Abstract
Objective
Genetic variations in the dopamine (DA) system are associated with cortical-striatal behavior in multiple populations. This study assessed associations of functional polymorphisms in the ankyrin repeat and kinase domain (ANKK1; Taq1a) and catechol-o-methyltransferase (COMT; Val158Met) genes with behavioral dysfunction following traumatic brain injury (TBI).
Participants
Prospective study of 90 survivors of severe TBI recruited from a level 1 trauma center.
Main Measures
The Frontal Systems Behavior Scale, a self or family report questionnaire evaluating behavior associated with frontal lobe dysfunction, was completed 6 and 12-months post-injury. Depression was measured concurrently with the Patient Health Questionnaire-9. Study participants were genotyped for Val158Met and Taq1a polymorphisms.
Results
No statistically significant behavioral differences were observed by Taq1a or Val158Met genotype alone. At 12-months, among those with depression, Met-homozygotes (Val158Met) self-reported worse behavior than Val-carriers (p=0.015) and A2-homozygotes (Taq1a) self-reported worse behavior than A1-carriers (p=0.028) in bivariable analysis. Multivariable models suggest an interaction between depression and genetic variation with behavior at 12-months post-TBI, and descriptive analysis suggests that carriage of both risk alleles may contribute to worse behavioral performance than carriage of either risk allele alone.
Conclusion
In the context of depression, Val158Met and Taq1a polymorphisms are individually associated with behavioral dysfunction 12-months following severe TBI with preliminary evidence suggesting cumulative, or perhaps epistatic, effects of COMT and ANKK1 on behavioral dysfunction.
Keywords: DRD2, ANKK1, COMT, TBI, Behavior, Rehabilomics, Frontal Lobe
Introduction
Understanding and managing traumatic brain injury (TBI) can be challenging as people with similar injury profiles can experience different cognitive, emotional, and behavioral outcomes1–4. Rehabilomics is a conceptual framework from which to investigate these diverse outcomes by examining the complex interplay between personal, biological, and psychosocial factors present in the context of TBI5,6. Rehabilomics is unique in its inclusion of personal biological factors, like genetic variation and serum biomarkers, which may contribute directly to TBI outcomes or interact with other biological and functional factors to affect outcomes. As such, the Rehabilomics framework can inform study designs for understanding biological mechanisms underlying various outcomes and neural recovery after TBI. The framework also provides a theoretical basis for developing personalized-medicine approaches to neurorehabilitation after TBI. As an example, our previous work has employed a Rehabilomics approach to study heterogeneity in cognitive deficits following TBI, incorporating personal (genetic) and biological (sex) factors7. Further, genetic variation influences on personal traits and psychiatric disorders is increasingly recognized as relevant to clinical practice8. Similar approaches can be used to study other complex TBI outcomes, including behavioral problems, with the goal of learning how best to design biologically tailored rehabilitation strategies.
Fifty-four percent of individuals with moderate/severe TBI report behavioral problems that persist for years post-injury,9,10 including aggression, disinhibition, amotivation, and difficulty planning and executing actions11,12. Behavioral problems result from the complex interactions among cognition (e.g. cognitive control), emotional state (e.g. depressive symptoms), and personal factors (e.g. genetics) manifesting primarily in response to environmental stimuli13. We have shown previously that the dopamine (DA) system is highly susceptible to dysfunction following TBI14. Clinically, DA agonists can improve outcome15,14 and have neurorestorative effects with in vivo experimental TBI models16,17 suggesting that TBI results in a functional hypodopaminergia, although mechanisms by which this occurs are still largely unknown. DA modulated processing can affect cognition via projections to the mesocortical system (prefrontal (PFC) and medial frontal cortices), emotion via amygdala and cingulate projections, and depression and amotivation by projections to the hippocampus and ventral striatum18,19,20. The DA system is under complex regulatory control by afferent regions and by the interplay between tonic and phasic elements of neurotransmission, where tonic DA levels modulate stimulus-driven phasic DA release21. The literature underscores the importance of DA systems, in humans and animal models, to PFC-centered behaviors like aggression22,23, impulsivity24,25, and executive function26,27. DA also influences other PFC-centered constructs such as cognition, which–when impaired–contributes to behavioral problems. Clinical DAergic therapy studies involving Attention Deficit Hyperactivity Disorder28 and Parkinson’s Disease29 demonstrate that DA system modulation can improve problematic behaviors.
The PFC uses cortical striatal afferents to govern elements of executive function that include aspects of social cognition and emotional regulation that influence behavior30. Furthermore, the PFC has the capacity for top-down regulatory control over ascending DA modulatory systems in a manner specific to the environmental stimuli and stressors at hand31,32, so examining variation within candidate genes involved in these connected DA regions could inform how these regions regulate behavior33,34. While numerous genes regulate DA system control, we have chosen two genes with well-described functional polymorphisms that have been highlighted as primary genes of importance in TBI specific outcomes research35. These two candidate genes are known as COMT (catechol-o-methyltransferase) and ANKK1. COMT is the enzyme primarily responsible for DA metabolism in the PFC and is linked to impulsivity and aggression among individuals with schizophrenia36. Within COMT, there exists a well-studied functional polymorphism called Val158Met (rs4680). The Val-allele has 4× greater enzyme function than the Met-allele, which leads to lower DA levels at cortical synapses but increased phasic responses at subcortical synapses37. Compared to standard mouse models, COMT overexpressing mice exhibit differences in DA release in the ventral striatum (VS), implicating a role for striatal DA metabolism as well38. The Taq1A polymorphism (rs1800497) in the ANKK1 gene, associated with DA type-2 receptor D239, is implicated with impulsivity in healthy populations40 and childhood aggression41 both of which are associated with DA system disruption. Taq1A polymorphisms also are associated with D2 pre-/post-synaptic receptor densities42, and are highly expressed in subcortical regions43. Studies report that Taq1A A1-carriers have lower receptor densities than A2-homozygotes42,44,45, but A2-carriers may have lower receptor densities in the context of depression46. Thus, the A2 allele, may actually impart a greater risk for poor DA modulation that differs from healthy populations. While other genetic components of the DA system may influence outcomes, COMT and ANKK1 are currently the only genes with both a strong mechanistic rationale and previously documented associations with other TBI outcomes (cognition) in the literature47,48,49.
Similarly, some studies indicate that genetic risk relationships between both Val158Met50,51 and Taq1A52 and behavior are only present in the context of a moderating stressor. Disrupted subcortical and PFC activity can occur in the presence of a chronic stressor, like Post-traumatic Stress Disorder (PTSD)53 or depression54 to decrease cognitive control over behavior, leading to increased reliance on emotion-based decision making and the emergence of poor behaviors similar to those observed with TBI. This phenomenon has been characterized as a switch from “top-down” cognitive control, in which the PFC controls subcortical regions to plan and execute a decision, to a state of “bottom-up” emotional control, where subcortical regions involving emotion and reward systems function with reduced PFC regulation55. This framework suggests that cognitive control interacts with emotional state to contribute to behavioral problems. Chronic rodent stress models lead to anxiety and despair-like behaviors that are associated with decreased DA neuron activity56. PTD rates are ~50% during the first year post-TBI57. PTD is associated with poor behavior post-injury9 and may trigger a positive feedback loop of behavioral problems and depressive symptoms across this time period58.
While previous work has focused on how DA genetics can influence cognition after TBI7,47 only one other study exists in the TBI literature examining DA system genetics and behavioral dysfunction, specifically aggression59. Since both Val158Met and Taq1A polymorphisms are associated with PFC and VS DA neurotransmission and related behavior, further TBI investigation is warranted. Thus, we examined how DA genetic variation influences behavior after TBI, both independently and in the context of a chronic stressor, specifically PTD. Based on previous literature, we hypothesized that Val158Met and Taq1A polymorphisms would be associated with PFC-centered behavior. Further, we hypothesized that genetic predilection to relatively increased PFC DA levels, associated with the COMT gene Met-allele, would be associated with poorer behavior post-TBI. Given ANKK1 gene associations with stress-inducing conditions like PTSD52,60, we hypothesized that ANKK1 genetic variation would influence behavior after TBI, particularly among those with PTD, wherein reduced mesostriatal DA neuron phasic firing and PFC over-activity56 may drive an imbalance with corticostriatal tonic-phasic DA modulation61. While A1-carriers can have worse behavioral and cognitive outcome in healthy and mTBI populations, our data characterizing those with severe TBI suggest A2-homozygotes have worse cognitive outcomes47. This study aims to clarify which alleles impart risk for poor behavioral outcomes in a moderate-to-severe TBI population. As PTD often emerges within the first 6-months post-injury,57 we expected the potential chronic stress effects of PTD on DA gene—behavior relationships to be most evident at 12-months post-injury.
Methods
Participants were recruited from inpatient and outpatient centers at the University of Pittsburgh Medical Center (UPMC) as part of a larger TBI study approved by our Institutional Review Board. Enrollment criteria included a non-penetrating severe TBI [admission Glasgow Coma Scale (GCS)≤8], a CT scan with evidence of intracranial injury, and age 16–75 years. Participants with documented evidence of hypoxia (>30 minutes) occurring prior to admission were excluded. Behavioral data at either 6-/12-months post-injury were available for 97 participants. Due to concerns regarding racial stratification62 and differences in allele frequency distribution between races in Taq1A63 and Val158Met64, this study was then restricted to self-reported white individuals, leaving a final cohort of 90 unique participants (6M: n=69; 12M: n=69; Both 6M and 12M: n=48). Supplemental Figure 1 shows further participant breakdown. Demographic information was obtained from medical records and/or participant/caregiver interview. The best GCS within 24 hours post-injury was used for analysis, given its discriminative ability when examining outcomes65. Assessors were blinded to genotype status.
Behavior Assessment
The Frontal Systems Behavior Scale (FrSBe) is a validated assessment of behaviors associated with damage to the frontal lobes and includes an overall score and three subscale scores: disinhibition, apathy, and executive dysfunction;66 both the self-report and family-report versions were administered when possible.67 Questions were scored with regard to current behavior (after injury) and pre-injury. Standardized FrSBe scoring yields norm-based T-scores adjusted for age, sex, and education. Higher T-scores indicate more problem behaviors.
Depression Assessment
The Patient Health Questionnaire-9 (PHQ9) is a validated self-report symptom-based questionnaire based on the DSM-IV criteria for depression and is validated for use after TBI68. Participants were categorized at 6 and 12 months as having PTD if endorsing ≥5 symptoms, at least one of which was a cardinal symptom of depression (depressed mood or anhedonia). History of premorbid psychiatric disorders, including depression, bipolar disorder, and/or anxiety disorder, was collected from interview or medical chart review.
Genotyping
DNA was isolated from blood using a simple salting out procedure69 and genotyped for COMT (rs4680, Val158Met) and ANKK1 (rs1800497, Taq1A variant). COMT Val158Met (rs4680) was genotyped using TaqMan allele discrimination technology and available 5′ exonuclease Assay-on-Demand TaqMan assays (Applied Biosystems). For ANKK1 Taq1A (rs1800497) genotyping, amplified DNA underwent 30 cycles of denaturation at 95°C for 1min., annealing at 58°C for 30s, and extension at 72°C for 1min., to amplify the 459bp product, which was then exposed to TaqI restriction endonuclease to perform restriction fragment length polymorphism (RFLP) analysis. Digested products were electrophoresed on a 3% agarose gel, stained with ethidium bromide for DNA band detection, and assigned a genotype based on presence/absence of original or cut DNA fragments. Primers used were 5′-CCGTCGACCCTTCCTGAGTGTCATCA-3′ and 5′-CCGTCGACGGCTGGCCAAGTTG TCTA-3′. Two individuals blinded to phenotype data69 called each genotype, and discrepancies were resolved by examining the raw data and re-running samples if necessary. We grouped participants into Met-Homozygotes vs. Val-Carriers and A1-Carriers vs. A2-homozygotes based on allele frequency, function, or previous studies in the literature.
Data Analysis
Statistical analyses were completed using SPSS (Version 22). Mean, median, standard deviation, and standard error were calculated when appropriate, and categorical data were reported as frequencies. Behavior and demographic data were compared using Mann-Whitney-U, ANOVA, Kruskall-Wallis, T-Tests, or Chi-square tests as appropriate. Group differences considering carrier and depression status were assessed using ANOVA and post-hoc analysis with Fisher’s LSD. We tested both Val158Met and ANKK1 in separate multiple regression models controlling for pre-injury psychiatric disorders, antidepressant use, and behavior before injury (FrSBe Before Total T-score). Partial eta squared values were reported to determine the amount of variance in behavior captured with each variable tested in each multivariable model.
Results
Demographic data for the entire sample are presented in Table 1. No significant differences by genotypes were identified for any descriptive factor. Bivariable analyses yielded no significant differences in self-reported behavior between Met-Homozygotes and Val-Carriers or between A1-Carriers and A2-homozygotes at 6-months or 12-months post-injury. The sample was in Hardy-Weinberg Equilibrium for each variant studied.
Table 1.
Sample (n=87) Demographic and Descriptive Characteristics
| Val-Carrier (n=63) | Met-Homozygotes (n=24) | p-Value | A1-Carrier (n=37) | A2 Homozygotes (n=50) | p-Value | |
|---|---|---|---|---|---|---|
| Age | 34.16 ± 13.99 | 37.38 ± 15.44 | 0.433 | 34.19 ± 15.10 | 35.00 ± 13.70 | 0.585 |
| Education | 13.11 ± 1.94 | 12.25 ± 1.60 | 0.140 | 12.86 ± 1.90 | 12.88 ± 1.88 | 0.897 |
| GCS | 8 | 7 | 0.903 | 8 | 7 | 0.305 |
| Gender (Female) | 14 (22.22%) | 3 (12.50%) | 0.378 | 10 (27.03%) | 7 (14.00%) | 0.173 |
| Pre-Morbid Psychiatric Disorder | 6/61 (9.84%) | 6/22 (27.27%) | 0.073 | 6/34 (17.65%) | 6/49 (12.25%) | 0.537 |
| PTD (Depressed-6mo) | 19/50 (38.00%) | 7/19 (35.00%) | 1.000 | 9/29 (31.03%) | 17/40 (42.50%) | 0.451 |
| PTD (Depressed-12mo) | 14/46 (30.40%) | 9/23 (39.13%) | 0.589 | 12/34 (35.30%) | 11/35 (31.43%) | 0.802 |
When examining relationships between genes and behavior by PTD status, we found a significant difference in behavior by Val158Met status only among those with PTD at 12 months (p=0.028), but not at 6-months (p=0.073) post-injury. We found similar results for Taq1A status at 12-months (p=0.028), but not 6-months (p=0.884) post-injury. Figure 1 illustrates no differences between Val-carriers and Met-homozygotes among those without PTD. Among those with PTD, Met-Homozygotes scored ~2 standard deviations higher (20 points FrSBe total T-score), indicating substantially more behavioral problems than Val-carriers. Similarly, among those with PTD, A2-homozygotes also reported scores that were ~2 standard deviations higher (20 points) than A1-carriers. Exploratory analyses suggested similar gene effects across all three FrSBe subscale scores, indicating no single subscale was driving these results (data not shown). Multivariable analysis (see table 2), controlling for pre-morbid psychiatric disorder, antidepressant use, and behavior before injury revealed a significant interaction between PTD status and COMT status at 12-months (n=64; p=0.007), but not at 6 months (n=64; p=0.427) post-injury. For ANKK1 status, this interaction was not significant at the 6 month (p=0.553) time point. However, at the 12 month time point, there was a trend towards significance for an interaction between the ANKK1 variant (A2/A2) and PTD (p=0.094). Compared to a base model without genetics or a PTD*Gene interaction, R2=0.436, adding a COMT*PTD interaction term accounted for 8% of observed variance (R2=0.516), while an ANKK1*PTD interaction term accounted for 2.9% of observed variance (R2=0.465). For a preliminary analysis of potential cumulative/epistatic effects of these two genes, we conducted group analyses within our PTD population only at 12 months (n=23). Assessment of individuals across both gene variants using ANOVA showed an overall effect of genotype grouping on behavior (F3=3.388; p=.039). Post-hoc pairwise comparisons show that Met-homozygotes/A2-homozygotes reported significantly worse behavior than Val-carrier/A2-homozygotes (p=0.044), and Val-carrier/A1-carriers (p=0.005); they also tended to perform worse than Met-homozygotes /A1-carriers (p=0.077); Figure 2).
Figure 1.
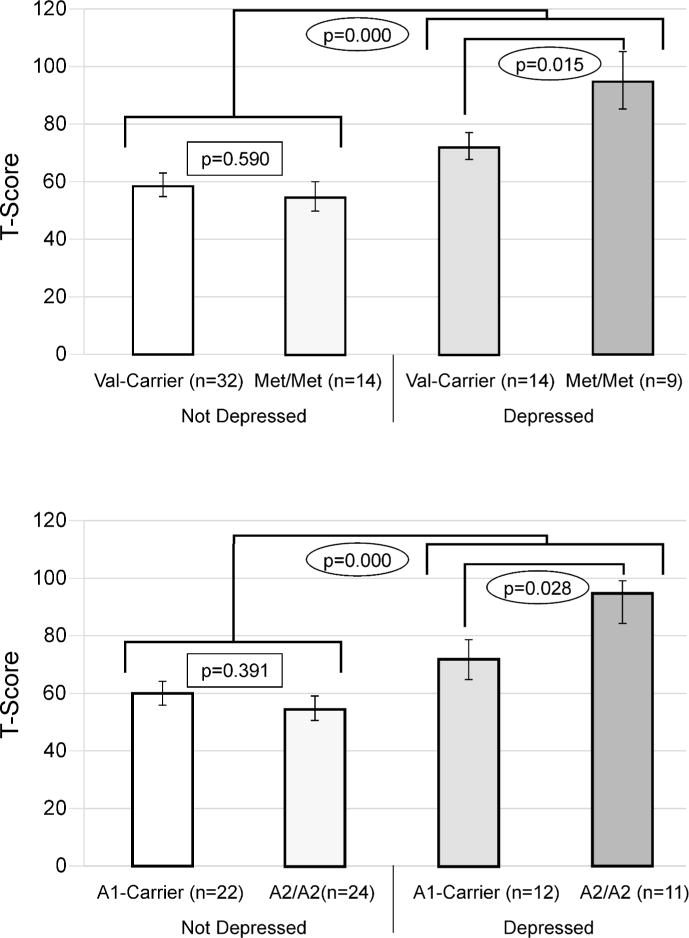
12-Month results. Carrier status and depression groups were compared separately for both Val158Met and Taq1a. Those with PTD exhibited significantly worse outcomes regardless of genetic status, and statistically significant differences by carrier status were only noted between those with PTD. Similar non-significant trends were also observed at 6 months (Data not shown).
Table 2.
Multivariate Regression for Self-Reported Behavior at 12 Months Post-Injury: ANKK1 and COMT
| ANKK1 | COMT | |||||
|---|---|---|---|---|---|---|
| Variable | F | Eta Squared | p | F | Eta Squared | p |
| Genetics (Taq1a or Val158Met) | .798 | 0.007 | 0.375 | 4.461 | 0.038 | 0.039 |
| PTD | 15.630 | 0.147 | 0.000 | 23.967 | 0.203 | 0.000 |
| Antidepressant Use | .024 | <.001 | 0.879 | .118 | 0.001 | 0.732 |
| Premorbid Psychiatric Disorder | .239 | 0.002 | 0.627 | .243 | 0.003 | 0.624 |
| FrSBe Before Injury | 13.499 | 0.127 | 0.001 | 16.577 | 0.141 | 0.000 |
| PTD* Gene Interaction | 2.900 | 0.027 | 0.094 | 7.947 | 0.067 | 0.007 |
| R2 =0.465 | <.001 | R2 =0.516 | <.001 | |||
| Base Model not including ANKK1 or PTD*ANKK1 Interaction: R2=0.436. ΔR=.029 This indicates that GRS Risk and PTD*GRS Risk interaction alone account for 2.9% of the variance in self-reported behavior 12 months post-injury, controlling for PTD, Antidepressant Use, and Premorbid Psychiatric Disorder. | Base Model not including COMT or PTD*COMT Interaction: R2=0.436. ΔR=.08 This indicates that COMT & PTD*COMT Risk interaction alone account for 8% of the variance in self-reported behavior 12 months post-injury, controlling for PTD, Antidepressant Use, and Premorbid Psychiatric Disorder. | |||||
Figure 2.
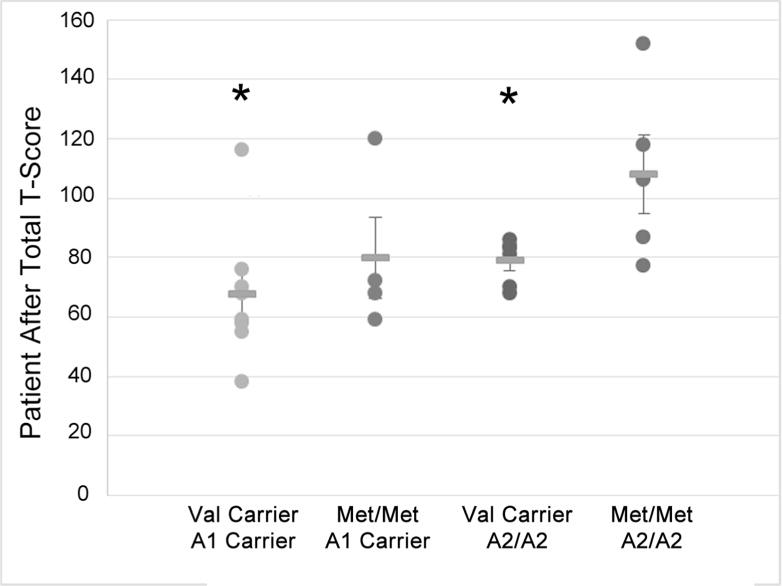
Individual FrSBe Self Total After scores and group means are presented for Val158Met by ANKK1 groups in a PTD only population (n=23). The groups who reported significantly better behavior (p<0.05) than Met/Met & A2/A2 are labeled with an #.
We conducted a secondary (bivariable) analysis using family reported behavior measures to assess the impact of self-awareness on behavioral outcome measures at the 12 month time point (n=57). The same group analyses were used as in the patient self-report (see Figure 1). A significant difference was once again observed between participants who were depressed and not depressed (p=0.005). Depressed Met-homozygotes performed significantly worse (~21 points) worse than their Val-carrier counterparts (p=0.040). No significant difference was observed between these genotypes in non-depressed populations (~7 points) (p=0.240). For Taq1A with PTD, there was no significant difference in family reported behavior between depressed (p=0.328) or non-depressed (p=0.827) A2-homozygotes and A1 carriers.
Discussion
Taking a Rehabilomics approach, this work represents the first study examining genetic associations with behavior post-TBI. We found no differences in behavior following TBI by Val158Met (COMT) or Taq1A (ANKK1) status alone; however, among those with PTD at 12-months, we found Met-homozygotes (Val158Met) and A2-homozygotes (Taq1A) self reported significantly more behavioral problems 12-months post-TBI, supporting previous evidence that a relationship between DA genetics and behavior emerges in the context of a moderating stressor like PTD. Even when corrected for perceived pre-injury behavior status, antidepressant usage, and pre-morbid mood disorder, a significant interaction between Val158Met and PTD was still present. Since depression may disrupt PFC areas highly associated with effective and efficient “top-down” control of cognitive-behavioral processes,56,70 individuals with PTD may be especially susceptible to genetically mediated differences in DA system function. Importantly, we observed genetic associations only at 12 months post-injury. Since PTD develops most frequently during the first 6-months post-injury57, its effects on DA-moderated behavior may require time beyond 6-month PTD onset to emerge. Alternatively, depression may emerge following resolution of a chronically stressful state (e.g. initial recovery from severe trauma).56 Regardless, temporal relationships between mood and behavior might explain why we observed some trends at 6-months, with statistically significant results at 12-months post-injury.
Our results suggest that the severity of poor behaviors among those with PTD is related to Val158Met and Taq1A variation, and these findings are not simply attributable to PTD alone (Figure 2). Our hypothesis was supported in that relative cortical DA system hyperfunction, presumably occurring among those homozygous for the COMT Met-allele (high PFC DA levels), was associated with worse behavior when occurring with PTD. Further, our hypothesis implicating the ANKK1 Taq1A variant in behavior within the context of PTD was also supported, although it did not hold up to multivariable correction. We also provide preliminary evidence for an interaction between ANKK1 and COMT in the context of PTD specific behavioral dysfunction. Although these findings need to be validated in an independent sample, the data demonstrate how this application of the Rehabilomics framework5 shows that DA genetics may contribute substantially to variability in behavior after TBI. Patients with identified DA genetic susceptibility could be monitored more closely for PTD development and managed with appropriate pharmaceutical, cognitive-behavioral, or other emerging therapies, and provide them earlier after injury to manage PTD and to prevent later development of severe behavioral symptoms.
Our previous work suggests that COMT Met-homozygosity is associated with relatively better cognitive performance after TBI among women7. Though the disparate relationship between Val158Met and cognition versus behavior at first seems paradoxical, these findings support a growing body of evidence highlighting that a simple global hypo-/hyper-dopaminergic model may not adequately describe complex cognitive-behavioral outcomes61,71. These phenomena may be modeled better by considering regional alterations in DA system regulation that result in relative states of either hyper/hypo-dopaminergia. To this point, experimental TBI research suggests subcortical (striatal) DA deficits17,72 and increased medial PFC DA synthesis73 following injury. These complex relationships may be further clarified when considering the tonic-phasic DA hypothesis,21 as it applies to “PFC and striatal stability versus flexibility” as articulated by Bilder and colleagues,61 for both cognitive and behavioral function after TBI. Together with previous work evaluating DA genetics and cognition after TBI7,47, this work represents the first clinical study supporting the tonic-phasic DA hypothesis.
The tonic-phasic theory of DA neurotransmission, as articulated by Grace21, states that “the dynamics of DA regulation within limbic striatal regions occurs via two processes: (1) high-amplitude transient, phasic DA release mediated by DA neuron burst firing, and (2) constant low-level ‘background’ tonic DA that is regulated by baseline DA neuron firing and corticostriatal glutamatergic afferents (pg. 1944)”. Building upon this framework, Bilder suggests that within subcortical systems, high-amplitude phasic DA is released in conjunction with behaviorally driven bursts of action potentials. Phasic DA release is modulated by subcortical tonic DA levels, via striatal presynaptic DA terminal D2 autoreceptors; glutamatergic corticostriatal afferents modulated by D1 receptor activity in the PFC also suppress phasic DA release. Since Met-homozygosity likely increases tonic DA cortically and subcortically, these individuals may exhibit a relative suppression in mesostriatal DA neuron phasic burst firing, which could be further amplified by previously observed DA transporter (DAT) reductions74,16 that limit DA clearance. Interestingly, preliminary evidence suggests that experimental TBI reduces mesostriatal DA neuron phasic firing and production of spontaneous DA transients.72 Striatal tonic/phasic DA modulation is distinct from DA actions in the PFC, due to limited DA autoreceptor modulation where Met-homozygosity (increased DA) leads to more cortical excitability and more cortical-striatal inhibition of striatal phasic DA61. Work by Dash73 demonstrates that experimental TBI can increase PFC DA tone through D1 receptor mechanisms. Thus, genetic variations in DA pathways may accentuate disruption of the tonic-phasic interplay between cortical and subcortical DA systems associated with TBI.
In this context, the tonic-phasic DA model suggests COMT activity could differentially influence “top-down” control (cortical based cognitive-behavioral stability) and “bottom-up” stimulus driven control of actions (subcortical cognitive-behavioral flexibility), which may be accentuated by depression. Clinically after TBI, higher cortical DA levels associated with Met-homozygosity may relatively preserve cortical DA system stability, important for neuropsychological performance, while simultaneously decreasing subcortical (phasic) DA transmission necessary for efficient changes in neural networks and flexibility in adapting to new situations and environments important for functional cognition and navigating the real-world environment75,76. Thus, the same DA levels that may be beneficial with neuropsychological test performance may lead to rigidity in thought and to fixation that are captured as relative deficits on behavior and functional cognition measures. Also, cortical suppression of subcortical phasic DA activity could further drive the depressive state56,70 that facilitated initial genetic associations with behavioral dysfunction after TBI.
D2 receptors primarily localized in the nucleus accumbens, caudate, and putamen, also significantly affect behavior. A2-homozygotes tend to report more behavioral problems than A1-carriers in the context of depression, which in the setting of the cognitive-behavioral interplay associated with top-down control, is consistent with our previously reported Taq1A associations with cognitive outcomes47. One interpretation of our data is that D2 receptor density among A2-homozygotes with depression leads to difficulty inhibiting maladaptive behaviors. Since D2 autoreceptor density can drive DA transporter expression77, the primary method for synaptic DA removal subcortically78, genetic variation within ANKK1, could also affect tonic DA availability subcortically after TBI74. With an injury+stress-induced inability to modulate PFC function, and its effect on “bottom-up” control, endogenous variations in striatal DA receptors may further blunt phasic responses and impair striatal flexibility to effectively manage behavior27. Our findings cannot elucidate the exact neurobiological mechanisms contributing to the apparent ANKK1 relationship with behavior, but the potential additive/epistatic effects of both Val158Met with PFC control of striatal DA systems and Taq1A related subcortical DA system control may together facilitate poor behavior after TBI. While an intriguing finding, our sample size for carriers of both risk allele is small, and the particularly poor behaviors among those who were Met and A2-homozygotes with PTD need to be replicated in larger sample sizes.
These data suggest a hypothesis-generating theoretical Rehabilomics-based framework for understanding the neurobiology of behavioral dysfunction in the context of a chronic stressor (PTD) (Figure 3) that may inform mechanisms of neural recovery and pathways for personalized-medicine approaches in neurorehabilitation. Individuals with relatively higher PFC DA activity and disrupted “top-down” PFC control associated with both TBI and PTD, have an inability to activate appropriate subcortical DA system responsivity to respond flexibly to novel or changing conditions. With PTD, blunted cognitive flexibility and emotional control manifests in problematic behaviors. COMT Met-homozygotes may have increased PFC DA levels and decreased subcortical phasic responses, and therefore, exhibit the proposed cortical response rigidity. A2-homozygotes with PTD have D2 function that magnifies the behavioral effects from decreased subcortical phasic DA responsivity that occurs in the context of COMT Met-homozygosity. Thus, we propose individuals who are both Met and A2 homozygotes with PTD would have both overactive PFC DA and decreased subcortical phasic DA responses that lead to greater susceptibility to dysregulated behavior. This hypothesis may help to clarify what are seemingly contradictory findings with how the same DA genetic variants paradoxically preserve cognition yet facilitate poor behavior.
Figure 3.
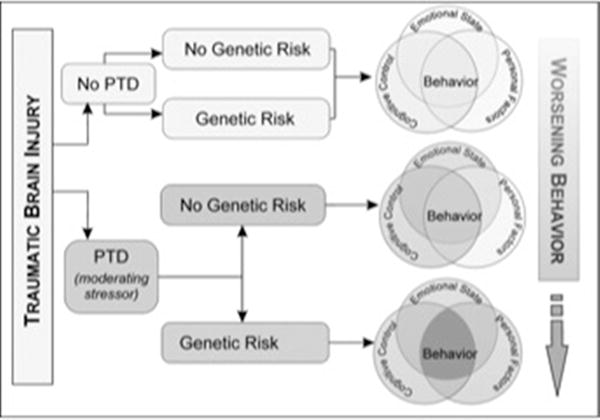
Behavior manifests as a result of cognitive control (cognition that can be measured with neuropsychological testing), emotional state (depression and anxiety), and personal factors (genetics or environment). Following TBI, an individual may exhibit deficits in none or all of these areas. For instance, individuals may have impairments in cognitive abilities, but no underlying mood disorder and a favorable genetic profile. They are expected to exhibit few behavioral problems. Those with impaired cognitive abilities and a mood disorder, but a favorable genetic profile may exhibit mild/moderate behavioral problems. However, those individuals with impaired cognitive control, a mood disorder, and an unfavorable genetic profile may exhibit the most severe behavioral problems. This work is just one example of how the Rehabilomics framework can be used to better characterize patient outcomes.
A clear and integrated framework for how personal biology within DA systems encompasses both cognition and behavior may enhance existing strategies for TBI neurorehabilitation and repair79. fMRI evidence suggests DA neuron dysfunction following TBI, with working memory activation related to Val158Met status80. This evidence of DA system dysfunction interacting with genetic susceptibility supports the notion that DA genetics inform clinical care. In rehabilitation, where strategies are needed to help individuals struggling with both chronic cognitive and behavioral disabilities, a Rehabilomics framework would address both biological changes (through pharmaceutical therapies) and psychosocial changes (through behavioral interventions), to guide personalized-medicine approaches after TBI. With known pharmacological targets for both COMT37 and D2 receptors81 available, understanding how to target DA systems based on individual genetics and symptom (cognitive vs. behavioral) profiles may provide more precision with effectively managing these individuals pharmacologically. Studies already show differential responses to antidepressant treatment by Val158Met status82,83. Furthermore, a study in schizophrenic populations showed Met-carriers may be more responsive to deficit-targeted computerized cognitive exercises to improve quality of life84. Future work may focus on using these same markers in determining which patients may benefit the most from cognitive rehabilitation and pharmaceutical therapies after TBI.
The population with severe TBI is a highly specialized rehabilitation population, with a relatively low incidence compared to other clinical populations. While our sample is small, compared to large population-based genetics studies, and validation in larger and more ethnically diverse samples are needed, numerous aspects of this study support the veracity of the findings. The literature strongly supports a functional role for the selected genetic polymorphisms; thus, we examined these known functional variants in the context of a new clinical population (TBI). Additionally, we demonstrate the potential clinical relevance of these functional polymorphisms in TBI with significant associations in cognitive7,47 and behavioral performance in this sample at the same assessment time-points.
Since our previous studies suggest that sex influences genetic relationships with cognitive outcomes, future work should investigate if sex moderates relationships between the risk groups identified in this study and behavior. Investigating temporal relationships between PTD onset and appearance of behavioral symptoms, particularly in response to acute stressors, within the context of DA genetics is also needed. Nonetheless, our work provides initial evidence that clinical studies examining DA system functional relationships to cognitive and behavior may identify appropriate and personalized management strategies more effectively than what is currently available, and these findings represent a robust early example of how Rehabilomics-based research applications may lead to personalized-approaches to TBI care.
Supplementary Material
Footnotes
Conflict of Interest Statement
The Author(s) have no conflicts of interest to disclose.
References
- 1.Carroll LJ, et al. Systematic Review of the Prognosis After Mild Traumatic Brain Injury in Adults: Cognitive, Psychiatric, and Mortality Outcomes: Results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil. 2014;95:S152–S173. doi: 10.1016/j.apmr.2013.08.300. [DOI] [PubMed] [Google Scholar]
- 2.Dikmen SS, et al. Cognitive outcome following traumatic brain injury. J Head Trauma Rehabil. 2009;24:430–438. doi: 10.1097/HTR.0b013e3181c133e9. [DOI] [PubMed] [Google Scholar]
- 3.Hesdorffer DC, Rauch SL, Tamminga CA. Long-term psychiatric outcomes following traumatic brain injury: a review of the literature. J Head Trauma Rehabil. 2009;24:452–459. doi: 10.1097/HTR.0b013e3181c133fd. [DOI] [PubMed] [Google Scholar]
- 4.Temkin NR, Corrigan JD, Dikmen SS, Machamer J. Social functioning after traumatic brain injury. J Head Trauma Rehabil. 2009;24:460–467. doi: 10.1097/HTR.0b013e3181c13413. [DOI] [PubMed] [Google Scholar]
- 5.Wagner AK. TBI translational rehabilitation research in the 21st Century: exploring a Rehabilomics research model. Eur J Phys Rehabil Med. 2010;46:549–556. [PubMed] [Google Scholar]
- 6.Wagner AK, Zitelli KT. A Rehabilomics focused perspective on molecular mechanisms underlying neurological injury, complications, and recovery after severe TBI. Pathophysiol Off J Int Soc Pathophysiol ISP. 2013;20:39–48. doi: 10.1016/j.pathophys.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Failla MD, Myrga JM. Sex x Gene Interaction Paper with GRS (UPDATE CITATION UPON ACCEPTANCE) [Google Scholar]
- 8.Kandel ER. A New Intellectual Framework for Psychiatry. Am J Psychiatry. 1998;155:457–469. doi: 10.1176/ajp.155.4.457. [DOI] [PubMed] [Google Scholar]
- 9.Juengst SB, Skidmore E, Wagner A. Behavioral Changes and Depression, Disability, and Life Satisfaction in Two Cohorts of Adults With TBI. Arch Phys Med Rehabil. 2014;95:e71. [Google Scholar]
- 10.Sabaz M, et al. Prevalence, comorbidities, and correlates of challenging behavior among community-dwelling adults with severe traumatic brain injury: a multicenter study. J Head Trauma Rehabil. 2014;29:E19–30. doi: 10.1097/HTR.0b013e31828dc590. [DOI] [PubMed] [Google Scholar]
- 11.Kelly G, Brown S, Todd J, Kremer P. Challenging behaviour profiles of people with acquired brain injury living in community settings. Brain Inj. 2008;22:457–470. doi: 10.1080/02699050802060647. [DOI] [PubMed] [Google Scholar]
- 12.Arciniegas DB, Wortzel HS. Emotional and Behavioral Dyscontrol After Traumatic Brain Injury. Psychiatr Clin North Am. 2014;37:31–53. doi: 10.1016/j.psc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56:267–283. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- 14.Bales JW, Wagner AK, Kline AE, Dixon CE. Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neurosci Biobehav Rev. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neurobehavioral Guidelines Working Group et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23:1468–1501. doi: 10.1089/neu.2006.23.1468. [DOI] [PubMed] [Google Scholar]
- 16.Wagner AK, et al. Chronic methylphenidate treatment enhances striatal dopamine neurotransmission after experimental traumatic brain injury. J Neurochem. 2009;108:986–997. doi: 10.1111/j.1471-4159.2008.05840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner AK, et al. Controlled cortical impact injury influences methylphenidate-induced changes in striatal dopamine neurotransmission. J Neurochem. 2009;110:801–810. doi: 10.1111/j.1471-4159.2009.06155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lisman JE, Grace AA. The Hippocampal-VTA Loop: Controlling the Entry of Information into Long-Term Memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Seo D, Patrick CJ, Kennealy PJ. Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress Violent Behav. 2008;13:383–395. doi: 10.1016/j.avb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 22.Miczek KA, Fish EW, de Bold JF, de Almeida RM. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and γ-aminobutyric acid systems. Psychopharmacology (Berl) 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- 23.Ferrari PF, Van Erp AMM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- 24.Economidou D, Theobald DE, Robbins TW, Everitt BJ, Dalley JW. Norepinephrine and Dopamine Modulate Impulsivity on the Five-Choice Serial Reaction Time Task Through Opponent Actions in the Shell and Core Sub-Regions of the Nucleus Accumbens. Neuropsychopharmacology. 2012;37:2057–2066. doi: 10.1038/npp.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cools R, Barker RA, Sahakian BJ, Robbins TW. l-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia. 2003;41:1431–1441. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 26.Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 27.Robbins TW, Arnsten AFT. The Neuropsychopharmacology of Fronto-Executive Function: Monoaminergic Modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav Brain Res. 2002;130:65–71. doi: 10.1016/s0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- 29.Weintraub D, Siderowf AD, Potenza MN, et al. ASsociation of dopamine agonist use with impulse control disorders in parkinson disease. Arch Neurol. 2006;63:969–973. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert SJ, Burgess PW. Executive function. Curr Biol. 2008;18:R110–R114. doi: 10.1016/j.cub.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Amat J, et al. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 32.Robbins TW. Controlling stress: how the brain protects itself from depression. Nat Neurosci. 2005;8:261–262. doi: 10.1038/nn0305-261. [DOI] [PubMed] [Google Scholar]
- 33.Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: Fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- 35.McAllister TW. Polymorphisms in Genes Modulating the Dopamine System: Do They Influence Outcome and Response to Medication After Traumatic Brain Injury? J Head Trauma Rehabil. 2009;24:65–68. doi: 10.1097/HTR.0b013e3181996e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strous RD, et al. Aggressive behavior in schizophrenia is associated with the low enzyme activity COMT polymorphism: A replication study. Am J Med Genet B Neuropsychiatr Genet. 2003;120B:29–34. doi: 10.1002/ajmg.b.20021. [DOI] [PubMed] [Google Scholar]
- 37.Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): Biochemistry, Molecular Biology, Pharmacology, and Clinical Efficacy of the New Selective COMT Inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 38.Simpson EH, et al. Genetic variation in COMT activity impacts learning and dopamine release capacity in the striatum. Learn Mem Cold Spring Harb N. 2014;21:205–214. doi: 10.1101/lm.032094.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blum K, et al. Dopamine D2 receptor gene variants: association and linkage studies in impulsive-addictive-compulsive behaviour. [Review] Pharmacogenetics. 1995;5:121–141. doi: 10.1097/00008571-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Eisenberg DTA, et al. Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 TaqI A and DRD4 48-bp VNTR association study. Behav Brain Funct BBF. 2007;3:2. doi: 10.1186/1744-9081-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zai CC, et al. Dopaminergic system genes in childhood aggression: possible role for DRD2. World J Biol Psychiatry Off J World Fed Soc Biol Psychiatry. 2012;13:65–74. doi: 10.3109/15622975.2010.543431. [DOI] [PubMed] [Google Scholar]
- 42.Thompson J, et al. D2 dopamine receptor gene (DRD2) Taql A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7:479–484. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Hall H, et al. Distribution of D1- and D2-Dopamine Receptors, and Dopamine and Its Metabolites in the Human Brain. 1994;11:245–256. doi: 10.1038/sj.npp.1380111. Publ Online 01 Dec 1994 Doi101038sjnpp1380111. [DOI] [PubMed] [Google Scholar]
- 44.Pohjalainen T, et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 45.Jönsson EG, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- 46.Savitz J, et al. DRD2/ANKK1 Taq1A polymorphism (rs1800497) has opposing effects on D2/3 receptor binding in healthy controls and patients with major depressive disorder. Int J Neuropsychopharmacol. 2013;16:2095–2101. doi: 10.1017/S146114571300045X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Failla MD, et al. Posttraumatic Brain Injury Cognitive Performance Is Moderated by Variation Within ANKK1 and DRD2 Genes. J Head Trauma Rehabil. doi: 10.1097/HTR.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yue JK, et al. Association of a common genetic variant within ANKK1 with six-month cognitive performance after traumatic brain injury. Neurogenetics. 2015;16:169–180. doi: 10.1007/s10048-015-0437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winkler EA, et al. COMT Val (158) Met polymorphism is associated with nonverbal cognition following mild traumatic brain injury. Neurogenetics. 2016;17:31–41. doi: 10.1007/s10048-015-0467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hygen BW, et al. Child Exposure to Serious Life Events, COMT, and Aggression: Testing Differential Susceptibility Theory. Dev Psychol. 2015 doi: 10.1037/dev0000020. [DOI] [PubMed] [Google Scholar]
- 51.Wagner S, et al. The catechol o-methyltransferase (COMT) val158met polymorphism modulates the association of serious life events (SLE) and impulsive aggression in female patients with borderline personality disorder (BPD) Acta Psychiatr Scand. 2010;122:110–117. doi: 10.1111/j.1600-0447.2009.01501.x. [DOI] [PubMed] [Google Scholar]
- 52.Young RM, et al. Harmful Drinking in Military Veterans with Post-Traumatic Stress Disorder: Association with the D2 Dopamine Receptor a1 Allele. Alcohol Alcohol. 2002;37:451–456. doi: 10.1093/alcalc/37.5.451. [DOI] [PubMed] [Google Scholar]
- 53.Shin LM, Rauch SL, Pitman RK. Amygdala, Medial Prefrontal Cortex, and Hippocampal Function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 54.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 55.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang C-h, Grace AA. Amygdala-ventral palidum pathway modulates the down-regulation of dopamine activity following unpredictable chronic mild stress in rats. Biol Psychiatry. 2014;76:223–230. doi: 10.1016/j.biopsych.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bombardier CH, et al. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA J Am Med Assoc. 2010;303:1938–1945. doi: 10.1001/jama.2010.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Juengst SB, Arenth PM, Wagner AK. Biopsychosocial outcomes in the first year after traumatic brain injury: behavior, depressive symptoms, and self-perception. Arch Phys Med Rehabil. 2015 In Press. [Google Scholar]
- 59.Pardini M, et al. Aggression, DRD1 polymorphism, and lesion location in penetrating traumatic brain injury. CNS Spectr. 2014;19:382–390. doi: 10.1017/S1092852914000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lawford BR, Young R, Noble EP, Kann B, Ritchie T. The D2 dopamine receptor (DRD2) gene is associated with co-morbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. Eur Psychiatry J Assoc Eur Psychiatr. 2006;21:180–185. doi: 10.1016/j.eurpsy.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Bilder RM, Volavka J, Lachman HM, Grace AA. The Catechol-O-Methyltransferase Polymorphism: Relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- 62.Freedman ML, et al. Assessing the impact of population stratification on genetic association studies. Nat Genet. 2004;36:388–393. doi: 10.1038/ng1333. [DOI] [PubMed] [Google Scholar]
- 63.Goldman D, et al. DRD2 Dopamine Receptor Genotype, Linkage Disequilibrium, and Alcoholism in American Indians and Other Populations. Alcohol Clin Exp Res. 1993;17:199–204. doi: 10.1111/j.1530-0277.1993.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 64.Hosák L. Role of the COMT gene Val158Met polymorphism in mental disorders: A review. Eur Psychiatry. 2007;22:276–281. doi: 10.1016/j.eurpsy.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 65.Udekwu P, Kromhout-Schiro S, Vaslef S, Baker C, Oller D. Glasgow Coma Scale score, mortality, and functional outcome in head-injured patients. J Trauma. 2004;56:1084–1089. doi: 10.1097/01.ta.0000124283.02605.a5. [DOI] [PubMed] [Google Scholar]
- 66.Carvalho JO, Ready RE, Malloy P, Grace J. Confirmatory factor analysis of the Frontal Systems Behavior Scale (FrSBe) Assessment. 2013;20:632–641. doi: 10.1177/1073191113492845. [DOI] [PubMed] [Google Scholar]
- 67.Malloy P, Grace J. A review of rating scales for measuring behavior change due to frontal systems damage. Cogn Behav Neurol Off J Soc Behav Cogn Neurol. 2005;18:18–27. doi: 10.1097/01.wnn.0000152232.47901.88. [DOI] [PubMed] [Google Scholar]
- 68.Fann JR, et al. Validity of the Patient Health Questionnaire-9 in assessing depression following traumatic brain injury. J Head Trauma Rehabil. 2005;20:501–511. doi: 10.1097/00001199-200511000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patton MH, Bizup BT, Grace AA. The infralimbic cortex bidirectionally modulates mesolimbic dopamine neuron activity via distinct neural pathways. J Neurosci Off J Soc Neurosci. 2013;33:16865–16873. doi: 10.1523/JNEUROSCI.2449-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry. 2010;15:918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, Zou H, Michael A, Wagner A. Chronic Methylphenidate Treatment Restored Spontaneous Dopamine Transients in the Striatum of Freely Moving Rats - Google Scholar. J Neurotrauma. 2011;28:A105. [Google Scholar]
- 73.Kobori N, Clifton GL, Dash PK. Enhanced catecholamine synthesis in the prefrontal cortex after traumatic brain injury: implications for prefrontal dysfunction. J Neurotrauma. 2006;23:1094–1102. doi: 10.1089/neu.2006.23.1094. [DOI] [PubMed] [Google Scholar]
- 74.Wagner AK, et al. The influence of genetic variants on striatal dopamine transporter and D2 receptor binding after TBI. J Cereb Blood Flow Metab. 2014;34:1328–1339. doi: 10.1038/jcbfm.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chaytor N, Schmitter-Edgecombe M. The Ecological Validity of Neuropsychological Tests: A Review of the Literature on Everyday Cognitive Skills. Neuropsychol Rev. 2003;13:181–197. doi: 10.1023/b:nerv.0000009483.91468.fb. [DOI] [PubMed] [Google Scholar]
- 76.Milders M, Fuchs S, Crawford JR. Neuropsychological Impairments and Changes in Emotional and Social Behaviour Following Severe Traumatic Brain Injury. J Clin Exp Neuropsychol. 2003;25:157–172. doi: 10.1076/jcen.25.2.157.13642. [DOI] [PubMed] [Google Scholar]
- 77.Lee FJS, et al. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 2007;26:2127–2136. doi: 10.1038/sj.emboj.7601656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ciliax BJ, et al. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guedes VA, Song S, Provenzano M, Borlongan CV. Understanding the pathology and treatment of traumatic brain injury and posttraumatic stress disorder: a therapeutic role for hyperbaric oxygen therapy. Expert Rev Neurother. 2016;16:61–70. doi: 10.1586/14737175.2016.1126180. [DOI] [PubMed] [Google Scholar]
- 80.McAllister TW, Flashman LA, McDonald BC, Saykin AJ. Mechanisms of Working Memory Dysfunction after Mild and Moderate TBI: Evidence from Functional MRI and Neurogenetics. J Neurotrauma. 2006;23:1450–1467. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- 81.Beaulieu J-M, Gainetdinov RR. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 82.Baune BT, et al. Association of the COMT val158met Variant with Antidepressant Treatment Response in Major Depression. Neuropsychopharmacology. 2007;33:924–932. doi: 10.1038/sj.npp.1301462. [DOI] [PubMed] [Google Scholar]
- 83.Benedetti F, et al. Effect of catechol-O-methyltransferase Val(108/158)Met polymorphism on antidepressant efficacy of fluvoxamine. Eur Psychiatry. 2010;25:476–478. doi: 10.1016/j.eurpsy.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 84.Bosia M, et al. Influence of catechol-O-methyltransferase Val158Met polymorphism on neuropsychological and functional outcomes of classical rehabilitation and cognitive remediation in schizophrenia. Neurosci Lett. 2007;417:271–274. doi: 10.1016/j.neulet.2007.02.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


