Abstract
Microglia-mediated clearance of amyloid beta-protein (Aβ) via Toll-like receptor 4 (TLR4) signaling may play an important role in the pathogenesis of Alzheimer’s disease (AD). However, as the disease progresses, activated microglia appear to become incapable of clearing Aβ deposits. Because repeated exposure to a TLR4 ligand leads to a diminished response of monocytes/macrophages to lipopolysaccharide (LPS) and because aggregated Aβ is a TLR4 ligand, we hypothesize that chronic exposure of microglia to Aβ deposits may induce a state of Toll-like receptor (TLR) signaling dysfunction, leading to decreased Aβ clearance and accelerated disease progression. LPS or phosphate-buffered saline (PBS) was injected into the hippocampus of AD-model (TgAPP/PS1) and wild-type (non-Tg) mice before and after the onset of Aβ deposition, at age 2 and 12 months, respectively. Brain specimens were collected 7 days post-injection and analyzed for microglial activation and Aβ load. While LPS-injected 2-month-old non-Tg mice showed 48-fold and 11-fold greater Iba1 immunoreactivity in the neocortex and hippocampus, respectively, compared with PBS-injected mice, LPS-injected 2-month-old TgAPP/PS1 mice had 61-fold and 13-fold increases in the neocortex and hippocampus, respectively. LPS injection activated microglia more strongly in TgAPP/PS1 mice than in non-Tg mice at 2 months of age. In contrast, at 12 months of age, Iba1 immunoreactivity of microglia was increased 541-fold and 38-fold in the neocortex and hippocampus, respectively, in LPS-injected non-Tg mice and 2.7-fold and 3.3-fold in the neocortex and hippocampus, respectively, in LPS-injected TgAPP/PS1 mice. Surprisingly, LPS injection decreased CD45 immunoreactivity in TgAPP/PS1 mice but increased it in non-Tg mice at 12 months. Although microglia in 12-month-old non-Tg mice showed stronger response to LPS than 2-month-old non-Tg mice, microglia in TgAPP/PS1 mice exhibited diminished immune response to LPS during aging. Our data indicate that microglial TLR4 signaling is altered in an AD mouse model and suggest that altered TLR4 signaling may contribute to Aβ accumulation in the brain.
Keywords: Alzheimer’s disease, microglia, amyloid, Toll-like receptor, animal model, lipopolysaccharide
1. Introduction
Recent genome-wide association studies (GWAS) on late-onset Alzheimer’s disease (AD) patients have identified approximately 10 genetic risk variants involved in immune/inflammatory responses, highlighting the importance of immune responses in the pathogenesis of AD [1]. Particularly, neuroinflammation in response to amyloid beta-protein (Aβ) accumulation contributes significantly to AD pathogenesis with activated microglia playing a prominent role [2]. Microglia can either clear Aβ and improve neuronal function or cause excess inflammation and damage neurons [3]. Growing evidence suggests that microglia-mediated clearance of Aβ depends on TLR signaling [4–6]. Certain TLR ligands such as lipopolysaccharide (LPS) or peptidoglycan are capable of modulating levels of inflammatory molecules and microglial uptake of Aβ in culture [5, 7]. Furthermore, TLR4-mutant AD-model mice accumulate more Aβ and exhibit more cognitive deficits as compared to their TLR4-wild-type counterparts [6], suggesting that the innate immune response is one of the body’s major defenses against AD. LPS, a TLR4 ligand, is normally a potent inducer of inflammation and septic shock in infected organisms. However, repeated exposure to LPS can result in altered TLR4 signaling or endotoxin/LPS tolerance [8]. Because Aβ aggregates activate microglia via TLR4 [9], it is possible that chronic exposure of microglia to Aβ induces alteration in TLR4 signaling. Early in AD pathogenesis, activated microglia still have the capability to clear Aβ deposits; later on however, signaling pathways in microglia may become altered, resulting in decreased Aβ clearance and accelerated disease progression [10]. Here we investigate the extent of microglial alteration in response to LPS in young mice (2 months) when Aβ deposition is absent and in old mice (12 months) after microglia have been exposed to extracellular Aβ deposition for several months.
2. Materials and Methods
2.1 Animals
A congenic C57BL/6J line of AD model mice, B6.Cg-Tg(APPswe,PSEN1dE9) 85Dbo/J strain (here referred to as TgAPP/PS1 mice), was purchased from Jackson Laboratory (Bar Harbor, ME) and maintained by crossing transgenic males with C57BL/6 females. TgAPP/PS1 mice possess a chimeric mouse/human amyloid precursor protein (APP) with the double mutations (K670N and M671L) and a humanized Aβ sequence. They also express human presenilin 1 (PS1) with an exon 9 deletion found in familial AD patients. At about 5 months TgAPP/PS1 mice begin to develop Aβ deposits. Non-transgenic C57BL/6 littermates (non-Tg mice) were used as controls. Brain specimens from males were used for histochemistry and immunohistochemistry (n = 5–8) while those from females were used for ELISA (n = 5) due to the difference in brain amyloid content between male and female TgAPP/PS1 mice [11, 12]. The animal protocols described here were prospectively approved by the Institutional Animal Care and Use Committee of the University of Illinois College of Medicine at Peoria.
2.2 Intrahippocampal injections and tissue preparation
This procedure was previously described in detail [13]. Briefly, mice were divided into treatment groups receiving LPS and control groups receiving phosphate-buffered saline (PBS). To test the influence of LPS on younger mice, 2-month-old mice received one single injection of 4µg LPS in 1µL volume into their right hippocampus. To test the effects of LPS on older mice, 12-month-old mice received one single injection of 2µg LPS in 1µL volume due to decreased survival with the original higher LPS dose (4µg). The same volume of PBS was given to control mice. The stereotaxic coordinates for the injection were 2.5 mm posterior to the bregma, 2.25 mm right from the midline, and 2.25 mm ventral. Experimental mice were allowed to recover and were terminated 7 days after injection due to prior studies showing that astrocyte and microglia responses to LPS stimulation peak at about 7 days [13]. Cerebrospinal fluid samples were collected prior to termination.
Males were deeply anesthetized with pentobarbital and then perfused transcardially with 4 % paraformaldehyde solution. Brains were removed and cryoprotected by sequentially increasing concentrations of sucrose solution. The left and right halves were separately frozen in Tissue-Tek optimal cutting temperature compound. The brains of female mice were harvested after euthanization and the right hemispheres were dounce-homogenized in a Bio-Plex cell lysis kit (Bio-Rad Laboratories, Hercules, CA) with added protease inhibitor and phenylmethane sulfonyl fluoride. Homogenates were then separated into soluble and insoluble fractions by sonication on ice for 1.5 minutes and centrifugation at a speed of 16,000xg for 30 minutes at 4 °C.
2.3 Quantification of Aβ and activated microglia by immunohistochemistry and histochemistry with histomorphometry
Male brain specimens were frozen and cut into 35µm sections by a microtome and stored in 10 % PBS + 1 % NaN3. As previously described [6], coronal brain sections were stained for Aβ load with MOAB-2 and microglial activity with Iba1 (1µg/ml) and CD45 (5µg/ml) using the avidin-biotin-peroxidase method (Vectastatin ABC Kit, Vector, Burlingame, CA). MOAB-2 antibody was generously provided by the laboratory of Dr. Mary Jo LaDu. Iba1 and CD45 antibodies were obtained from Abcam (Cambridge, MA) and Serotec (Raleigh, NC), respectively. Thioflavin S was used to identify fibrillar Aβ by fluorescence.
For each mouse, 3 coronal brain sections within 1 mm from the injection site, each section separated by >300µm intervals, were imaged using an Olympus BX61 automated microscope and Olympus Fluoview system and then analyzed using Image Pro Plus v5.1 image analysis software (MediaCybernetics, Silver Spring, MD) capable of color segmentation and automation via programmable macros. Both neocortex and hippocampus were found in all the brain sections and analyzed separately. Background staining was excluded from morphometric analysis, that is, only pixels darker than the uniform background staining across large areas were included in measurements. Stained area was expressed as a percentage of total area.
2.4 Quantification of protein and Aβ by ELISA
Protein concentrations of the right brain supernatant and cerebrospinal fluid samples of female mice were determined beforehand so that a standardized amount of protein could be added to each well. A commercial ELISA kit (Invitrogen, Carlsbad, CA) was used to quantify Aβ40 and Aβ42 levels in the supernatants and cerebrospinal fluid samples according to manufacturer’s instructions.
2.5 Statistical analysis
SPSS version 19 was used for analysis. Each outcome variable was tested for normality. Log transformations were performed for outcome variables that were not normally distributed. Spearman or Pearson correlations of the predictor and outcome variables were inspected and general linear models were used to test the differences for Aβ load (using the markers MOAB-2 and thioflavin S) and inflammation (using the markers Iba1 and CD45). Two-tailed student’s t-tests were utilized to generate p values for comparisons involving one predictor and one outcome variable. A significance level of p≤ 0.05 was accepted as significant.
3. Results
3.1 LPS injection did not induce Aβ deposition in the brain at age 2 months and decreased Aβ deposits in the hippocampus at 12 months in an AD mouse model
Previous studies reported that LPS injection prematurely induced thioflavin-positive Aβ deposition [14] or accumulation of intracellular Aβ and C-terminal APP fragments [15, 16] in AD mouse models. However, most antibodies against Aβ react with APP and its Aβ-bearing APP-derivatives. In order to avoid such potential confounders, the MOAB-2 antibody that specifically reacts with Aβ but not with APP or C-terminal APP fragments was used [17]. As was the case with the PBS injection, Aβ accumulation was not discernible by MOAB-2 immunohistochemistry in the brains of 2-month-old TgAPP/PS1 and non-Tg mice 7 days after intra-hippocampal injection of LPS (data not shown). Thioflavin staining failed to detect Aβ fibril formation after LPS injection, also (data not shown).
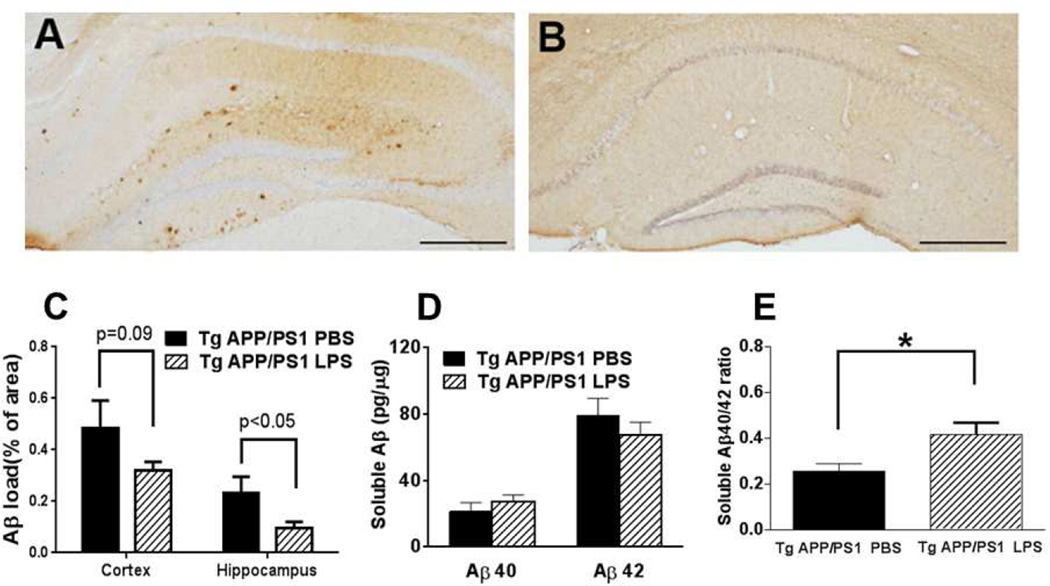
When two 12-month-old non-Tg mice were initially subjected to 4µg LPS injection, they did not survive 4 days past the procedure. Although two 12-month-old TgAPP/PS1 mice survived 7 days after 4µg LPS injection, one did not. The brains from these mice were not analyzed for this study. As a result, 12-month-old mice assigned to LPS treatment received 2µg LPS injection for this study. LPS injection into the right hippocampus decreased local Aβ load in 12-month-old TgAPP/PS1 mice within 7 days post-injection as seen by MOAB-2 immunostaining in coronal brain slices (Fig. 1A-C). The reduction in MOAB-2 staining was significant in the hippocampus (P = 0.038). There was a trend toward decreased MOAB-2 immunoreactivity in the neocortex (P = 0.096) with the majority of Aβ clearance occurring at the cortical site near the LPS injection. Thus, the effect of LPS on Aβ clearance was most striking near the injection site. While total amyloid on average was decreased by LPS injection, thioflavin S immunofluorescence was not significantly changed (data not shown), indicating that LPS injection stimulates clearance of mainly diffuse rather than fibrillar Aβ. This is consistent with previously published results [13, 18].
Fig. 1. Intra-hippocampal LPS injection decreases immunereactive Aβ deposits in the hippocampus and increases cerebral buffer-soluble Aβ40/42 ratio in 12-month-old TgAPP/PS1 mice.
A-C, Twelve-month-old TgAPP/PS1 mice were euthanized 7 days after LPS or PBS (control) injection into the right hippocampus. Immunohistochemistry was performed using MOAB-2 antibody to visualize Aβ deposits. As compared with the PBS group (A), Aβ deposits were decreased in the brains of LPS-treated mice (B). Average percentages of stained areas are shown as a bar graph in (C). D, E, The buffer-soluble Aβ40 and Aβ42 contents in the right cerebra of TgAPP/PS1 mice were quantified by ELISA at age 12 months. While the individual amounts of soluble Aβ40 and 42 did not differ significantly between treatment groups (D), the ratio of Aβ40/42 was significantly increased in the brains of mice treated with LPS (E). The scale bars in the graph indicate 250µm. (means + SEM), *P < 0.05.
Buffer-soluble Aβ40 and Aβ42 levels in 12-month-old TgAPP/PS1 mice were determined by ELISA. While LPS injection did not significantly alter Aβ40 (21.34 ± 5.36 and 27.53 ± 3.80 pg/µg protein for PBS and LPS injection, respectively) and Aβ42 levels (79.48 ± 10.18 and 67.40 ± 7.88 pg/µg protein for PBS and LPS injection, respectively) in the soluble fraction of right cerebral homogenates (Fig. 1D), it did significantly increase the Aβ40/42 ratio (0.258 ± 0.032 and 0.415 ± 0.053 for PBS and LPS injection, respectively, Fig. 1E; P = 0.050). This suggests that LPS stimulation of the TLR4 pathway increases clearance of the more pathogenic Aβ42 species of amyloid. There was no significant difference in Aβ40, Aβ42, or ratio in the cerebrospinal fluid (data not shown), indicating that cerebrospinal fluid clearance is not the major mechanism for alteration of buffer-soluble Aβ40/42 ratio in the brain.
3.2 Microglial activation by LPS injection was dampened in the presence of Aβ
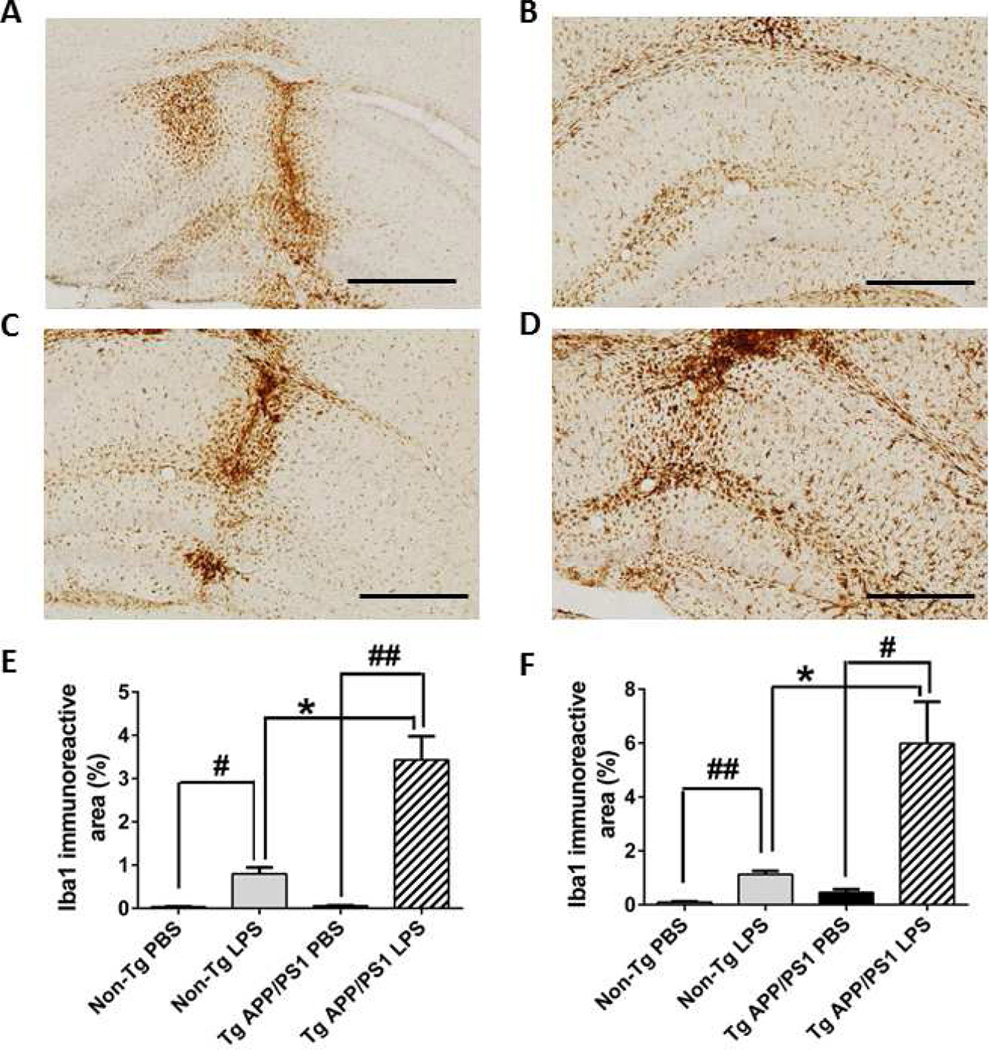
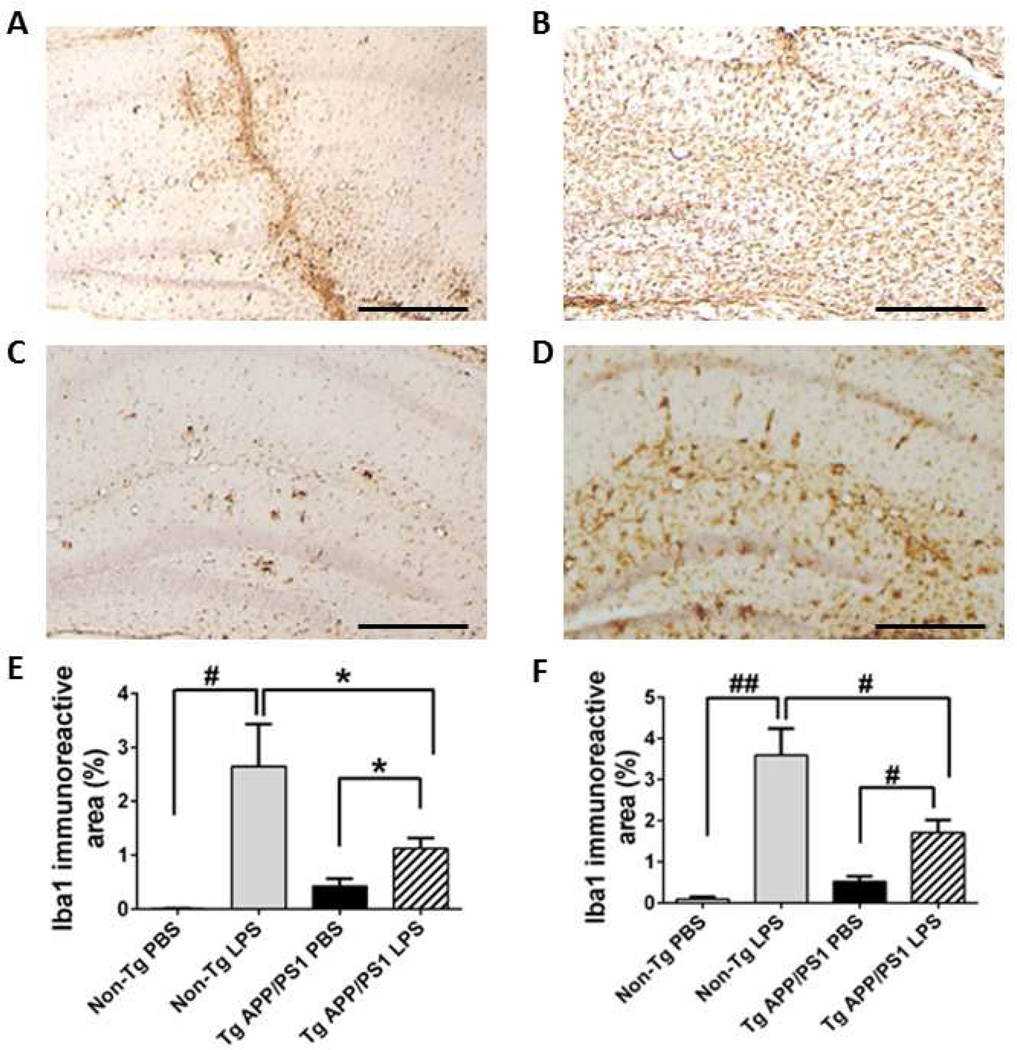
A previous study found that a significant increase of microglial activation occurred 3 days after intrahippocampal LPS injection and the activity assessed by microglial markers (CD45 and Fcγ receptor) reached the peak on day 7 in an AD mouse model (Tg2576 mice) [13]. Therefore, we determined levels of microglial markers in our experimental animals 7 days after intrahippocampal LPS injection. As expected, LPS injection increased Iba1 expression in microglia/monocytes in non-Tg and TgAPP/PS1 mice at 2 and 12 months when compared to PBS-injected controls (Fig. 2 and 3). In the absence of Aβ pathology in non-Tg mice, LPS injection elicited abundant Iba1 expression at 2 and 12 months compared to PBS injection: 48-fold (P < 0.01) and 11-fold (P < 0.001) increases in Iba1 immunoreactive neocortical and hippocampal areas, respectively, at 2 months (Fig. 2E and F) and 541-fold (P < 0.01) and 38-fold (P < 0.001) increases in the neocortex and hippocampus, respectively, at 12 months (Fig. 3E and F). However, in the presence of Aβ deposition in Tg-APP/PS1 mice at 12 months, there was a clearly blunted increase in Iba1 immunoreactivity in LPS-compared to PBS-treated mice: 61-fold (P < 0.001) and 13-fold (P < 0.01) increases in the neocortex and hippocampus, respectively, at 2 months (Fig. 2E and F) and only 2.7-fold (P < 0.05) and 3.3-fold (P < 0.01) increases in the neocortex and hippocampus, respectively, at 12 months (Fig. 3E, F). Thus, enhancement of Iba1 immunoreactivity by LPS injection was greater in 2-month-old TgAPP/PS1 mice than in their 12-month-old counterparts, suggesting dampened response to LPS in mice chronically exposed to Aβ deposits (Fig. 2 and 3; P < 0.05). Although enhancement of LPS-induced Iba1 immunoreactivity at 12-months (541-fold increase in the neocortex) was greater than that in 2-months (48-fold) in non-Tg mice in spite of a lower LPS dose at 12 months, TgAPP/PS1 mice appeared hypersensitive to LPS before Aβ deposition at 2 months (61-fold in the neocortex) (Fig. 2E and F, P < 0.05) but then hyposensitive to LPS after Aβ deposition occurred at 12 months (2.7-fold) (Fig. 3E and F, P <0.05).
Fig. 2. LPS injection activates microglia.
A-D, Activated microglia were immunostained with Iba1 antibody 7 days after LPS (B, D) or PBS (A, C) injection into TgAPP/PS1 (C, D) and non-Tg (A, B) mice at 2 months of age. E, F, Average percentages of stained areas in the neocortex (E) and hippocampus (F) are shown as a bar graph (means + SEM). LPS injection induced much stronger Iba1 expression in TgAPP/PS1 mice than in non-Tg mice (E, F). The scale bars in the graph indicate 250µm. *P < 0.05, #P < 0.01, and ##P < 0.001.
Fig. 3. Aβ deposition alters Iba1 expression in microglia.
A-D Activated Iba1-positive microglia were visualized in brain sections at the injection site from TgAPP/PS1 (C,D) and non-Tg (A,B) mice at age 12 months. While the TgAPP/PS1 mice injected with PBS (C) exhibit Iba1 immunoreactivity presumably associated with Aβ deposits, Iba1 immunoreactivity in non-Tg mice treated with PBS (A) is mostly found along the needle track. Compared to their non-Tg counterparts (B), TgAPP/PS1 mice (D) injected with LPS are unable to achieve the same level of microglial activation. E, F, Average percentages of Iba1-immunoreactive areas in the neocortex (E) and hippocampus (F) are shown as a bar graph (means + SEM). The scale bars in the graph indicate 250µm. *P < 0.05, #P < 0.01, and ##P < 0.001.
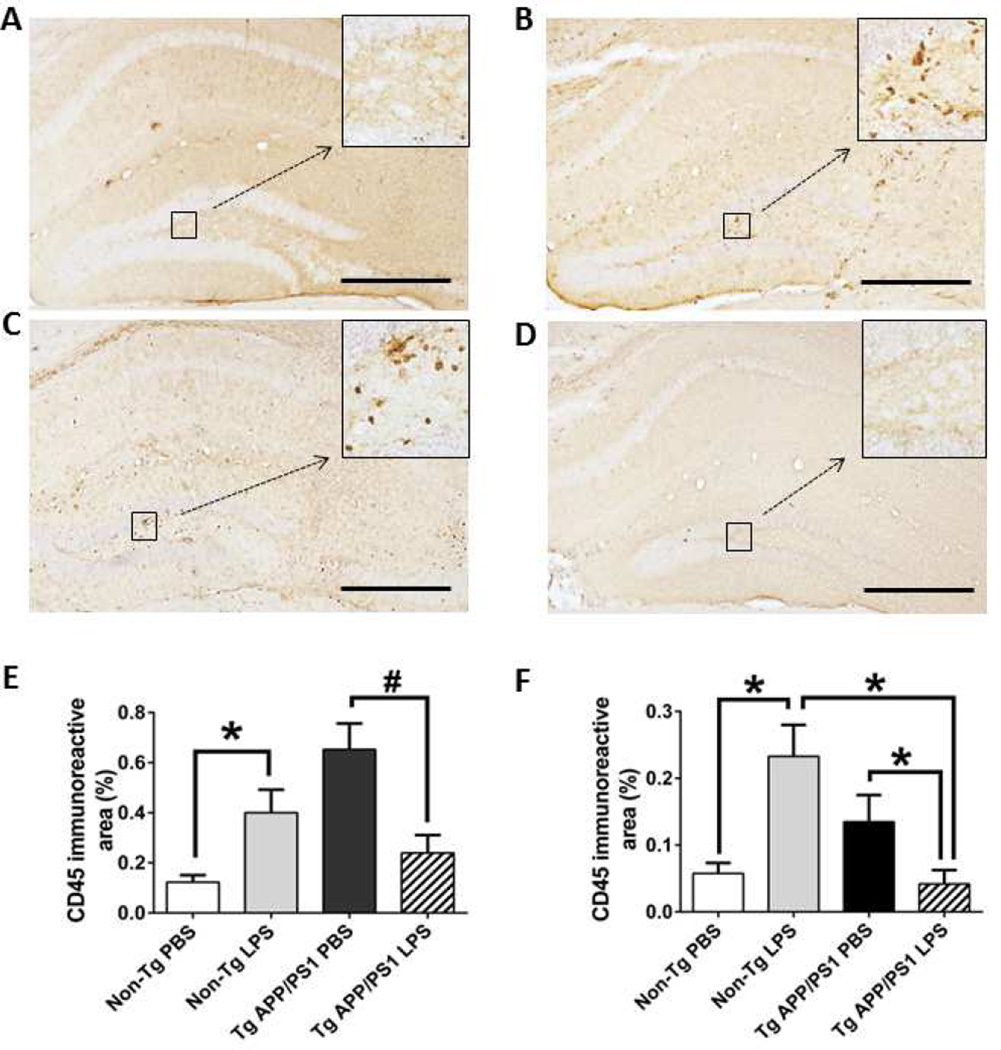
As expected, LPS injection increased CD45 expression in the neocortex (P = 0.033) and hippocampus (P = 0.014) compared to PBS injection in 12-month-old non-Tg mice (Fig. 4A,B,E and F). While TgAPP/PS1 mice injected with PBS exhibited discretely clustered CD45 staining (Fig. 4C), LPS injection decreased CD45 staining compared to PBS injection in TgAPP/PS1 mice at 12 months (Fig. 4C-F: P = 0.004 for neocortex and P = 0.036 for hippocampus). LPS injection induced more CD45 expression in non-Tg mice than in TgAPP/PS1 mice at 12 months.
Fig. 4. Aβ deposition alters CD45 expression in microglia.
A-D, Activated CD45-positive microglia were visualized in brain sections from TgAPP/PS1 (C, D) and non-Tg (A, B) mice at 12 months. Increased CD45 expression can be seen 7 days after LPS injection in non-Tg mice (B) compared to PBS-injected non-Tg mice (A). TgAPP/PS1 mice injected with PBS exhibit discretely clustered CD45 expression (C). In contrast, TgAPP/PS1 mice injected with LPS exhibit only a low level of CD45 staining (D). The inset images (A through D) are a higher magnification of the areas indicated by the squares. E,F, Average percentages of CD45-immunoreactive areas in the neocortex (E) and hippocampus (F) are shown as a bar graph (means + SEM). The scale bars in the graph indicate 250µm. *P < 0.05, #P < 0.01.
4. Discussion
We have demonstrated that microglial responses to LPS increased in wild-type mice during aging but decreased in TgAPP/PS1 mice. LPS activated more microglia in young TgAPP/PS1 mice without Aβ deposition than in young wild-type mice but activated less microglia in old TgAPP/PS1 mice with Aβ deposition than in old wild-type mice. Thus, TLR4 signaling is altered in TgAPP/PS1 mice, demonstrating the remarkable contrast in TLR4 signaling between wild-type and TgAPP/PS1 mice as well as before and after Aβ deposition in the brain.
Although the previous studies found intracellular accumulation of Aβ by 4G8 antibody after LPS injection in APPs we transgenic mice [15, 16], we were unable to detect such Aβ-immunoreactivity by MOAB-2 antibody and thioflavin positivity in our AD mouse model at 2 months even after LPS injection. Because most antibodies against Aβ including 4G8 antibody also react with cellular APP and its derivatives and because MOAB-2 antibody does not react with cellular APP and its C-terminal fragments, we used MOAB-2 antibody in order to circumvent potential staining of cellular non-Aβ components. However, it is possible that minuscule amounts of soluble Aβ oligomers may already exist even at 2 months and directly or indirectly prime microglia [19, 20] because super-low-dose LPS can prime monocytes [21, 22]. Consistent with this notion, LPS injection induced stronger microglial activation in TgAPP/PS1 mice than in non-Tg mice at 2 months of age. On the other hand, the results presented here from 12-month-old TgAPP/PS1 mice are consistent with the findings of other groups showing that intrahippocampal LPS injection reduces diffuse but not fibrillar Aβ deposits [13, 18]. While prior studies have investigated LPS stimulation in AD mouse models [3], our study is the first to demonstrate that microglia in TgAPP/PS1 mice with Aβ deposition are less responsive to LPS stimulation than those in non-Tg control mice when the degrees of microglial activation are assessed by Iba1 and CD45 immunoreactivity.
The basal inflammatory response increase with age and most aged tissues including the brain are characterized by low-level chronic inflammation [23]. Increasing lines of evidence indicate that aged microglia develop an increased pro-inflammatory phenotype (primed) characterized by a lower threshold to inflammatory stimuli and an exaggerated inflammatory response to them [24]. In line with this concept, inflammatory responses to LPS treatment in old non-Tg mice were stronger than those in young non-Tg mice when assessed by microglial Iba1 staining. However, microglia in old-TgAPP/PS1 mice showed decreased Iba1 staining in response to LPS treatment. Thus, the activation of TLR4 signaling by LPS is diminished in old TgAPP/PS1 mice with numerous Aβ deposits. Because LPS can stimulate Aβ clearance by microglia in vitro [5, 7] and in vivo [13, 18], as Aβ deposits accumulate in the brain, diminished TLR4 signaling may compromise Aβ clearance by microglia and further promote Aβ accumulation and neurodegeneration. The molecular mechanism by which Aβ deposition induces diminished TLR4 signaling in microglia remains to be determined.
Although altered TLR signaling seems to occur during the progression of AD, these results show that Aβ clearance can still be optimized through the activation of the TLR4 signaling pathway in microglia with LPS. Super-stimulation with LPS clears mostly diffuse Aβ deposits. It is unclear whether this effect would be clinically beneficial, and the results should be correlated with behavioral and functional studies. A promising LPS derivative, monophosphoryl lipid A, was peripherally administered to an AD mouse model and was shown to effectively activate microglia to clear Aβ through a minimally inflammatory pathway and improve cognitive function [25]. Additionally, certain CpG oligodeoxynucleotides, TLR9 ligands, were shown to ameliorate Aβ and tau pathology and cognitive deficits in AD mouse models [26, 27]. Although the pathway for increased Aβ clearance in response to such TLR ligands is unknown, these are exciting findings that support the use of TLR agonists, especially those with favorable side effect profiles, as potential therapy for AD. Our findings are concordant with the concept of altered TLR signaling in the AD pathogenesis.
The link between inflammation and AD has been studied for decades [28, 29]. Recently, major advances in the areas of immunology and genetics have accumulated more evidence supporting the central role of immune modulation in AD pathogenesis and its potential therapies [1]. Accordingly, it is possible that altered TLR4 signaling may implicate genetic risk variants of AD in the pathogenesis. Indeed, inositol polyphosphate-5-phosphatase D (INPP5D) suppresses TLR4-mediated LPS responses [30, 31] and its gene is one of AD risk genes identified by GWAS. Further studies are needed to investigate the possible involvement of AD risk genes in the development of altered TLR4 signaling in microglia during AD progression.
Supplementary Material
Highlights.
Microglia are hypersensitive to LPS before Aβ deposition in Alzheimer model mice
Microglia become hyposensitive to LPS after Aβ deposition in Alzheimer model mice
Microglia become hypersensitive to LPS during aging in wild-type control mice
Microglial TLR4 signaling is altered in Alzheimer model mice
Acknowledgments
We thank Dr. Mary Jo LaDu, University of Illinois at Chicago for donation of MOAB-2 antibody, Danielle Nelson and Tad Maguire for laboratory assistance, and Linda Walter for assistance in manuscript preparation. This research was supported in part by National Institutes of Health grants AG030399 and AG042082.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reitz C. Genetic diagnosis and prognosis of Alzheimer's disease: challenges and opportunities. Expert Rev Mol Diagn. 2015;15:339–348. doi: 10.1586/14737159.2015.1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 3.Morgan D, Gordon MN, Tan J, et al. Dynamic complexity of the microglial activation response in transgenic models of amyloid deposition: implications for Alzheimer therapeutics. J Neuropathol Exp Neurol. 2005;64:743–753. doi: 10.1097/01.jnen.0000178444.33972.e0. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Liu Y, Hao W, et al. TLR2 is a primary receptor for Alzheimer's amyloid beta peptide to trigger neuroinflammatory activation. J Immunol. 2012;188:1098–1107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- 5.Tahara K, Kim HD, Jin JJ, et al. Role of toll-like receptor signalling in A(beta) uptake and clearance. Brain. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song M, Jin J, Lim JE, et al. TLR4 mutation reduces microglial activation, increases Abeta deposits and exacerbates cognitive deficits in a mouse model of Alzheimer's disease. J Neuroinflammation. 2011;8:92. doi: 10.1186/1742-2094-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen K, Iribarren P, Hu J, et al. Activation of toll-like receptor 2 on microglia promotes cell up-take of Alzheimer's disease-associated amyloid beta peptide. J Biol Chem. 2006;281:3651–3659. doi: 10.1074/jbc.M508125200. [DOI] [PubMed] [Google Scholar]
- 8.Broad A, Jones DE, Kirby JA. Toll-like receptor (TLR) response tolerance: a key physiological "damage limitation" effect and an important potential opportunity for therapy. Curr Med Chem. 2006;13:2487–2502. doi: 10.2174/092986706778201675. [DOI] [PubMed] [Google Scholar]
- 9.Capiralla H, Vingtdeux V, Zhao H, et al. Resveratrol mitigates lipopolysaccharide- and Abeta-mediated microglial inflammation by inhibiting the TLR4/NF-kappaB/STAT signaling cascade. J Neurochem. 2012;120:461–472. doi: 10.1111/j.1471-4159.2011.07594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Tanila H, Puolivali J, et al. Gender differences in the amount and deposition of amyloidbeta in APPswe and PS1 double transgenic mice. Neurobiol Dis. 2003;14:318–327. doi: 10.1016/j.nbd.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Sierksma AS, Prickaerts J, Chouliaras L, et al. Behavioral and neurobiological effects of prenatal stress exposure in male and female APPswe/PS1dE9 mice. Neurobiol Aging. 2013;34:319–337. doi: 10.1016/j.neurobiolaging.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Herber DL, Roth LM, Wilson D, et al. Time-dependent reduction in Abeta levels after intracranial LPS administration in APP transgenic mice. Exp Neurol. 2004;190:245–253. doi: 10.1016/j.expneurol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Qiao X, Cummins DJ, Paul SM. Neuroinflammation-induced acceleration of amyloid deposition in the APPV717F transgenic mouse. Eur J Neurosci. 2001;14:474–482. doi: 10.1046/j.0953-816x.2001.01666.x. [DOI] [PubMed] [Google Scholar]
- 15.McAlpine FE, Lee JK, Harms AS, et al. Inhibition of soluble TNF signaling in a mouse model of Alzheimer's disease prevents pre-plaque amyloid-associated neuropathology. Neurobiol Dis. 2009;34:163–177. doi: 10.1016/j.nbd.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheng JG, Bora SH, Xu G, et al. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice. Neurobiol Dis. 2003;14:133–145. doi: 10.1016/s0969-9961(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 17.Youmans KL, Tai LM, Nwabuisi-Heath E, et al. APOE4-specific changes in Abeta accumulation in a new transgenic mouse model of Alzheimer disease. J Biol Chem. 2012;287:41774–41786. doi: 10.1074/jbc.M112.407957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiCarlo G, Wilcock D, Henderson D, et al. Intrahippocampal LPS injections reduce Abeta load in APP+PS1 transgenic mice. Neurobiol Aging. 2001;22:1007–1012. doi: 10.1016/s0197-4580(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 19.Bayer TA, Wirths O. Intracellular accumulation of amyloid-Beta - a predictor for synaptic dysfunction and neuron loss in Alzheimer's disease. Front Aging Neurosci. 2010;2:8. doi: 10.3389/fnagi.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda S, Hashimoto T, Roe AD, et al. Brain interstitial oligomeric amyloid beta increases with age and is resistant to clearance from brain in a mouse model of Alzheimer's disease. FASEB J. 2013;27:3239–3248. doi: 10.1096/fj.13-229666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10:217–224. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- 22.Morris MC, Gilliam EA, Button J, et al. Dynamic modulation of innate immune response by varying dosages of lipopolysaccharide (LPS) in human monocytic cells. J Biol Chem. 2014;289:21584–21590. doi: 10.1074/jbc.M114.583518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norden DM, Muccigrosso MM, Godbout JP. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology. 2015;96:29–41. doi: 10.1016/j.neuropharm.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michaud JP, Halle M, Lampron A, et al. Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer's disease-related pathology. Proc Natl Acad Sci U S A. 2013;110:1941–1946. doi: 10.1073/pnas.1215165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scholtzova H, Kascsak RJ, Bates KA, et al. Induction of toll-like receptor 9 signaling as a method for ameliorating Alzheimer's disease-related pathology. J Neurosci. 2009;29:1846–1854. doi: 10.1523/JNEUROSCI.5715-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scholtzova H, Chianchiano P, Pan J, et al. Amyloid beta and Tau Alzheimer's disease related pathology is reduced by Toll-like receptor 9 stimulation. Acta Neuropathol Commun. 2014;2:101. doi: 10.1186/s40478-014-0101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cras P, Kawai M, Siedlak S, et al. Neuronal and microglial involvement in beta-amyloid protein deposition in Alzheimer's disease. Am J Pathol. 1990;137:241–246. [PMC free article] [PubMed] [Google Scholar]
- 29.Haga S, Akai K, Ishii T. Demonstration of microglial cells in and around senile (neuritic) plaques in the Alzheimer brain. An immunohistochemical study using a novel monoclonal antibody. Acta Neuropathol. 1989;77:569–575. doi: 10.1007/BF00687883. [DOI] [PubMed] [Google Scholar]
- 30.Xiong Y, Medvedev AE. Induction of endotoxin tolerance in vivo inhibits activation of IRAK4 and increases negative regulators IRAK-M, SHIP-1, and A20. J Leukoc Biol. 2011;90:1141–1148. doi: 10.1189/jlb.0611273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An H, Xu H, Zhang M, et al. Src homology 2 domain-containing inositol-5-phosphatase 1 (SHIP1) negatively regulates TLR4-mediated LPS response primarily through a phosphatase activity- and PI-3K-independent mechanism. Blood. 2005;105:4685–4692. doi: 10.1182/blood-2005-01-0191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.