Abstract
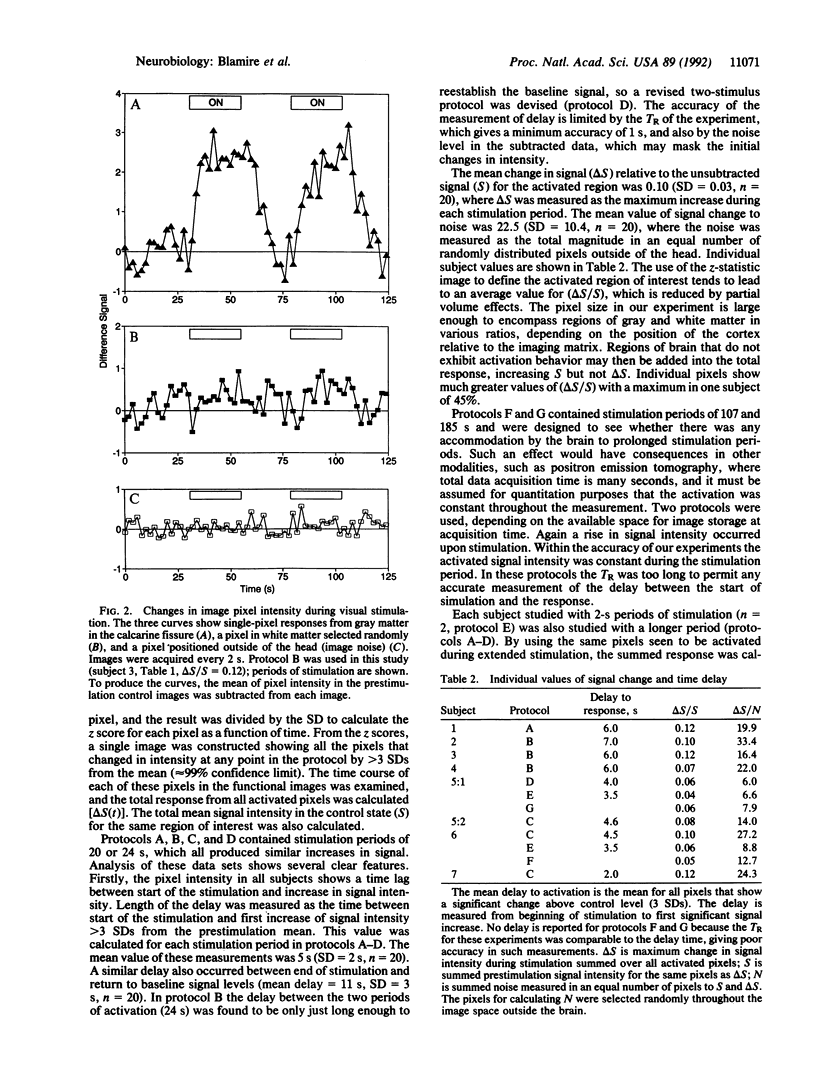
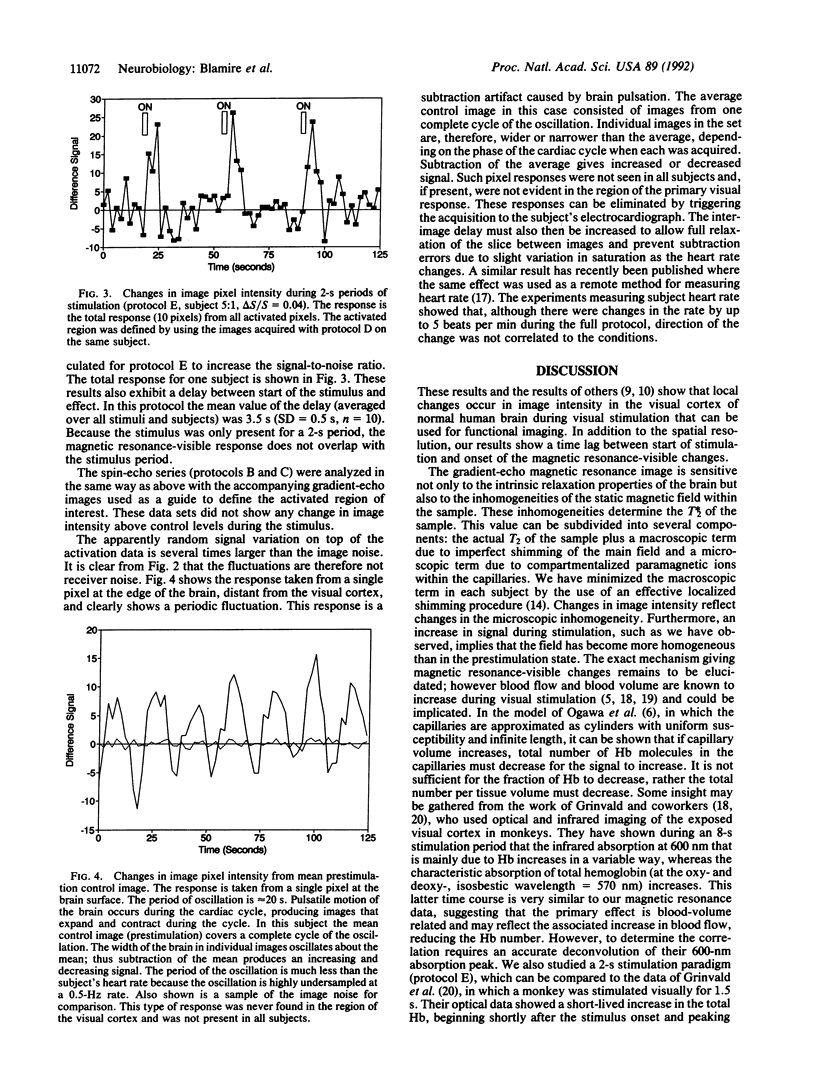
We report the use of high-speed magnetic resonance imaging to follow the changes in image intensity in the human visual cortex during stimulation by a flashing checkerboard stimulus. Measurements were made in a 2.1-T, 1-m-diameter magnet, part of a Bruker Biospec spectrometer that we had programmed to do echo-planar imaging. A 15-cm-diameter surface coil was used to transmit and receive signals. Images were acquired during periods of stimulation from 2 s to 180 s. Images were acquired in 65.5 ms in a 10-mm slice with in-plane voxel size of 6 x 3 mm. Repetition time (TR) was generally 2 s, although for the long flashing periods, TR = 8 s was used. Voxels were located onto an inversion recovery image taken with 2 x 2 mm in-plane resolution. Image intensity increased after onset of the stimulus. The mean change in signal relative to the prestimulation level (delta S/S) was 9.7% (SD = 2.8%, n = 20) with an echo time of 70 ms. Irrespective of the period of stimulation, the increase in magnetic resonance signal intensity was delayed relative to the stimulus. The mean delay measured from the start of stimulation for each protocol was as follows: 2-s stimulation, delay = 3.5 s (SD = 0.5 s, n = 10) (the delay exceeds stimulus duration); 20- to 24-s stimulation, delay = 5 s (SD = 2 s, n = 20).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandettini P. A., Wong E. C., Hinks R. S., Tikofsky R. S., Hyde J. S. Time course EPI of human brain function during task activation. Magn Reson Med. 1992 Jun;25(2):390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Belliveau J. W., Kennedy D. N., Jr, McKinstry R. C., Buchbinder B. R., Weisskoff R. M., Cohen M. S., Vevea J. M., Brady T. J., Rosen B. R. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991 Nov 1;254(5032):716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E., Mintun M. A., Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988 Jul 22;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E. Stimulus rate determines regional brain blood flow in striate cortex. Ann Neurol. 1985 Mar;17(3):303–305. doi: 10.1002/ana.410170315. [DOI] [PubMed] [Google Scholar]
- Frostig R. D., Lieke E. E., Ts'o D. Y., Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Lieke E., Frostig R. D., Gilbert C. D., Wiesel T. N. Functional architecture of cortex revealed by optical imaging of intrinsic signals. 1986 Nov 27-Dec 3Nature. 324(6095):361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Kim W. S., Cho Z. H. Cardiac cycle extraction from projection data using static signal suppression. Magn Reson Med. 1992 Mar;24(1):182–188. doi: 10.1002/mrm.1910240121. [DOI] [PubMed] [Google Scholar]
- Kwong K. K., Belliveau J. W., Chesler D. A., Goldberg I. E., Weisskoff R. M., Poncelet B. P., Kennedy D. N., Hoppel B. E., Cohen M. S., Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEIER P., ZIERLER K. L. On the theory of the indicator-dilution method for measurement of blood flow and volume. J Appl Physiol. 1954 Jun;6(12):731–744. doi: 10.1152/jappl.1954.6.12.731. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Lee T. M., Kay A. R., Tank D. W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Lee T. M., Nayak A. S., Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990 Apr;14(1):68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Tank D. W., Menon R., Ellermann J. M., Kim S. G., Merkle H., Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S. E., Fox P. T., Posner M. I., Mintun M., Raichle M. E. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988 Feb 18;331(6157):585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Rosen B. R., Belliveau J. W., Vevea J. M., Brady T. J. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990 May;14(2):249–265. doi: 10.1002/mrm.1910140211. [DOI] [PubMed] [Google Scholar]
- Turner R., Le Bihan D., Moonen C. T., Despres D., Frank J. Echo-planar time course MRI of cat brain oxygenation changes. Magn Reson Med. 1991 Nov;22(1):159–166. doi: 10.1002/mrm.1910220117. [DOI] [PubMed] [Google Scholar]



