with clinically diagnosed psychotic disorders and youth at clinical risk.
Structural brain abnormalities in PS youth
We examined gray matter volume in PS youth at multiple scales, including tissue class, lobar, and voxelwise analyses. Throughout, we maximized sensitivity by using advanced image processing techniques: regional volumes were estimated using cutting-edge multi-atlas segmentation,58 while voxelwise analyses used a highly accurate deformable registration60 in combination with a study-specific template. These analyses provided convergent results, and demonstrated that PS youth have diminished GM volume, with lobar deficits that were most prominent in frontal cortex. It should be noted that as in prior meta-analyses of adult clinical samples,15,20 PS youth also had significantly lower ICV; all regional and voxelwise analyses co-varied for ICV and thus represent volumetric decrements above and beyond such a global effect. Voxelwise analyses revealed significant areas of lower volume that were maximal in the medial temporal lobe, and also present in ventromedial prefrontal cortex, orbitofrontal cortex, posterior cingulate, and dorsolateral prefrontal cortex.
Many of the regions impacted are part of the default mode network, a large-scale functional network that is critical for internally directed attention, theory of mind, social cognition, and memory.73,74 Both functional75–77 and structural deficits13,20,78 of default mode regions have been widely documented in psychosis. Indeed, in a prior report from this cohort we described default mode hyper-connectivity in PS youth.39 The current results thus provide convergent evidence for multi-modal structural and functional abnormalities of default mode regions in PS youth.
These results show substantial concordance with other studies of psychosis and risk across the lifespan, including adults with chronic schizophrenia,13,15,16,20 childhood-onset schizophrenia,79,80 and first-episode psychosis.10,12,31–33 Our results indicate that structural abnormalities seen in clinical populations are also present in youth with PS symptoms, and suggest that abnormalities of structural brain maturation may arise relatively early in the course of development. Early loss of gray matter is consistent with prior reports of “accelerated aging” in schizophrenia.81–83 While expansion of white matter observed here is also consistent with premature acceleration of brain development, this finding has not typically been reported in clinical samples. To our knowledge, only one prior study has examined structural brain abnormalities in youth with PS symptoms: Jacobson et al. (2010) documented increases in gray matter density in a small sample (n=11) of children ages 11–13.42 Differences among these results may be accounted for by the difference in dependent measure (density versus volume) and the small sample size of the prior work.
Medial temporal lobe volume deficits in PS youth
Volumetric deficits in PS youth were observed in the medial temporal lobe, including the hippocampal head, amygdala, and parahippocampal cortex. Critically, reduced volume in these regions also correlated with symptom severity, in particular positive symptoms. These results are concordant with a large literature of both structural18,20,26,27,84–90 and functional24,91–96 medial temporal deficits in psychosis. Medial temporal volume loss has previously been documented in samples including adults with chronic schizophrenia,17,18,20,24,88 first episode psychosis,17,18,32,33 and youth at clinical risk for psychosis,26,27,32,97 but has not previously been reported in association with PS symptoms in a population-based sample. While the exact mechanism of injury to the hippocampus in psychosis is as yet unknown, recently Small et al. documented hypermetabolism at baseline in hippocampus CA1 in youth at clinical high risk for psychosis, which was linked to both elevated levels of extracellular glutamate and subsequent volume loss.98 Such changes may potentially be linked to GABA-ergic deficits in the medial temporal lobe in psychosis.99 In the present data, medial temporal lobe volumes showed a marked nonlinear developmental pattern, with volumetric deficits only becoming apparent in mid-adolescence. While speculative, the nonlinear pattern of medial temporal volume reduction in the PS youth seen in our data may be consistent with the ongoing effects of glutamate-related toxicity following a period of both higher metabolism and volume. Potentially, both structural and functional changes could be phase specific,100,101 and represent an series of allosteric compensatory responses to an initial deficit.100 Future studies employing dedicated imaging techniques sensitive to hippocampal neurogenesis and injury may provide further insight.91
Community-based research of PS symptoms: Opportunities and limitations
In this study we examined PS symptoms present in the community. In comparison with other strategies, such as studying help-seeking youth at clinical high risk, this approach comes with both advantages and disadvantages. The community-based approach allowed us to study younger participants who were largely unmedicated, and also to accrue a substantially larger sample size at a single site and scanner. However, the effect sizes of observed effects are relatively small (i.e., d=0.4 or below) and large samples may be required to detect such effects. Further, it should be noted that the correspondence with abnormalities in adult clinical samples is descriptive, and observed abnormalities are partially, though not completely, similar to those identified in meta-analysis of schizophrenia.11,87,101
Additionally, the present cross-sectional analysis does not allow us to evaluate the degree to which observed abnormalities are driven by individual participants who will later become overtly psychotic.34 Given the higher prevalence of PS symptoms compared to population rates of schizophrenia, it seems likely that PS symptoms are themselves associated with structural brain abnormalities. This would support a dimensional view of the psychosis spectrum, and accord closely with the NIMH Research Domain Criteria approach.48,49 As suggested by such a multi-dimensional view of psychopathology, it should be noted that participants with PS symptoms also have elevated levels of co-morbid symptoms from other psychopathology dimensions.36 While specificity analyses established that youth with other non-PS psychopathology did not have the same deficits as PS youths, additional work will be necessary to evaluate the unique impact of PS symptoms in the context of other symptom dimensions. The necessity of further research to establish specificity is underlined by prior reports of cortical and medial temporal lobe abnormalities in both other psychiatric disorders102,103 and in association with cognitive deficits such as those seen in PS youth.51,104 Finally, it should be noted that despite our efforts to control for relevant covariates, un-modeled confounding variables remain a persistent concern in psychiatric neuroimaging.
Conclusions
These results establish that community youths with PS symptoms have similar patterns of structural brain abnormalities seen in clinically ascertained samples from the psychosis spectrum. Together with recent reports from this sample regarding cognitive deficits,36,37,51 reduced executive activation,40 exaggerated amygdala threat-responsivity,40 and functional network dysconnectivity in PS youths,39 these findings suggest that brain abnormalities are associated with PS symptoms at a young age, before clinical high-risk symptoms are typically identified. Such deficits are not dependent on disease chronicity or the confounding influence of psychotropic medication. These brain phenotypes may become a biomarker that can be used in genomic studies, drug discovery, and clinical trials of novel therapeutics.34,105–108 Especially when used in combination with cognitive testing and measures of polygenic risk109–112 imaging phenotypes may help evaluate risk and target interventions for youth with PS symptoms before frank psychosis occurs and negative outcomes accrue. Moving forward, development of data-driven analytic techniques to parse heterogeneity113 within PS youth will accelerate translation to clinical practice.
Supplementary Material
Psychosis spectrum youth have volumetric deficits that impact all lobes of the brain and progress with age. For all analyses, sex, race, maternal education, and ICV are included as covariates. Shaded regions represent 95% confidence intervals.
Acknowledgments
Thanks to the acquisition and recruitment team: Karthik Prabhakaran, Ryan Hopson, Jeff Valdez, Raphael Gerraty, Marisa Riley, Jack Keefe, Elliott Yodh, and Rosetta Chiavacci. Thanks to Harsha Battapady for data processing, and Tianhao Zhang for discussion regarding analytics. Thanks to Chad Jackson and Larry Macy for data management and systems support. Supported by RC2 grants from the National Institute of Mental Health MH089983 and MH089924 and P50MH096891. Additional support was provided by R01MH107703 and K23MH098130 to TDS, R01MH101111 to DHW, K01MH102609 to DRR, K08MH079364 to MEC, R01NS085211 to RTS, T32MH065218 to SNV, and the Dowshen Program for Neuroscience. Support for developing statistical analyses (RTS & TDS) was provided by a seed grant by the Center for Biomedical Computing and Image Analysis (CBICA) at Penn. TDS had full access to all of the data in the study, takes responsibility for the integrity of the data, and the accuracy of the data analysis. REG participated in an advisory board for Otsuka Pharmaceuticals and RCG receives royalties from the Brain Resource Centre and serves without compensation on an advisory board for Lumosity; all other authors have no disclosures.
Footnotes
Disclosures: REG participated in an advisory board for Otsuka Pharmaceuticals and RCG receives royalties from the Brain Resource Centre and serves without compensation on an advisory board for Lumosity; all other authors have no disclosures.
References
- 1.Freedman R. Schizophrenia. N Engl J Med. 2003;349:1738–1749. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Avenevoli S, Costello EJ, et al. Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2012;69:372–380. doi: 10.1001/archgenpsychiatry.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler RC, Avenevoli S, Costello J, et al. Severity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2012;69:381–389. doi: 10.1001/archgenpsychiatry.2011.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey BJ, Oliveri ME, Insel T. A Neurodevelopmental Perspective on the Research Domain Criteria (RDoC) Framework. Biol Psychiatry. 2014;76:350–353. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Di Martino A, Fair DA, Kelly C, et al. Unraveling the Miswired Connectome: A Developmental Perspective. Neuron. 2014;83:1335–1353. doi: 10.1016/j.neuron.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 7.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGlashan TH, Johannessen JO. Early detection and intervention with schizophrenia: rationale. Schizophr Bull. 1996;22:201–222. doi: 10.1093/schbul/22.2.201. [DOI] [PubMed] [Google Scholar]
- 9.Addington J, Cadenhead KS, Cannon TD, et al. North American Prodrome Longitudinal Study: a collaborative multisite approach to prodromal schizophrenia research. Schizophr Bull. 2007;33:665–672. doi: 10.1093/schbul/sbl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruggeri M, Bonetto C, Lasalvia A, et al. Feasibility and Effectiveness of a Multi-Element Psychosocial Intervention for First-Episode Psychosis: Results From the Cluster-Randomized Controlled GET UP PIANO Trial in a Catchment Area of 10 Million Inhabitants. Schizophr Bull. 2015;41:1192–1203. doi: 10.1093/schbul/sbv058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bora E, Fornito A, Radua J, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127:46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Chan RCK, Di X, McAlonan GM, Gong Q-Y. Brain Anatomical Abnormalities in High-Risk Individuals, First-Episode, and Chronic Schizophrenia: An Activation Likelihood Estimation Meta-analysis of Illness Progression. Schizophr Bull. 2011;37:177–188. doi: 10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-Analysis of Gray Matter Anomalies in Schizophrenia: Application of Anatomic Likelihood Estimation and Network Analysis. Biol Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry. 1998;155:1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- 15.Haijma SV, Van Haren N, Cahn W, Koolschijn PCMP, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 17.Gur RE, Cowell P, Turetsky BI, et al. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55:145–152. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- 18.Gur RE, Turetsky BI, Cowell PE, et al. Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry. 2000;57:769–775. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- 19.Thompson PM, Stein JL, Medland SE, et al. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014 doi: 10.1007/s11682-013-9269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Erp TGM, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- 22.Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004;9:609–620. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- 23.Heckers S, Rauch S, Goff D, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 24.Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- 25.Gao X-M, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- 26.Ganzola R, Maziade M, Duchesne S. Hippocampus and amygdala volumes in children and young adults at high-risk of schizophrenia: research synthesis. Schizophr Res. 2014;156:76–86. doi: 10.1016/j.schres.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 27.Dean DJ, Orr JM, Bernard JA, et al. Hippocampal Shape Abnormalities Predict Symptom Progression in Neuroleptic-Free Youth at Ultrahigh Risk for Psychosis. Schizophr Bull. 2015 doi: 10.1093/schbul/sbv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boos HBM, Aleman A, Cahn W, Pol HH, Kahn RS. Brain Volumes in Relatives of Patients With Schizophrenia: A Meta-analysis. Arch Gen Psychiatry. 2007;64:297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- 29.McDonald C, Dineen B, Hallahan B. Meta-analysis of brain volumes in unaffected first-degree relatives of patients with schizophrenia overemphasizes hippocampal deficits. Arch Gen Psychiatry. 2008;65:603–4. doi: 10.1001/archpsyc.65.5.603. author reply 604–5. [DOI] [PubMed] [Google Scholar]
- 30.Roalf DR, Vandekar SN, Almasy L, et al. Heritability of Subcortical and Limbic Brain Volume and Shape in Multiplex-Multigenerational Families with Schizophrenia. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palaniyappan L, Maayan N, Bergman H, Davenport C, Adams CE, Soares-Weiser K. Voxel-based morphometry for separation of schizophrenia from other types of psychosis in first episode psychosis. Cochrane Database Syst Rev. 2015;8:CD011021. doi: 10.1002/14651858.CD011021.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nenadic I, Dietzek M, Schönfeld N, et al. Brain structure in people at ultra-high risk of psychosis, patients with first-episode schizophrenia, and healthy controls: a VBM study. Schizophr Res. 2015;161:169–176. doi: 10.1016/j.schres.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 33.Bois C, Levita L, Ripp I, et al. Hippocampal, amygdala and nucleus accumbens volume in first-episode schizophrenia patients and individuals at high familial risk: A cross-sectional comparison. Schizophr Res. 2015;165:45–51. doi: 10.1016/j.schres.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Cannon TD, Chung Y, He G, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–157. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 36.Calkins ME, Moore TM, Merikangas KR, et al. The psychosis spectrum in a young U.S. community sample: findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 2014;13:296–305. doi: 10.1002/wps.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calkins ME, Merikangas KR, Moore TM, et al. The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry. 2015 doi: 10.1111/jcpp.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGlashan TH, Miller TJ, Woods SW. Pre-onset detection and intervention research in schizophrenia psychoses: current estimates of benefit and risk. Schizophr Bull. 2001;27:563–570. doi: 10.1093/oxfordjournals.schbul.a006896. [DOI] [PubMed] [Google Scholar]
- 39.Satterthwaite TD, Vandekar SN, Wolf DH, et al. Connectome-wide network analysis of youth with Psychosis-Spectrum symptoms. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf DH, Satterthwaite TD, Calkins ME, et al. Functional Neuroimaging Abnormalities in Youth With Psychosis Spectrum Symptoms. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2014.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Hanlon E, Leemans A, Kelleher I, et al. White Matter Differences Among Adolescents Reporting Psychotic Experiences: A Population-Based Diffusion Magnetic Resonance Imaging Study. JAMA Psychiatry. 2015;72:668–677. doi: 10.1001/jamapsychiatry.2015.0137. [DOI] [PubMed] [Google Scholar]
- 42.Jacobson S, Kelleher I, Harley M, et al. Structural and functional brain correlates of subclinical psychotic symptoms in 11–13 year old schoolchildren. Neuroimage. 2010;49:1875–1885. doi: 10.1016/j.neuroimage.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Satterthwaite TD, Elliott MA, Ruparel K, et al. Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage. 2014;86:544–553. doi: 10.1016/j.neuroimage.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelleher I, Cannon M, Cannon M. Psychotic-like experiences in the general population: characterizing a high-risk group for psychosis. Psychol Med. 2011;41:1–6. doi: 10.1017/S0033291710001005. [DOI] [PubMed] [Google Scholar]
- 45.Kelleher I, Connor D, Clarke MC, Devlin N, Harley M, Cannon M. Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychol Med. 2012;42:1857–1863. doi: 10.1017/S0033291711002960. [DOI] [PubMed] [Google Scholar]
- 46.Kelleher I, Murtagh A, Molloy C, et al. Identification and characterization of prodromal risk syndromes in young adolescents in the community: a population-based clinical interview study. Schizophr Bull. 2012;38:239–246. doi: 10.1093/schbul/sbr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelleher I, Corcoran P, Keeley H, et al. Psychotic symptoms and population risk for suicide attempt: a prospective cohort study. JAMA Psychiatry. 2013;70:940–948. doi: 10.1001/jamapsychiatry.2013.140. [DOI] [PubMed] [Google Scholar]
- 48.Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13:28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 50.Gur RE, Kaltman D, Melhem ER, et al. Incidental findings in youths volunteering for brain MRI research. AJNR Am J Neuroradiol. 2013;34:2021–2025. doi: 10.3174/ajnr.A3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gur RC, Calkins ME, Satterthwaite TD, et al. Neurocognitive Growth Charting in Psychosis Spectrum Youths. JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2013.4190. [DOI] [PubMed] [Google Scholar]
- 52.Miller TJ, Cicchetti D, Markovich PJ, McGlashan TH, Woods SW. The SIPS screen: a brief self-report screen to detect the schizophrenia prodrome. Schizophr Res. 2004;70:s78. [Google Scholar]
- 53.Kobayashi H, Nemoto T, Koshikawa H, et al. A self-reported instrument for prodromal symptoms of psychosis: testing the clinical validity of the PRIME Screen-Revised (PS-R) in a Japanese population. Schizophr Res. 2008;106:356–362. doi: 10.1016/j.schres.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 54.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 55.Satterthwaite TD, Connolly JJ, Ruparel K, et al. The Philadelphia Neurodevelopmental Cohort: A publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2015.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doshi J, Erus G, Ou Y, Gaonkar B, Davatzikos C. Multi-atlas skull-stripping. Acad Radiol. 2013;20:1566–1576. doi: 10.1016/j.acra.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li C, Gore JC, Davatzikos C. Multiplicative Intrinsic Component Optimization (MICO) for MRI Bias Field Estimation and Tissue Segmentation. Magn Reson Imaging. 2014 doi: 10.1016/j.mri.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doshi JJ, Erus G, Ou Y, Davatzikos C. Ensemble-based medical image labeling via sampling morphological appearance manifolds. MICCAI Challenge Workshop on Segmentation: Algorithms, Theory and Applications; Nagoya, Japan. 2013. [Google Scholar]
- 59.Davatzikos C, Genc A, Xu D, Resnick SM. Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage. 2001;14:1361–1369. doi: 10.1006/nimg.2001.0937. [DOI] [PubMed] [Google Scholar]
- 60.Ou Y, Sotiras A, Paragios N, Davatzikos C. DRAMMS: Deformable registration via attribute matching and mutual-saliency weighting. Med Image Anal. 2011;15:622–639. doi: 10.1016/j.media.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erus G, Battapady H, Satterthwaite TD, et al. Imaging Patterns of Brain Development and their Relationship to Cognition. Cereb Cortex. 2014 doi: 10.1093/cercor/bht425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 64.Vandekar SN, Shinohara RT, Raznahan A, et al. Topologically dissociable patterns of development of the human cerebral cortex. J Neurosci. 2015;35:599–609. doi: 10.1523/JNEUROSCI.3628-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wood SN. Stable and efficient multiple smoothing parameter estimation for generalized additive models. Journal of the American Statistical Association. 2004:99. [Google Scholar]
- 66.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2011;73:3–36. [Google Scholar]
- 67.Satterthwaite TD, Shinohara RT, Wolf DH, et al. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1400178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanfilipo MP, Benedict RHB, Zivadinov R, Bakshi R. Correction for intracranial volume in analysis of whole brain atrophy in multiple sclerosis: the proportion vs. residual method. Neuroimage. 2004;22:1732–1743. doi: 10.1016/j.neuroimage.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 69.Voevodskaya O, Simmons A, Nordenskjöld R, et al. The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer’s disease. Front Aging Neurosci. 2014;6:264. doi: 10.3389/fnagi.2014.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 71.Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ho D, Imai K, King G, Stuart E. MatchIt: Nonparametric preprocessing for parametric casual inference. R package version. 2006;2:2–11. [Google Scholar]
- 73.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 75.Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 76.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ongür D, Lundy M, Greenhouse I, et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183:59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salgado-Pineda P, Fakra E, Delaveau P, McKenna PJ, Pomarol-Clotet E, Blin O. Correlated structural and functional brain abnormalities in the default mode network in schizophrenia patients. Schizophr Res. 2011;125:101–109. doi: 10.1016/j.schres.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 79.Giedd JN, Jeffries NO, Blumenthal J, et al. Childhood-onset schizophrenia: progressive brain changes during adolescence. Biol Psychiatry. 1999;46:892–898. doi: 10.1016/s0006-3223(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 80.Rapoport JL, Gogtay N. Childhood onset schizophrenia: support for a progressive neurodevelopmental disorder. Int J Dev Neurosci. 2011;29:251–258. doi: 10.1016/j.ijdevneu.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koutsouleris N, Davatzikos C, Borgwardt S, et al. Accelerated brain aging in schizophrenia and beyond: a neuroanatomical marker of psychiatric disorders. Schizophr Bull. 2014;40:1140–1153. doi: 10.1093/schbul/sbt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thompson PM, Vidal C, Giedd JN, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun D, Stuart GW, Jenkinson M, et al. Brain surface contraction mapped in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Mol Psychiatry. 2008;14:976–986. doi: 10.1038/mp.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zipursky RB, Marsh L, Lim KO, et al. Volumetric MRI assessment of temporal lobe structures in schizophrenia. Biol Psychiatry. 1994;35:501–516. doi: 10.1016/0006-3223(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 85.Marsh L, Harris D, Lim KO, et al. Structural magnetic resonance imaging abnormalities in men with severe chronic schizophrenia and an early age at clinical onset. Arch Gen Psychiatry. 1997;54:1104–1112. doi: 10.1001/archpsyc.1997.01830240060009. [DOI] [PubMed] [Google Scholar]
- 86.Altshuler LL, Bartzokis G, Grieder T, et al. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- 87.Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meisenzahl EM, Koutsouleris N, Bottlender R, et al. Structural brain alterations at different stages of schizophrenia: a voxel-based morphometric study. Schizophr Res. 2008;104:44–60. doi: 10.1016/j.schres.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 89.Meisenzahl EM, Seifert D, Bottlender R, et al. Differences in hippocampal volume between major depression and schizophrenia: a comparative neuroimaging study. Eur Arch Psychiatry Clin Neurosci. 2010;260:127–137. doi: 10.1007/s00406-009-0023-3. [DOI] [PubMed] [Google Scholar]
- 90.Wheeler AL, Voineskos AN. A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Front Hum Neurosci. 2014;8:653. doi: 10.3389/fnhum.2014.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ho NF, Hooker JM, Sahay A, Holt DJ, Roffman JL. In vivo imaging of adult human hippocampal neurogenesis: progress, pitfalls and promise. Mol Psychiatry. 2013;18:404–416. doi: 10.1038/mp.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holt DJ, Weiss AP, Rauch SL, et al. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol Psychiatry. 2005;57:1011–1019. doi: 10.1016/j.biopsych.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 93.Holt DJ, Kunkel L, Weiss AP, et al. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr Res. 2006;82:153–162. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 94.Hall J, Whalley HC, Marwick K, et al. Hippocampal function in schizophrenia and bipolar disorder. Psychol Med. 2010;40:761–770. doi: 10.1017/S0033291709991000. [DOI] [PubMed] [Google Scholar]
- 95.Seidman LJ, Rosso IM, Thermenos HW, et al. Medial temporal lobe default mode functioning and hippocampal structure as vulnerability indicators for schizophrenia: A MRI study of non-psychotic adolescent first-degree relatives. Schizophr Res. 2014;159:426–434. doi: 10.1016/j.schres.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 96.Allen P, Seal ML, Valli I, et al. Altered prefrontal and hippocampal function during verbal encoding and recognition in people with prodromal symptoms of psychosis. Schizophr Bull. 2011;37:746–756. doi: 10.1093/schbul/sbp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meisenzahl EM, Koutsouleris N, Gaser C, et al. Structural brain alterations in subjects at high-risk of psychosis: a voxel-based morphometric study. Schizophr Res. 2008;102:150–162. doi: 10.1016/j.schres.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 98.Schobel SA, Chaudhury NH, Khan UA, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heckers S, Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res. 2015;167:4–11. doi: 10.1016/j.schres.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krystal JH, Anticevic A. Toward Illness Phase-Specific Pharmacotherapy for Schizophrenia. Biol Psychiatry. 2015;78:738–740. doi: 10.1016/j.biopsych.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 101.Fornito A, Yücel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104–113. doi: 10.1016/j.schres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 102.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mathew I, Gardin TM, Tandon N, et al. Medial temporal lobe structures and hippocampal subfields in psychotic disorders: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. JAMA Psychiatry. 2014;71:769–777. doi: 10.1001/jamapsychiatry.2014.453. [DOI] [PubMed] [Google Scholar]
- 104.Weinberger DR, Radulescu E. Finding the Elusive Psychiatric “Lesion” With 21st-Century Neuroanatomy: A Note of Caution. Am J Psychiatry. 2015 doi: 10.1176/appi.ajp.2015.15060753. appiajp201515060753. [DOI] [PubMed] [Google Scholar]
- 105.Anticevic A, Hu X, Xiao Y, et al. Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci. 2015;35:267–286. doi: 10.1523/JNEUROSCI.2310-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Anticevic A, Tang Y, Cho YT, et al. Amygdala Connectivity Differs Among Chronic, Early Course, and Individuals at Risk for Developing Schizophrenia. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chung Y, Cannon TD. Brain imaging during the transition from psychosis prodrome to schizophrenia. J Nerv Ment Dis. 2015;203:336–341. doi: 10.1097/NMD.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cannon TD, Sun F, McEwen SJ, et al. Reliability of neuroanatomical measurements in a multisite longitudinal study of youth at risk for psychosis. Hum Brain Mapp. 2014;35:2424–2434. doi: 10.1002/hbm.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stein JL, Medland SE, Vasquez AA, et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet. 2012;44:552–561. doi: 10.1038/ng.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hibar DP, Stein JL, Renteria ME, et al. Common genetic variants influence human subcortical brain structures. Nature. 2015;520:224–229. doi: 10.1038/nature14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ripke S, O’Dushlaine C, Chambert K, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–1159. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang T, Koutsouleris N, Meisenzahl E, Davatzikos C. Heterogeneity of Structural Brain Changes in Subtypes of Schizophrenia Revealed Using Magnetic Resonance Imaging Pattern Analysis. Schizophr Bull. 2014 doi: 10.1093/schbul/sbu136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Psychosis spectrum youth have volumetric deficits that impact all lobes of the brain and progress with age. For all analyses, sex, race, maternal education, and ICV are included as covariates. Shaded regions represent 95% confidence intervals.