Abstract
Background & Aims
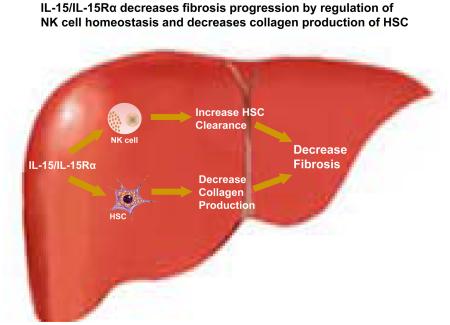
Interleukin-15 (IL-15) and its high affinity receptor interleukin-15 receptor alpha (IL-15Rα) are widely expressed in immune cells and hepatic resident cells. IL-15 signaling has important functions in homeostasis of natural killer (NK), natural killer T (NKT) and cytotoxic T (CD8+T) cells, and in liver regeneration. We hypothesized that IL-15 has a protective role in liver fibrosis progression by maintaining NK cell homeostasis.
Methods
Fibrosis was induced using two mechanistically distinct models. Congenic bone marrow transplantation was used to evaluate the contribution of IL-15 signaling from various compartments to NK, CD8+T and NKT cell homeostasis and fibrogenesis. The gene expression profile of hepatic stellate cell (HSC) from IL-15Rα knockout (IL-15RαKO) mice and wild type mice were captured using microarray analysis and validated in isolated HSC. Quantitative real-time PCR was used to assess repressors of collagen transcription.
Results
IL-15RαKO mice exhibited more fibrosis in both models. IL-15 signaling from specific types of hepatic cells had divergent roles in maintaining liver NK, CD8+T and NKT cells, with a direct and protective role on radio-resistant non-parenchymal cells beyond the control of NK homeostasis. HSCs isolated from IL-15RαKO mice demonstrated up-regulation of collagen production. Finally, IL-15RαKO HSC with or without transforming growth factor beta (TGF-β) stimulation exhibited increased expression of fibrosis markers and decreased collagen transcription repressors expression.
Conclusions
IL-15Rα signaling has a direct anti-fibrotic effect independent of preserving NK homeostasis. These findings establish a rationale to further explore the anti-fibrotic potential of enhancing IL-15 signaling in HSCs.
Keywords: NK cells, NKT cells, collagen, hepatic stellate cells, interleukin 15, fibrosis, mice
Introduction
Hepatic fibrosis is a wound healing response to chronic injury due to a range of insults including viral infections, alcohol and metabolic diseases, which results in extracellular matrix deposition, distortion of the normal liver structure and impaired liver function [1]. Liver fibrosis remains an important cause for morbidity and mortality worldwide [2], and there is still an urgent need to uncover key mechanisms underlying fibrosis and develop novel therapies to halt disease progression.
Interleukin-15 (IL-15) is a pleiotropic cytokine that is a member of the common gamma chain family, which also includes IL-2, IL-4, IL-7, IL-9 and IL-21. It plays crucial roles in the development, homeostasis and physiology of a wide range of lymphoid cells including memory cytotoxic CD8+ T cells [3,4], natural killer (NK) cells [5,6], natural killer T (NKT) cells [7] and interferon (IFN) producing killer dendritic cells (IKDC) [8]. IL-15 signals are delivered uniquely; as opposed to other cytokines that are secreted, IL-15 primarily exists bound to the high affinity IL-15 receptor α subunit (IL-15Rα) that is shuttled to the cell surface, where it stimulates opposing cells through the β/γ receptor complex [9]. In the murine liver, IL-15 is expressed by bone marrow (BM)-derived cells, such as dendritic cells (DCs) [10], monocytes and Kupffer cells [5], and also by hepatic resident cells, including hepatocytes, oval cells [11] and hepatic stellate cells (HSCs) [12].
HSCs play a prominent role in fibrogenesis; upon activation following liver injury, HSCs differentiate into myofibroblast-like cells that are contractile, proliferative and fibrogenic [13]. HSCs express IL-15Rα/IL-15 and play an important role in NKT cell proliferation [12].
There has been increasing progress in defining the roles of immune cells in controlling fibrosis, in particular NK cells [14,15], NKT cells [16,17], CD8+T cells [18], B cells [19], macrophages [20] and DCs [21]. NK cells limit fibrosis progression by killing activated HSCs through granzyme, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and the production of IFN-γ [14,15,22].
Although IL-15 signaling plays a pivotal role in the development of NK, NKT and CD8+ T cells, all of which can regulate liver fibrosis, there have been no studies investigating the direct role of IL-15 signaling in fibrogenesis. Its only reported activity to date in liver has been to stimulate proliferation of mature hepatocytes by increasing oval cell numbers and hepatocyte mitosis in a murine model of liver regeneration [11].
In order to clarify the role of IL-15 signaling in liver fibrosis progression, we subjected IL-15RαKO mice to two mechanistically distinct models of fibrosis: chronic carbon tetrachloride (CCl4) administration and bile duct ligation (BDL). Furthermore, using bone marrow transplantation (BMT), we have dissected the contribution of IL-15 signaling in different hepatic cellular compartments in regulating hepatic NK, NKT and CD8+T cell homeostasis and fibrosis progression. We have also uncovered an unexpected NK independent direct anti-fibrogenic role of IL-15Rα in HSCs in vitro associated with down-regulation of collagen transcriptional repressors.
Results
Mice deficient in IL-15Rα have enhanced fibrosis progression
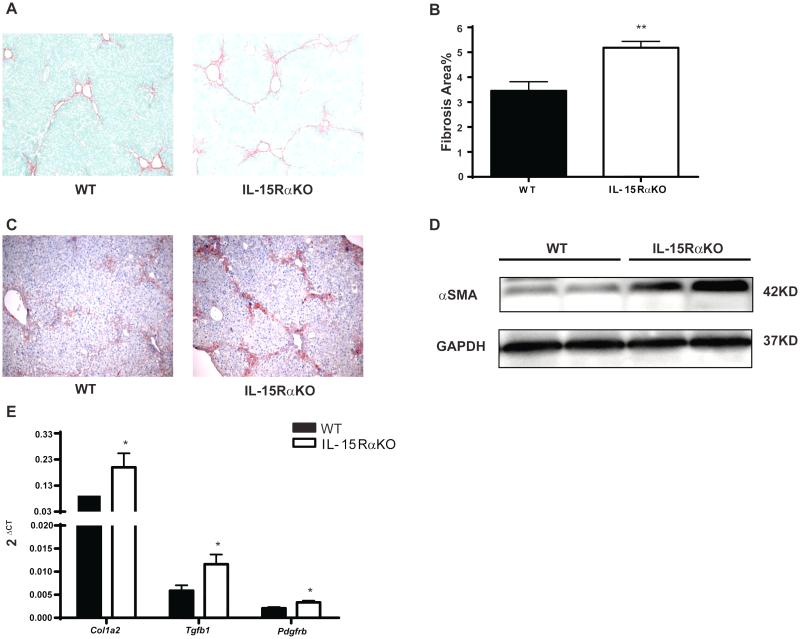
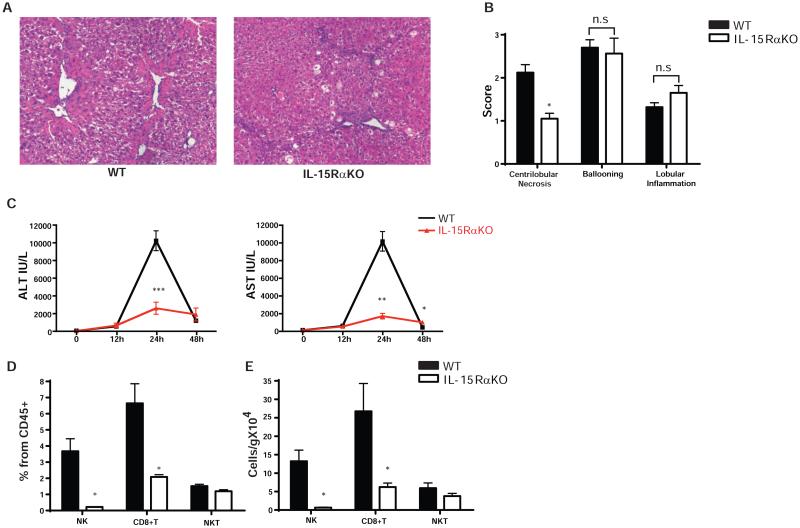
Consistent with earlier reports [23], IL-15Rα knockout mice were confirmed to be deficient in NK, NKT, and CD8+ T cells (Supplementary Fig.1 and 2). We first investigated whether the absence of IL-15Rα alters fibrosis progression in the CCl4-induced fibrosis model. Increased fibrosis was observed in IL-15RαKO mice compared to WT controls, with more collagen deposition quantified by morphometry of Sirius Red collagen staining (Fig. 1A-B) In addition to increased fibrosis, there were increased numbers of activated HSCs in IL-15RαKO mice based on alpha smooth muscle actin (α-SMA) immunohistochemical staining (Fig. 1C) and Western Blotting (Fig. 1D). Enhanced fibrogenesis in IL-15RαKO mice was further confirmed by real-time PCR of the fibrogenic markers collagen1A2 (Col1a2), transforming growth factor beta 1 (Tgfb1) and platelet-derived growth factor receptor beta (Pdgfrb) which were increased compared to WT controls (Fig. 1E). Increased fibrosis in IL-15RαKO mice was not the result of more injury or inflammation, as determined by blind pathologic evaluation (Fig. 2A-B), and by serum ALT and AST measurements at multiple time points after CCl4 administration. In fact, the peak ALT and AST values observed in IL-15RαKO mice were only one-fifth of those in WT mice (Fig. 2C), suggesting a possible role of IL-15 signaling in amplifying liver injury. The deficiency of NK and CD8+T cells in IL-15RαKO mice relative to control mice persisted under the chronic inflammatory conditions induced by CCl4, whereas there was no longer a significant difference in NKT cells (Fig. 2D). To validate the phenotype in a second model of murine fibrosis, we induced cholestatic liver injury by BDL. Similar to chronic CCl4 administration, BDL also induced more fibrosis in IL-15RαKO mice compared with control mice (Supplementary Fig.3).
Figure 1. IL-15RαKO mice show increased fibrosis compared to WT mice in the chronic CCl4 model.
Mice were treated with CCl4 as described in the Materials and Methods. Results are mean ±SE (n=5, repeated twice) (A-C) Type I collagen deposition was visualized by Sirius Red staining (A), and quantified by morphometry (B). (C-E) IL-15RαKO mice had more activated stellate cells as determined by immunostaining (C) and Western blotting (D) for α-SMA. (E) Hepatic mRNA levels of Col1a2, Tgfb1 and Pdgfrb were measured by qPCR and normalized to GAPDH.
Figure 2. CCl4 administration does not increase liver injury but partially restores hepatic NKT cell population in IL-15RαKO mice.
(A-B) HE staining (A) and histological grading (B) indicates less necrosis in IL-15RαKO liver after chronic CCl4 exposure while ballooning and lobular inflammation did not differ from WT controls. (Original magnification×100 [A]) (C) Peak serum ALT and AST levels in IL-15RαKO mice were significantly much lower than those in WT mice. (D-E) IL-15RαKO mice continue to display a deficiency in liver NK cells and CD8+T cells following chronic CCl4 administration as determined by flow cytometry and quantified by percentage of CD45+ cells (D) and absolute number (E). Liver leukocytes were isolated as described in Materials and Methods and gated using SSC/FSC properties, 4',6-Diamidino-2-Phenylindole (DAPI)– (to exclude dead cells), single cell population (to exclude doublets) and CD45+ (to exclude non-hematopoietic cells). NK cells were identified as NK1.1+CD3e-. CD8+T cells were identified as NK1.1-CD3e+CD8+ while NKT cells are indicated as NK1.1+CD3e+. *p<0.05, **p<0.01, ***p<0.001.
Opposite to these models, exogenous administration of IL-15 has an anti-fibrotic effect in CCl4 induced liver fibrosis (Supplementary Fig. 4A and 4B).
IL-15Rα on both BM-derived and hepatic resident cells are required for hepatic NK and CD8+ T cell homeostasis
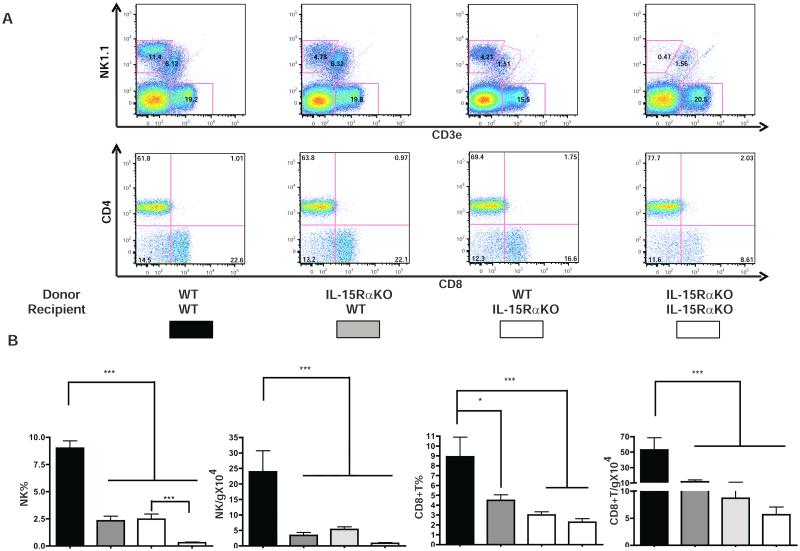
As noted previously, the deficiency of NK cells and CD8+ T cells in IL-15RαKO mice cells persists following chronic CCl4 injection. Since CD8+ T cells have pro-fibrogenic properties [18] while NK cells can limit fibrosis progression [14,15], we hypothesized that the enhanced fibrogenesis in IL-15RαKO mice was primarily the result of NK cell deficiency. In order to address this hypothesis, we first evaluated whether it was IL-15 signaling in BM-derived cells or in hepatic resident cells that regulates NK and CD8+ T cell development. We used lethal irradiation and BMT to generate groups of chimeric mice that lacked IL-15Rα expression in either radio-resistant cells (hepatocytes, endothelial cells, sessile Kupffer cells and HSC) or radio-sensitive cells (all hematopoietic-derived liver cells) (Supplementary Fig. 5A). Evaluation of intrahepatic leukocyte populations 12 weeks after BMT revealed that the absence of IL-15Rα on hematopoietic derived cells resulted, as expected, in a deficiency of NK and CD8+ T cells. However the reduced frequency of hepatic NK and CD8+ T cells was not as severe as that observed in the complete absence of IL-15Rα on all cells (Fig. 3A-B). This observation suggests a contribution of IL-15Rα from resident cells to hepatic NK and CD8+ T cells homeostasis (Fig. 3A-B). In the reciprocal experiment, transplanting IL-15Rα wild type bone marrow partially corrected the NK cell deficiency observed in IL-15RαKO mice (Fig. 3B), indicating that trans-cellular IL-15 presentation by radio-sensitive hematopoietic derived cells is also required for normal liver NK and CD8+T cell homeostasis.
Figure 3. L-15 signaling from both BM-derived and hepatic resident cell compartments are required for NK and CD8+T cell homeostasis.
Liver leukocytes were isolated as described in Materials and Methods and NK, CD8+T and NKT cells were characterized by flow cytometry (A) and quantified by percentage of CD45+ cells and absolute number (B). NKT cell quantification is shown in Supplementary Fig. 4B, C.
In contrast to the effects on NK cells, the absence of IL-15Rα on the hepatic resident radio-resistant cells was associated with a deficit of NKT cells regardless of whether donor mice were WT or IL-15RαKO (Supplementary Fig. 5B-C). Reciprocally, the selective absence of IL-15Rα on hematopoietic cells did not significantly reduce NKT cells relative to mice with a wild type hematopoietic compartment. These data support the concept that hepatic NKT cell homeostasis is primarily regulated by IL-15 presentation by resident hepatic cells rather than other intrahepatic leukocytes.
IL-15Rα on both BM-derived cells and hepatic resident cells decreases fibrosis progression
In order to dissect the physiological compartment in which IL-15 signaling contributes to fibrosis progression, liver fibrosis was induced in chimeric mice selectively lacking IL-15Rα on either radio-resistant or radio-sensitive cells. 12 weeks after BMT, the chimeric mice received CCl4 injections for four weeks (Supplementary Fig. 5A) and fibrosis was evaluated as described in the Materials and Methods.
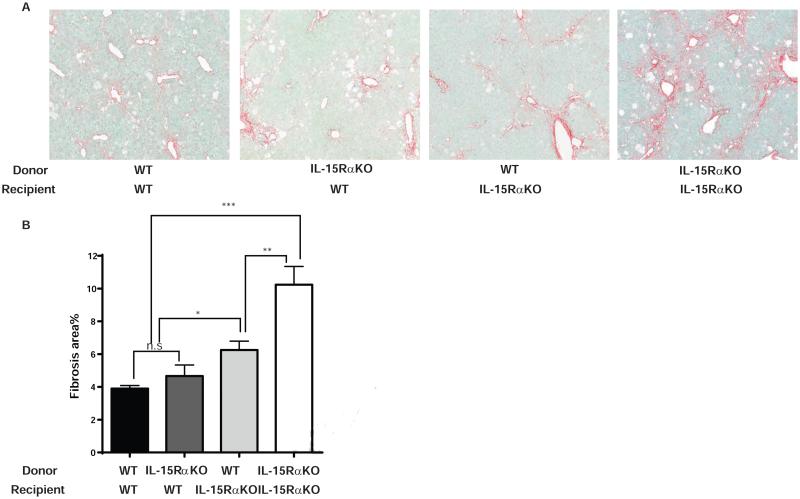
As shown in Fig. 4, IL-15RαKO mice transplanted with WT BM developed less fibrosis than IL-15RαKO mice transplanted with IL-15RαKO. These mice with WT bone marrow had a similar number of NKT cells but more NK cells, suggesting that the partially restored NK cell population was responsible for the lower level of fibrosis in these mice compared to IL-15RαKO mice transplanted with IL-15RαKO bone marrow. The anti-fibrotic role of NK cells in this model is confirmed by NK cell transfer experiments that decrease fibrosis progression in IL-15RαKO mice (Supplementary Fig. 6), as previously reported by other authors [14,15].
Figure 4. IL-15Rα on both BM-derived and hepatic resident cells decreased fibrosis progression.
Four groups of chimeric mice were generated by BMT with different combinations of donors and recipients. Fibrillar collagen deposition was determined by Sirius Red staining (A) and quantified by morphometry (B) Results are shown by mean ±SE. (n=5, repeated twice) (Original magnification ×100 [A]). *p<0.05, **p<0.01, ***p<0.001.)
WT mice transplanted with IL-15RαKO BM developed similar levels of fibrosis compared with WT mice transplanted with WT BM (Fig. 4) despite the former group having markedly fewer NK cells (Fig. 3), suggesting that IL-15 presentation by hepatic resident cells has a protective effect and limits fibrosis progression despite the deficiency in NK cells. Further analysis showed that IL-15RαKO mice that received WT BM had greater fibrosis than WT mice that received IL-15RαKO BM (Fig. 4B) despite the two types of mice having similar numbers of NK and CD8+T cells (Fig. 3). While IL-15RαKO mice transplanted with WT BM have fewer NKT cells than WT mice transplanted with IL-15RαKO BM, work by others has shown that NKT cell deficiency does not affect fibrosis after four weeks of CCl4 treatment [16]. Taken together, these findings support the hypothesis that IL-15Rα expression may control fibrosis progression by a mechanism independent of the effect on NK cell homeostasis.
Trans-presentation of IL-15 to HSCs down-regulates collagen production upon TGFβ stimulation
We hypothesized that IL-15 signaling in hepatic resident cells in the liver protects against liver fibrosis progression. As shown in Fig. 2A-C, chronic CCl4 does not lead to greater liver injury in IL-15RαKO compared to WT mice, which suggests that the protective role of IL-15 signaling is not the result of less hepatocyte injury. HSCs are a radio-resistant cell population in the liver that is known to play a central role in fibrogenesis. We isolated and cultured HSCs from WT and IL-15RαKO mice, and the purity of activated cultured HSCs was validated by 93-95% expression of α-SMA (Supplementary Fig. 7A-B) and absence of CD45 expression (Supplementary Fig. 7C). As previously reported, immunofluorescence staining and Western blotting confirmed that IL-15Rα (Supplementary Fig. 8A) and IL-15 (Supplementary Fig. 8B) were expressed on cultured activated HSCs.
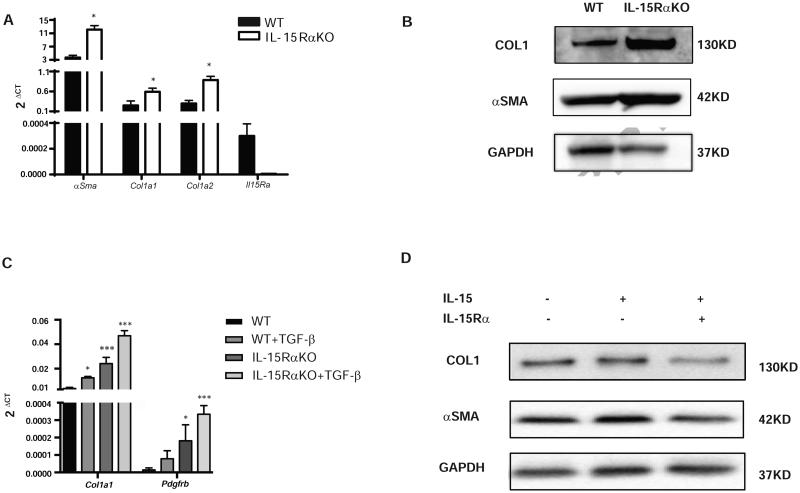
The analysis of collagen1A1 (Col1a1), Col1a2 and α-SMA mRNA expression by real-time PCR confirmed an increase of α-SMA and collagen-1 transcripts in HSCs isolated from IL-15RαKO mice (Fig. 5A). This increase was validated at the level of protein for collagen and α-SMA (Fig. 5B), suggesting a critical role for IL-15Rα in the control of HSC fibrogenic potential. Moreover, in vitro, after TGF-β stimulation, IL-15RαKO HSC expressed more Col1a1 and Pdgfrb than WT (Fig. 5C)
Figure 5. IL-15 signaling controls fibrogenic potential of HSCs.
(A-B) Increased expression of fibrogenic markers α-Sma, Col1a1 and Col1a2 was observed in IL-15RαKO culture activated HSCs than in WT as measured by qPCR (A) and Western Blot (B). (C) IL-15RαKO HSCs has increased expression of Col1a1 and Pgdfrb at baseline and after TGF-β stimulation. (D) Delivery IL-15/IL-15Rα complex reduced collagen I and α-SMA production in IL-15RαKO HSCs. *p<0.05, ***p<0.001
To further dissect the type of presentation involved during this anti-fibrogenic effect, we determined whether collagen production in IL-15Rα KO HSC could be restored by trans-presentation of IL-15 upon TGF-β stimulation. Indeed, adding the complex of IL-15/IL-15Rα to HSC down-regulated collagen production after TGF-β stimulation, while IL-15 alone had no effect, supporting the role of trans-presentation (Fig. 5D).
IL-15Rα controls the fibrogenic potential of HSCs by down-regulating collagen transcription repressors
Next, we compared the mRNA expression profiles of purified HSC isolated from murine livers of IL-15RαKO and wild type mice after in vivo CCl4 induced activation. Gene set enrichment analysis (GSEA) of differentially expressed transcripts revealed enrichment in IL-15RαKO HSC of proliferation/survival pathways (RAS, RHO, MAPK, AKT) and also cell death pathways (TNFR2, TNF signaling via NFκB and signal death through JNK pathways) (Supplementary Table 1).
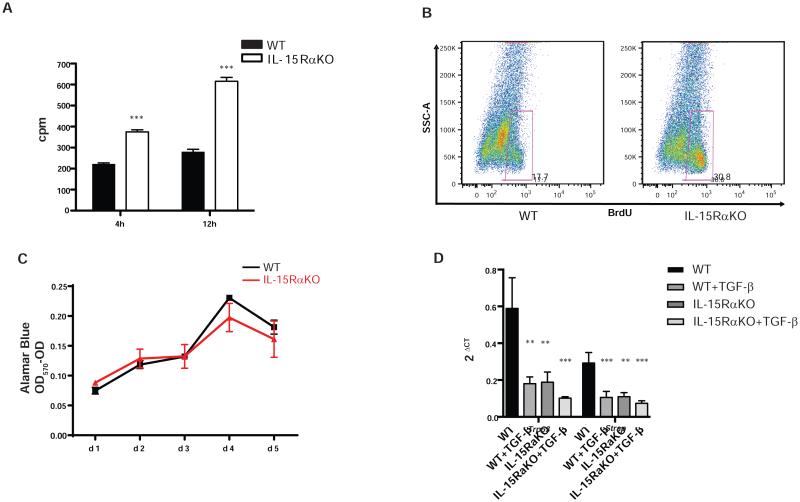
IL-15Rα signaling has an important effect on hematopoietic cell homeostasis by affecting their cell cycle [24]. Unexpectedly, transcriptomic analysis suggested that both the HSC proliferative capacity and their survival were affected by the absence of IL-15Rα. Analysis of proliferation of culture-activated HSC (passage 1) by 3H-thymidine incorporation indicated that IL-15RαKO HSC had an increased proliferation rate compared with WT HSC (Fig. 6A); this was also confirmed by detecting BrdU incorporation in HSC when they are activated in vivo (Fig. 6B). However, there was no difference in the growth curves of HSCs isolated from IL-15RαKO and WT mice (Fig. 6C), strongly suggesting up-regulation of apoptosis and proliferation in IL-15RαKO HSCs.
Figure 6. IL-15Rα controls the fibrogenic potential of HSCs involving down-regulation of collagen transcription repressors.
(A-C) IL-15Rα deficiency resulted in increased proliferation in HSCs as shown by thymidine incorporation assay (A) and BrdU flow cytometry analysis (B) without affecting the cell growth curve (C). (D) Reduction of collagen transcription repressor Trp53 and Strap in the absence of IL-15Rα at the baseline and after TGF-β stimulation. *p<0.05, **p<0.01, ***p<0.001
Finally, we analyzed suppressors of collagen production that may be down-regulated in the absence of IL-15Rα. Analysis of microarrays data suggested that p53 (Trp53) [25] and serine-threonine kinase receptor-associated protein (Strap) [26,27] are decreased in HSC without IL-15Rα. Quantitative real-time PCR data confirmed that absence of IL-15Rα in HSC results in a reduction in the expression these suppressors of collagen transcription at the baseline and after TGF-β stimulation (Fig. 6D) strongly supporting the role of IL-15Rα signaling in suppressing collagen production at the transcription level.
Human HSC express IL-15Rα
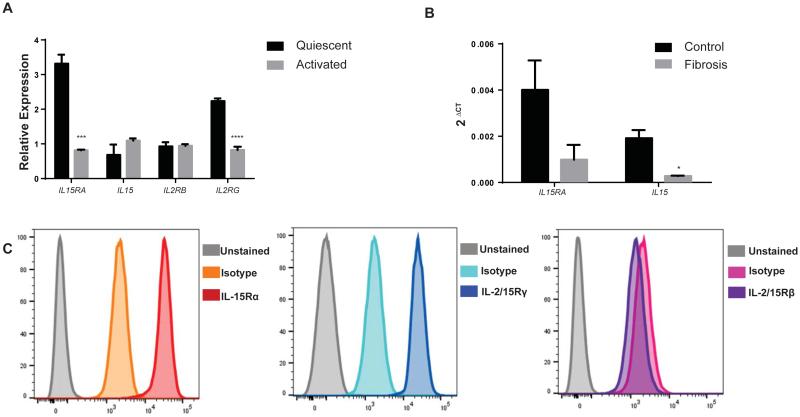
No data exists on whether IL-15 is expressed by human HSC and changed during fibrogenesis. First we investigated mRNA expression of the IL-15 signaling complex (IL-15 and IL-15R subunits) in already publicly available transcriptome microarrays of normal and fibrotic liver. There is an upregulation of the IL-15 signaling complex during fibrogenesis, most likely related to the recruitment of cells enriched in these transcripts: monocytes, macrophages and DC (Supplementary Fig. 9). However when we looked specifically at quiescent and activated HSC isolated from human livers, the relative expression of IL-15Rα was significantly down-regulated during the activation process of HSC, supporting the suppressive role of IL-15 signaling on collagen (Fig. 7A). We further validated these findings in HSC freshly isolated (as reported previously [28]) from normal and fibrotic livers (Fig. 7B). There is less IL-15 transcript (p<0.05) and a trend to less IL-15Rα mRNA (p=0.08) in activated HSC isolated from fibrotic livers (Fig. 7B) when compared with HSC isolated from healthy control livers. Our analysis confirms the findings reported in human transcriptome HSC database analysis.
Figure 7. Expression of IL-15R subunits in human HSCs.
(A-B) Decreased expression of IL-15Rα in activated human HSCs as obtained from publically available dataset GSE 68001 (A) and by real time PCR of HSCs isolated from normal and cirrhotic livers (B). (C) Expression of IL-15Rα, IL-2/15Rγ but not IL-2/15Rβ in human HSCs cell line LX-1 characterized by flow cytometry. *p<0.05, **p<0.01, ***p<0.001
Additionally we analyzed LX-1 cells, a human HSC cell line [29] for the expression of IL-15 receptor signaling subunits. Using flow cytometry we found human HSC expressing IL-15Rα and the common gamma receptor subunit (Fig. 7C) suggesting a non-IL-2/15Rβ dependent pathway of signaling in HSC, a pathway reported to be active in non-lymphoid cells [30].
Discussion
Our study establishes that IL-15Rα plays a protective role in murine liver fibrosis. Firstly, we show that the absence of IL-15Rα is associated with worse fibrosis in two different models of liver injury. Secondly, using BM chimeric mice we confirm that the presence of IL-15Rα on both radio-sensitive BM-derived intrahepatic leukocytes and radio-resistant hepatic resident cells control hepatic NK and CD8+T cell homeostasis. Thirdly, we observe that mice that express IL-15Rα on hematopoietic-derived cells but not on radio-resistant hepatic resident cells have increased fibrogenesis that cannot be explained by the NK cell defect resulting from IL-15 signaling required for their homeostasis and we identify an increased fibrogenic potential of HSCs in the absence of IL-15Rα. Lastly, we demonstrate that this fibrogenic potential is mediated in part by reduced levels of suppressors of collagen production.
IL-15RαKO mice have already been characterized as having deficiencies in NK, NKT and memory CD8+T cell populations [23]. Although, CD8+T cells have pro-fibrogenic properties [18], we observed enhanced fibrosis progression after chronic CCl4-induced liver injury in IL-15RαKO mice despite having significantly fewer CD8+T cells. Reports of the role of NKT cells during fibrosis progression are controversial [16]. Interestingly, in our model, the baseline NKT cell deficiency in IL-15RαKO mice is no longer apparent after chronic liver injury. Taking into account this observation and the lack of effect of NKT cells on fibrosis after four weeks of CCl4 [16], we did not focus our attention on the role of NKT cells. Instead, considering the widely published and consistent reports on the protective role of NK cells [14,15,31] in fibrosis progression, we focused primarily on the effect of IL-15 from different sources on NK cell development and the impact on fibrosis progression.
IL-15 signaling plays an important role in NK cell development and maturation [32]. IL-15 delivery expands NK cell numbers and enhances their cytolytic activity in targeting YAC-1 cells [33]. Using congenic BMT, we confirmed that hematopoietic- and hepatic resident-derived IL-15 signaling pathways are both equally important for liver NK cell development, as has previously been reported [34]. This situation contrasts with NKT cell homeostasis, which primarily requires the presence of IL-15Rα on radio-resistant hepatic resident cells. Simultaneous signals through IL-15Rα on hepatic resident cells and liver hematopoietic-derived cells provide an explanation for the enrichment of NK and NKT cells in the liver.
The importance of IL-15Rα on non-hematopoietic cells for fibrosis progression was determined using chimeric mice obtained by BMT. The transplant of WT BM into IL-15RαKO recipients partially reduces the severity of fibrosis seen in IL-15RαKO mice, and suggests an NK cell dependent mechanism. However, although transferring IL-15RαKO BM into WT mice reduces the number of NK cells, the degree of fibrosis does not differ from mice that received WT BM, suggesting an NK cell independent protective role of IL-15Rα in hepatic radio-resistant resident cells.
Hepatocytes comprise the main population of radio-resistant cells in the liver that express IL-15Rα. Since signaling through IL-15Rα mostly provides a survival signal [35– 37] , we expected to observe decreased hepatocyte survival and increased necrosis in IL-15RαKO mice. However, the opposite effect was observed. IL-15RαKO mice exhibited an almost 5-fold decrease in peak ALT and AST levels after CCl4 administration. This effect is not totally unexpected considering that NK, NKT and CD8+ T cells increase the severity of liver injury in a variety of models [38–40] and in IL-15RαKO mice all these cell types are deficient. From these observations we can exclude the possibility that increased fibrosis in IL-15RαKO mice is due to increased hepatocyte injury. Moreover, this finding is consistent with the clinical observation that there is little correlation between the severity of necrosis/liver injury and fibrosis progression in patients [41,42].
Next, we focused on the main population of fibrogenic cells, represented by HSCs. We have reported and validated that HSCs express IL-15Rα and IL-15. GSEA analysis indicated that IL-15Rα changes proliferation/survival pathways and TNFR and JNK mediated signals, suggesting that differences in HSC proliferation may offset differences in apoptosis, which would account for the fact we did not observe changes in the growth curves of HSCs in the absence of IL-15Rα. We observed an increased fibrogenic potential at the level of collagen production in IL-15Rα deficient HSCs compared with WT HSCs. Similarly, IL-15 has been reported to counteract TGF-β-mediated fibrogenesis in human fetal lung fibroblasts [43], despite a previous description of an association between serum IL-15 levels and the extent of pulmonary fibrosis [44]. Also, in a coxsackievirus B3-induced myocarditis model, treatment with IL-15 prevented myocardial fibrosis and improved cardiac function while inhibition of intrinsic IL-15 tended to aggravate the disease [45]. In HSC, the absence of IL-15Rα results in more collagen mRNA at baseline and after TGF-β stimulation. Moreover, our results suggest that IL-15 signaling controls suppressors of collagen transcription such as Trp53 and Strap. However, although wild-type cells treated with TGF-β produce similar levels of Trp53 and Strap as IL-15RαKO cells, IL-15RαKO cells produce more collagen than the wild-type cells treated with TGF-β. This suggests that additional mechanisms are involved. Furthermore, our finding that treatment of IL-15RαKO cells with both IL-15Rα and IL-15 decreases collagen production supports our theory that trans-presentation is involved in this effect.
From the translational perspective our study is important as human HSC express IL-15Rα, which is down-regulated during hepatic fibrogenesis. The trend of decreasing fibrosis progression by IL-15 treatment supports the idea that treatment with complexes of IL-15Rα/IL-15 or/and IL-15Rα will provide further additional benefits by stimulating HSC in trans, similar to physiological IL-15 signaling.
Our data show that IL-15 signaling from both hepatic resident and BM-derived cells plays a protective role in liver fibrosis progression. Given that IL-15 and IL-15Rα/IL-15 complex administration have been found to be effective and safe therapies for different tumor models, including metastatic hepatocellular carcinoma [46], melanoma, colorectal cancer [47] and lymphoma [48], IL-15 might serve as a potential therapy for liver fibrosis by enhancing the cytolytic effect of NK cells [46] or, as suggested by our findings, decreasing the fibrogenic potential of HSC.
These findings, especially the role of IL-15Rα on HSC, shed new light on the novel function of IL-15Rα in the biology of cells of non-hematopoietic origin. Furthermore, we provide a basis for the use of this molecule as a potential therapeutic target.
Materials and Methods
Animals
Six-week-old female IL-15RαKO and wild type (WT) B6/129 mice were purchased from Jackson Laboratories (Bar Harbor, ME). All procedures were performed in accordance with the Institutional Animal Care and Use Committee Protocols of the Icahn School of Medicine at Mount Sinai and University of Illinois at Chicago.
Materials
All reagents were obtained from Sigma-Aldrich unless stated otherwise.
Hepatic fibrosis models
CCl4-induced fibrosis was generated by intraperitoneal injections of 0.5 µl CCl4/g body weight in corn oil (10%), three times per week for 4 weeks, and the mice were sacrificed 1 day after the last CCl4 administration. BDL-induced liver fibrosis was performed by placing a metallic surgical vascular clip across the lower end of the common bile duct [49]. Fibrosis severity was evaluated 14 days after the surgery.
Grading and staging of liver injury
An expert liver pathologist (M.F.) who was blindled to the experimental protocol scored 5 random areas per slide for centrilobular necrosis, ballooning and lobular inflammation. Paraffin-embedded liver sections were stained with Picrosirius red to measure collagen content as described previously [18], using the Bioquant computerized morphometry program.
Flow cytometry
Total intrahepatic leukocyte populations were isolated using the protocol published by Wintermeyer et al. [17]. Multi-parameter analyses of stained cell suspensions were performed on an LSR II (Becton Dickinson) and analyzed with FlowJo software (Tree Star). The intrahepatic leukocytes were gated using SSC/FSC properties, DAPI– (to exclude dead cells), single cells population (to exclude doublets), CD45+ (to exclude non-hematopoietic cells). NK cells were identified as NK1.1+CD3e-. CD8+T cells were gated as NK1.1-CD3e+CD8+ while NKT cells were indicated as NK1.1+CD3e+ (Supplementary Fig. 1).
HSC isolation, culture and in vitro treatment
Mouse HSCs were isolated from WT and IL-15RαKO mice using an established method [50] with modifications. Briefly, the mouse liver was perfused in situ with pronase and collagenase, followed by Percoll (GE) density gradient centrifugation. HSC cultures were maintained in Dulbecco's Modified Eagle Medium with 20% Fetal Bovine Serum. Purity of HSC isolations was validated by expression of α-SMA (Supplementary Fig. 7A-B) and the absence of CD45 expression (Supplementary Fig. 7C). Purified HSC (first passage after isolation) were cultured for 48 hours in 6 well plates to achieve 80% confluence and treated for 48 hours with IL-15 (50 ng/mL), IL-15Rα (350 ng/mL), IL-15/IL-15Rα (50 ng/mL+350 ng/mL), or TGF-β (5 ng/mL).
Immunohistochemistry
To stain for α-SMA, paraffin-embedded liver sections were incubated with rabbit anti-α-SMA primary antibody (Abcam, 1:50) and visualized with Histostain Plus kit (Invitrogen).
Microarray analysis, normalization and data analysis
RNA was isolated from primary mouse hepatic stellate cells (Passage 0) with TRIzol reagent (Invitrogen) followed by a cleanup procedure using QIAGEN RNeasy mini columns according to manufacturer’s protocol. RNA samples were processed for hybridization on the MouseRef-8 v2.0 Expression Beadchip (Illumina) by the Genomics Core Facility of Mount Sinai School of Medicine. Raw data were taken from iScan Control Software (Illumina) and analyzed using Genomestudio Software (Illumina) with quantile normalization.
Statistics and analysis were performed on log normalized data using the module “Multiplot” found in GenePattern (www.broadinstitute.org/genepattern) [51]. The data set was filtered for transcripts with a coefficient of variation (CV) < 0.5 within population replicates (4 replicates each group). Molecular pathways associated with IL-15RαKO were surveyed by applying Gene Set Enrichment Analysis (GSEA) [52] implemented in GenePattern on Molecular Signature Database (MSigDB) (www.broadinstitute.org/msigdb).
Bioinformatics Analysis
Gene expression profiling datasets were obtained from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database (accession numbers: GSE 68001 and GSE 25097).
Statistical analysis
Results are presented as mean ±SE and were analyzed by Student’s t-test, Mann-Whiney U test or ANOVA analysis, as appropriate, using Prism 4 software (GraphPad Software, Inc.). P-values <0.05 were considered significant.
Supplementary Material
Lay Summary.
We investigated how a cellular protein, Interleukin-15 (IL-15), decreases the amount of scar tissue that is formed upon liver injury. We found that IL-15 and its receptor decreases the amount of scar tissue that is created by specialized liver cells (called stellate cells) and increases the number of a specific subgroup of immune cells (natural killer cells) that are known to eliminate stellate cells.
Acknowledgment
We are very grateful to Dr. Virginia Hernandez-Gea, Dr. Feng Hong, and Huazhi Han (PhD) for assistance in HSC isolation and purification, and Dr. Tung Ming Leung for AST and ALT measurement.
Financial Support: This work was supported by funds from NIH K08DK088954-01A1 (to CA); NIH DK56621 and 1K05AA018408-01 (to SLF); CA112100 and HL086899 (to MM); NIH/NIDDK (DK099558), Irma T. Hirschl Trust, Dr. Harold and Golden Lamport Research Award (to YH). This work was partially supported by funds from UTHealth Innovation in Cancer Prevention Research Training Program Post-doctoral Fellowship (Cancer Prevention and Research Institute of Texas grant # RP140103 (to JJ). Part of this work was presented in abstract form at the 2011 Annual Meeting of the American Association for the Study of Liver Diseases.
Transcript profiling accession number: GSE45612, GSE 68001 and GSE 25097.
List of abbreviations
- IL-15RαKO
IL-15Rα knockout
- IL-15
Interleukin-15
- CD8+T
cytotoxic T
- NK
natural killer
- NKT
natural killer T
- DC
dendritic cell
- BM
bone marrow
- HSC
hepatic stellate cell
- CCl4
carbon tetrachloride
- BDL
bile duct ligation
- BMT
Bone Marrow Transplantation
- WT
wild type
- α- SMA
alpha smooth muscle actin
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- RNA
Ribonucleic acid
- DAPI
4',6-Diamidino-2-Phenylindole
- MHC
major histocompatibility complex
- BrdU
Bromodeoxyuridine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare no conflict of interest.
Author contributions:
JJ: study concept and design, data acquisition, analysis and interpretation of data, drafting the manuscript, statistical analysis;
KO: study concept and design, data acquisition, analysis, interpretation of data, statistical analysis, drafting the manuscript;
HF: data acquisition and analysis, interpretation of data, drafting the manuscript;
MIF: analysis and interpretation of data;
AHR: analysis and interpretation of data, drafting the manuscript;
KK, YH and XC: analysis and interpretation of data;
TdP: data acquisition, analysis and interpretation of data;
DV: data acquisition, analysis and interpretation of data, technical support;
DS: data acquisition;
KHL: data acquisition;
YL: data acquisition;
MB: data acquisition
SLF: interpretation of data, drafting the manuscript, critical revision of the manuscript for important intellectual content, obtained funding, administrative support;
MM: interpretation of data, drafting the manuscript, critical revision of the manuscript for important intellectual content, obtained funding, technical and material support;
CA: study concept and design, data acquisition, analysis and interpretation of data, drafting the manuscript, statistical analysis, obtained funding, study supervision.
References
- [1].Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7:425–36. doi: 10.1038/nrgastro.2010.97. doi:10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- [2].Lim Y-S, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. 2008;12:733–46. doi: 10.1016/j.cld.2008.07.007. vii. doi:10.1016/j.cld.2008.07.007. [DOI] [PubMed] [Google Scholar]
- [3].Su Y-C, Lee C-C, Kung JT. Effector function-deficient memory CD8+ T cells clonally expand in the liver and give rise to peripheral memory CD8+ T cells. J Immunol. 2010;185:7498–506. doi: 10.4049/jimmunol.1002606. doi:10.4049/jimmunol.1002606. [DOI] [PubMed] [Google Scholar]
- [4].Correia MP, Cardoso EM, Pereira CF, Neves R, Uhrberg M, Arosa FA. Hepatocytes and IL-15: a favorable microenvironment for T cell survival and CD8+ T cell differentiation. J Immunol. 2009;182:6149–59. doi: 10.4049/jimmunol.0802470. doi:10.4049/jimmunol.0802470. [DOI] [PubMed] [Google Scholar]
- [5].Golden-Mason L, Kelly AM, Doherty DG, Traynor O, McEntee G, Kelly J, et al. Hepatic interleuklin 15 (IL-15) expression: implications for local NK/NKT cell homeostasis and development. Clin Exp Immunol. 2004;138:94–101. doi: 10.1111/j.1365-2249.2004.02586.x. doi:10.1111/j.1365-2249.2004.02586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. doi:10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Castillo EF, Acero LF, Stonier SW, Zhou D, Schluns KS. Thymic and peripheral microenvironments differentially mediate development and maturation of iNKT cells by IL-15 transpresentation. Blood. 2010;116:2494–503. doi: 10.1182/blood-2010-03-277103. doi:10.1182/blood-2010-03-277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ullrich E, Bonmort M, Mignot G, Jacobs B, Bosisio D, Sozzani S, et al. Trans-presentation of IL-15 dictates IFN-producing killer dendritic cells effector functions. J Immunol. 2008;180:7887–97. doi: 10.4049/jimmunol.180.12.7887. [DOI] [PubMed] [Google Scholar]
- [9].Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol Lett. 2010;127:85–92. doi: 10.1016/j.imlet.2009.09.009. doi:10.1016/j.imlet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Saxena V, Shivakumar P, Sabla G, Mourya R, Chougnet C, Bezerra JA. Dendritic cells regulate natural killer cell activation and epithelial injury in experimental biliary atresia. Sci Transl Med. 2011;3:102ra94. doi: 10.1126/scitranslmed.3002069. doi:10.1126/scitranslmed.3002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Suzuki A, McCall S, Choi SS, Sicklick JK, Huang J, Qi Y, et al. Interleukin-15 increases hepatic regenerative activity. J Hepatol. 2006;45:410–8. doi: 10.1016/j.jhep.2006.04.008. doi:10.1016/j.jhep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- [12].Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–29. doi: 10.1016/j.immuni.2006.11.011. doi:10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- [13].Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–69. doi: 10.1053/j.gastro.2008.03.003. doi:10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Melhem A, Muhanna N, Bishara A, Alvarez CE, Ilan Y, Bishara T, et al. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol. 2006;45:60–71. doi: 10.1016/j.jhep.2005.12.025. doi:10.1016/j.jhep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- [15].Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–52. doi: 10.1053/j.gastro.2005.10.055. doi:10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- [16].Park O, Jeong W-I, Wang L, Wang H, Lian Z-X, Gershwin ME, et al. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–94. doi: 10.1002/hep.22813. doi:10.1002/hep.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wintermeyer P, Cheng C-W, Gehring S, Hoffman BL, Holub M, Brossay L, et al. Invariant natural killer T cells suppress the neutrophil inflammatory response in a mouse model of cholestatic liver damage. Gastroenterology. 2009;136:1048–59. doi: 10.1053/j.gastro.2008.10.027. doi:10.1053/j.gastro.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Safadi R, Ohta M, Alvarez CE, Fiel MI, Bansal M, Mehal WZ, et al. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology. 2004;127:870–82. doi: 10.1053/j.gastro.2004.04.062. [DOI] [PubMed] [Google Scholar]
- [19].Holt AP, Stamataki Z, Adams DH. Attenuated liver fibrosis in the absence of B cells. Hepatology. 2006;43:868–71. doi: 10.1002/hep.21155. doi:10.1002/hep.21155. [DOI] [PubMed] [Google Scholar]
- [20].Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. doi:10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jiao J, Sastre D, Fiel MI, Lee UE, Ghiassi-Nejad Z, Ginhoux F, et al. Dendritic cell regulation of carbon tetrachloride-induced murine liver fibrosis regression. Hepatology. 2012;55:244–55. doi: 10.1002/hep.24621. doi:10.1002/hep.24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jeong W-I, Park O, Radaeva S, Gao B. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology. 2006;44:1441–51. doi: 10.1002/hep.21419. doi:10.1002/hep.21419. [DOI] [PubMed] [Google Scholar]
- [23].Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- [24].Liu K, Catalfamo M, Li Y, Henkart PA, Weng N. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc Natl Acad Sci USA. 2002;99:6192–7. doi: 10.1073/pnas.092675799. doi:10.1073/pnas.092675799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ghosh AK. Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Exp Biol Med (Maywood) 2002;227:301–14. doi: 10.1177/153537020222700502. [DOI] [PubMed] [Google Scholar]
- [26].Datta PK, Moses HL. STRAP and Smad7 synergize in the inhibition of transforming growth factor beta signaling. Mol Cell Biol. 2000;20:3157–67. doi: 10.1128/mcb.20.9.3157-3167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vukmirovic M, Manojlovic Z, Stefanovic B. Serine-threonine kinase receptor-associated protein (STRAP) regulates translation of type I collagen mRNAs. Mol Cell Biol. 2013;33:3893–906. doi: 10.1128/MCB.00195-13. doi:10.1128/MCB.00195-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hong F, Tuyama A, Lee TF, Loke J, Agarwal R, Cheng X, et al. Hepatic stellate cells express functional CXCR4: role in stromal cell-derived factor-1alpha-mediated stellate cell activation. Hepatology. 2009;49:2055–67. doi: 10.1002/hep.22890. doi:10.1002/hep.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xu L, Hui AY, Albanis E, Arthur MJ, O’Byrne SM, Blaner WS, et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–51. doi: 10.1136/gut.2004.042127. doi:10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. doi:10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- [31].Gur C, Doron S, Kfir-Erenfeld S, Horwitz E, Abu-Tair L, Safadi R, et al. NKp46-mediated killing of human and mouse hepatic stellate cells attenuates liver fibrosis. Gut. 2012;61:885–93. doi: 10.1136/gutjnl-2011-301400. doi:10.1136/gutjnl-2011-301400. [DOI] [PubMed] [Google Scholar]
- [32].Pek EA, Chan T, Reid S, Ashkar AA. Characterization and IL-15 dependence of NK cells in humanized mice. Immunobiology. 2011;216:218–24. doi: 10.1016/j.imbio.2010.04.008. doi:10.1016/j.imbio.2010.04.008. [DOI] [PubMed] [Google Scholar]
- [33].Arina A, Murillo O, Dubrot J, Azpilikueta A, Gabari I, Perez-Gracia JL, et al. Interleukin-15 liver gene transfer increases the number and function of IKDCs and NK cells. Gene Ther. 2008;15:473–83. doi: 10.1038/gt.2008.4. doi:10.1038/gt.2008.4. [DOI] [PubMed] [Google Scholar]
- [34].Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrançois L, Aguila HL. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proc Natl Acad Sci USA. 2004;101:5616–21. doi: 10.1073/pnas.0307442101. doi:10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huntington ND, Puthalakath H, Gunn P, Naik E, Michalak EM, Smyth MJ, et al. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat Immunol. 2007;8:856–63. doi: 10.1038/ni1487. doi:10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zheng X, Wang Y, Wei H, Ling B, Sun R, Tian Z. Bcl-xL is associated with the anti-apoptotic effect of IL-15 on the survival of CD56(dim) natural killer cells. Mol Immunol. 2008;45:2559–69. doi: 10.1016/j.molimm.2008.01.001. doi:10.1016/j.molimm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- [37].Malamut G, El Machhour R, Montcuquet N, Martin-Lannerée S, Dusanter-Fourt I, Verkarre V, et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J Clin Invest. 2010;120:2131–43. doi: 10.1172/JCI41344. doi:10.1172/JCI41344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zou Y, Chen T, Han M, Wang H, Yan W, Song G, et al. Increased killing of liver NK cells by Fas/Fas ligand and NKG2D/NKG2D ligand contributes to hepatocyte necrosis in virus-induced liver failure. J Immunol. 2010;184:466–75. doi: 10.4049/jimmunol.0900687. doi:10.4049/jimmunol.0900687. [DOI] [PubMed] [Google Scholar]
- [39].Kennedy NJ, Russell JQ, Michail N, Budd RC. Liver damage by infiltrating CD8+ T cells is Fas dependent. J Immunol. 2001;167:6654–62. doi: 10.4049/jimmunol.167.11.6654. [DOI] [PubMed] [Google Scholar]
- [40].Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci USA. 2000;97:5498–503. doi: 10.1073/pnas.040566697. doi:10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Boyacioğlu S, Gür G, Yilmaz U, Korkmaz M, Demirhan B, Bilezikçi B, et al. Investigation of possible clinical and laboratory predictors of liver fibrosis in hemodialysis patients infected with hepatitis C virus. Transplant Proc. 2004;36:50–2. doi: 10.1016/j.transproceed.2003.11.066. doi:10.1016/j.transproceed.2003.11.066. [DOI] [PubMed] [Google Scholar]
- [42].Uslusoy HS, Nak SG, Gülten M. Noninvasive predictors for liver fibrosis in patients with nonalcoholic steatohepatitis. World J Hepatol. 2011;3:219–27. doi: 10.4254/wjh.v3.i8.219. doi:10.4254/wjh.v3.i8.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wuttge DM, Wildt M, Scheja A, Westergren-Thorsson G. Interleukin-15 attenuates transforming growth factor-β1-induced myofibroblast differentiation in human fetal lung fibroblasts. Eur Cytokine Netw. 2010;21:165–76. doi: 10.1684/ecn.2010.0202. doi:10.1684/ecn.2010.0202. [DOI] [PubMed] [Google Scholar]
- [44].Wuttge DM, Wildt M, Geborek P, Wollheim FA, Scheja A, Akesson A. Serum IL-15 in patients with early systemic sclerosis: a potential novel marker of lung disease. Arthritis Res Ther. 2007;9:R85. doi: 10.1186/ar2284. doi:10.1186/ar2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bigalke B, Schwimmbeck PL, Haas CS, Lindemann S. Effect of interleukin-15 on the course of myocarditis in Coxsackievirus B3-infected BALB/c mice. Can J Cardiol. 2009;25:e248–54. doi: 10.1016/s0828-282x(09)70511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chang C-M, Lo C-H, Shih Y-M, Chen Y, Wu P-Y, Tsuneyama K, et al. Treatment of hepatocellular carcinoma with adeno-associated virus encoding interleukin-15 superagonist. Hum Gene Ther. 2010;21:611–21. doi: 10.1089/hum.2009.187. doi:10.1089/hum.2009.187. [DOI] [PubMed] [Google Scholar]
- [47].Bessard A, Solé V, Bouchaud G, Quéméner A, Jacques Y. High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. Mol Cancer Ther. 2009;8:2736–45. doi: 10.1158/1535-7163.MCT-09-0275. doi:10.1158/1535-7163.MCT-09-0275. [DOI] [PubMed] [Google Scholar]
- [48].Kishida T, Asada H, Itokawa Y, Cui F-D, Shin-Ya M, Gojo S, et al. Interleukin (IL)-21 and IL-15 genetic transfer synergistically augments therapeutic antitumor immunity and promotes regression of metastatic lymphoma. Mol Ther. 2003;8:552–8. doi: 10.1016/s1525-0016(03)00222-3. [DOI] [PubMed] [Google Scholar]
- [49].Kirkland JG, Godfrey CB, Garrett R, Kakar S, Yeh BM, Corvera CU. Reversible surgical model of biliary inflammation and obstructive jaundice in mice. J Surg Res. 2010;164:221–7. doi: 10.1016/j.jss.2009.08.010. doi:10.1016/j.jss.2009.08.010. [DOI] [PubMed] [Google Scholar]
- [50].Friedman SL, Roll FJ. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal Biochem. 1987;161:207–18. doi: 10.1016/0003-2697(87)90673-7. [DOI] [PubMed] [Google Scholar]
- [51].Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nat Genet. 2006;38:500–1. doi: 10.1038/ng0506-500. doi:10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- [52].Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. doi:10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.